Helium Properties & Uses Report
advertisement

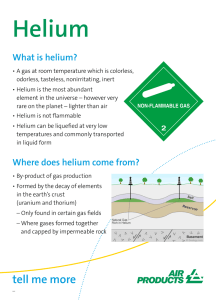
Abbie Donald 8/29/19 5th METAL, NONMETAL, METALLOID, OR GAS? • Helium is a gas. • Helium can be bought in these tanks. ATOMIC MASS AND NUMBER • Atomic mass- 4.0026 • Atomic number- 2 PROTONS, ELECTRONS, AND NEUTRONS • Protons- 2 • Electrons- 2 • Neutrons- 2 ON THE PERIODIC TABLE… • Helium is located in period 1 and group 18. ELECTRON CONFIGURATION • 1s2 HELIUM AT ROOM TEMPERATURE • Helium is odorless, tasteless, and colorless. • Helium is the only element that does not solidify under ordinary pressures and remains a liquid even at absolute 0. WHERE IS IT FOUND? • The ground • Helium can be found in certain parts of the world, notably in Texas, as a minor component in some sources of natural gas. WHO DISCOVERED IT AND HOW? • Pierre Janssen and Norman Lockyer • The first evidence of helium was observed on August 18, 1898, as a bright yellow line with a wavelength of 587.49 nanometers in the spectrum of the chromosphere of the Sun. HELIUM USES AND COMPOUNDS • Helium gas is used to inflate blimps, scientific balloons, refridgeration, as an inhert shield for arc welding, and it is used for its coolant properties because of its boiling point being close to 0. • Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons or join with anything to make covalent compounds. • Helium is the most unreactive element. HEAT CAPACITY • 3.1156 WHAT APPLICATIONS DOES IT HAVE IN INDUSTRY? • Cryogenics, cooling of superconducting magnets, and in MRI scanners. HOW IS IT USED IN THE MEDICAL WORLD? • Liquid helium is used for magnetic resonance imaging devices. HEALTH EFFECTS • Inhalation of excessive amounts can cause dizziness, nausea, vomiting, loss of consciousness, and even death. FLAMMABLE? • Helium is extremely flammable. INTERESTING FACTS • Helium is the second most abundant element in the universe. • In 1928 helium became available on the open market for the first time. • Helium is so light that Earth’s gravity is not strong enough to hold on to it. When helium atoms are released into the atmosphere, they rise until they escape into space. • Helium was discovered in the Sun’s atmosphere before it was found on Earth.