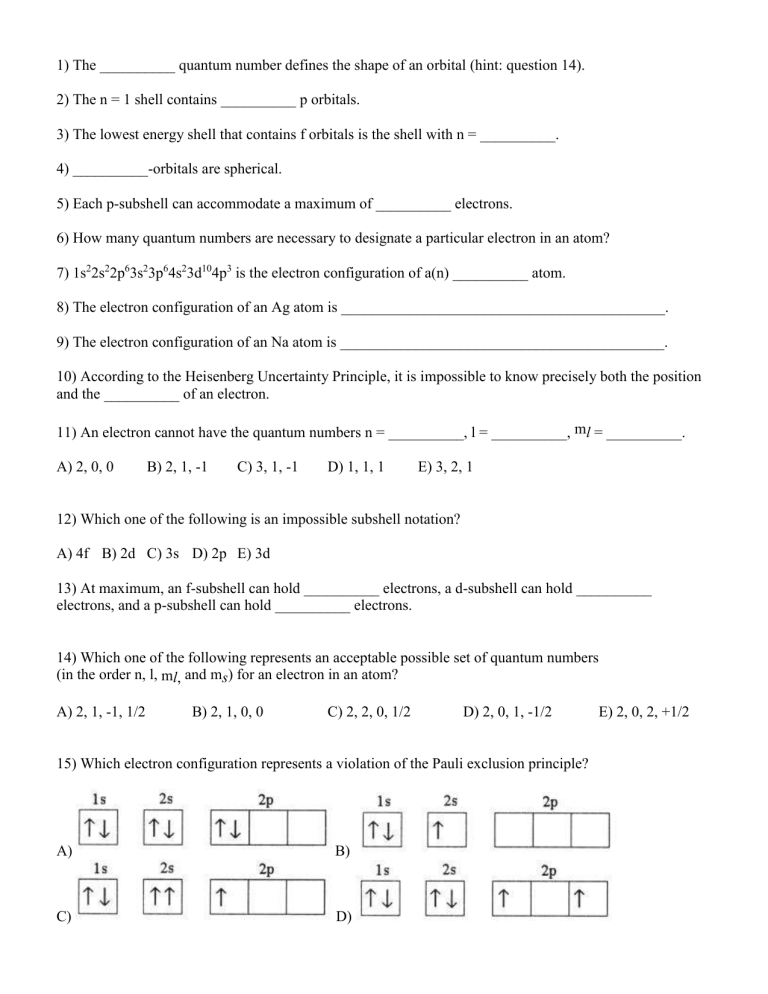
1) The __________ quantum number defines the shape of an orbital (hint: question 14). 2) The n = 1 shell contains __________ p orbitals. 3) The lowest energy shell that contains f orbitals is the shell with n = __________. 4) __________-orbitals are spherical. 5) Each p-subshell can accommodate a maximum of __________ electrons. 6) How many quantum numbers are necessary to designate a particular electron in an atom? 7) 1s22s22p63s23p64s23d104p3 is the electron configuration of a(n) __________ atom. 8) The electron configuration of an Ag atom is ___________________________________________. 9) The electron configuration of an Na atom is ___________________________________________. 10) According to the Heisenberg Uncertainty Principle, it is impossible to know precisely both the position and the __________ of an electron. 11) An electron cannot have the quantum numbers n = __________, l = __________, ml = __________. A) 2, 0, 0 B) 2, 1, -1 C) 3, 1, -1 D) 1, 1, 1 E) 3, 2, 1 12) Which one of the following is an impossible subshell notation? A) 4f B) 2d C) 3s D) 2p E) 3d 13) At maximum, an f-subshell can hold __________ electrons, a d-subshell can hold __________ electrons, and a p-subshell can hold __________ electrons. 14) Which one of the following represents an acceptable possible set of quantum numbers (in the order n, l, ml, and ms) for an electron in an atom? A) 2, 1, -1, 1/2 B) 2, 1, 0, 0 C) 2, 2, 0, 1/2 D) 2, 0, 1, -1/2 15) Which electron configuration represents a violation of the Pauli exclusion principle? A) B) C) D) E) 2, 0, 2, +1/2 16) Which one of the following is the correct electron configuration for a nitrogen atom? A) B) C) D) 17) Draw the orbital diagram (boxes) of Ca ______________________________________________. 18) Which electron configuration represents a violation of Hund's rule for an atom in its ground state? A) B) C) D) 19) What does the Aufbau Principle state? 20) Why was the Bohr model of the atom modified/rejected? Bonus: (+1 each) 1) Of the following transitions in the Bohr hydrogen atom, the __________ transition results in the emission of the highest-energy photon. A) n = 1 → n = 6 B) n = 6 → n = 1 C) n = 6 → n = 3 D) n = 3 → n = 6 2) Write the electron configurations for the following Ions. Mg2+ O23) What are Laymen, Balmer, and Paschen Series? 4) What 2 elements have exceptions to the expected electron configuration pattern? 5) How far can a dog run into the woods? E) n = 1 → n = 4




