PHY 3513 Fall 1999 – Homework 2
advertisement

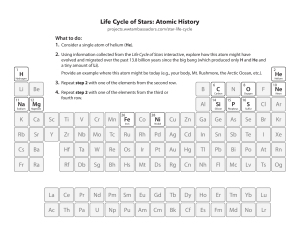
PHY 3513 Fall 1999 – Homework 2 Due at the start of class on Friday, September 10. Answer both questions. To obtain full credit, you must explain your reasoning and show all working. Please write neatly and include your name on the front page of your answers. 1. One mole of an ideal gas undergoes the following closed cycle, composed of three steps, starting from pressure P0 and volume V0 : (i) An isobaric contraction from V0 to a volume V1 = V0 /2. (ii) An isothermal expansion to a volume V2 . (iii) An adiabatic return to the starting point of the cycle. (a) Draw a P -V diagram (horizontal axis = V ) showing the path followed by the system as it undergoes the thermodynamic cycle. Be sure to label the three steps (i, ii, and iii) and draw arrows to show the direction that the system moves on the diagram. (b) Calculate the value of V2 in terms of other variables introduced above and the adiabatic exponent γ. (c) Calculate the work performed by the system during each step of the cycle and hence the total work performed during the complete cycle. (d) Evaluate the highest temperature reached during the cycle and the total work performed, given that P0 = 2.0 × 105 Pa and V0 = 1.0 × 10−2 m3 . Assume γ = 5/3. 2. Consider a container of helium gas in thermal equilibrium at a temperature of 100 K. For the purposes of this question, assume that helium is an ideal gas and take the mass of a helium atom to be 6.7 × 10−27 kg. (a) Calculate the r.m.s. velocity of the helium atoms in the gas. (b) Calculate the kinetic energy of an atom which is traveling at four times the r.m.s. velocity. (c) How far vertically above the earth’s surface would you have to raise the atom from part (b) to change its gravitational potential energy by an amount equal to the atom’s kinetic energy? Take the acceleration due to gravity to be 9.8 m/s2 (independent of height).