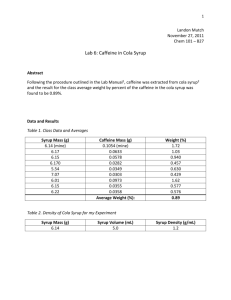
Emaree Stone Organic Chemistry October 10, 2019 Lab Partners: Cheyanne Childress, Brandon Tyler INTRODUCTION: The goal of this experiment is to extract caffeine from the cola syrup, this will be accomplished using the microscale procedure. After adding combining 5 mL of cola syrup with 5 mL of water into a centrifuge tube, 1 mL of concentrated ammonium hydroxide will beaded to the mixture. After these substances are combined, they will be allowed to separate into different layers. This process will be repeated 2 more times with the addition of trichloromethane. After allowing the mixture to separate out into layers, a pipette will be used to extract the caffeine, and the resulting caffeine will be measured to gain a rough weight (page 17). NOTEBOOK REFERENCE: Pages 17-19 CHEMICAL EQUATIONS: There were no chemical equations in this experiment. OBSERVATIONS: When combining 5 mL of water, 5 mL of cola syrup, 1 mL of ammonium hydroxide, and 1.5 mL of trichloromethane into a centrifuge tube, there was an immediate separation of substances that could be identified. The substance that rose to the top of the centrifuge tube was the dark tinted cola syrup, while a clear substance sank to the bottom of the centrifuge tube was a combination of the ammonium hydroxide and trichloromethane. DATA: Chemical Name Ammonium Hydroxide Trichloromethane Cola Syrup Water Caffeine (Page 17, 19) Starting Amount 1.0 mL 1.5 mL 5.0 mL 5.0 mL Not Measurable Ending Amount Not required to measure Not required to measure Not required to measure Not required to measure 0.3 mL CALCULATIONS: There were no calculations preformed in this experiment. DISCUSSION OF RESULTS: The amount of caffeine extracted from 5 mL of cola syrup was a total of 0.3 mL, and these results were not necessarily expected. With that amount of cola syrup, the expectation was that a greater amount of caffeine would be able to be extracted and measured; however, that was not the case. With a ending amount of 0.3 mL of caffeine, there were several humans errors that could have led to the low amount of caffeine gathered such as: sucking up syrup into the pipette, completing the extraction process a total of 4 times instead of 3, and the anhydrons chloride calcium pellets being absorbed by the mixture. Each of these factors have the potential to cause the data to be skewed (page 19).