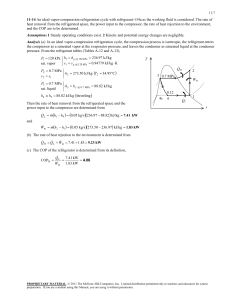
Lab Handout 2019 05 26 Chun-Ti Chang Performance Assessment for a Refrigerated Water Bath Introduction This experiment examines the performance of a refrigerated water bath. The machine is constructed based on vapor-compression cycle, and the speed of its compressor is adjustable. In experiment, you will tune the cooling load for each different compressor speeds to settle the machine to steady state. Then you measure the refrigerant’s temperature and pressure at designated locations to quantify the state of the refrigerant. Together with input power and cooling load, you can then deduce the coefficient of performance (COP) and mass flow rate of the refrigerant. Finally, you will comment on the machine’s performance based on your thermodynamic analysis. Equipment A refrigerated water bath, a motor controller, a personal computer, a variable AC transformer (variac), a heating rod, a submersible water pump, 4 thermocouples, a voltmeter, 2 thermometers, 4 pressure gauges, a clamp meter and a power analyzer. Description Our refrigerated water bath runs a vapor compression cycle, as figure 1 shows. In the cycle, the refrigerant is compressed to its highest pressure and temperature (P1, T1) in the cycle as it leaves the compressor at location 1. Then the refrigerant is chilled and condensed as it flows through the condenser. The refrigerant becomes a saturated liquid when it reaches location 2. For absorbing heat, the refrigerant passes through an expansion device and reaches its lowest pressure and temperature (P3, T3) in the cycle at location 3. The refrigerant absorbs heat as it flows through the evaporator. It vaporizes as it exits the evaporator, at location 4. The cold, low-pressure refrigerant is then pressurized by the compressor again, and the cycle repeats. This experiment quantifies the machine’s performance when both the room temperature Tr and the water temperature Tw are fixed. The air conditioners in the lab will hold Tr fixed, and the machine reaches a steady state when Tw is also kept constant. In experiment, the machine’s performance is quantified as you successfully maintain Tw constant. This is done by adjusting the compressor power Win and cooling load. Practically, you can control Win by tuning the speed of the compressor. The faster the compressor spins, the faster it consumes energy, and the faster it removes heat. As the compressor runs, Tw will keep dropping if no heat is supplied. To keep Tw constant, you will supply heat to the water bath as the cooling load to the machine. The heat is applied with a regular heating rod. You will adjust the heating power by tuning the AC voltage through a variac. Meanwhile, you will monitor the actual heating power through a power analyzer. Your heating power equals QL when the machine is settled to a steady state. Then you calculate the machine’s COP as COP = QL ÷ Win Intuitively, QL grows with Win. But how does COP vary with Win? You will figure this out from your measurement and analysis. 1 Lab Handout 2019 05 26 Chun-Ti Chang You can run the experiment in two ways. You can prescribe a series of compressor speeds first. Then you find the corresponding heating power that balances the machine’s refrigeration capacity for each compressor speed. Alternatively, you can prescribe several heating powers and figure out how fast the compressor has to spin to remove the injected heat. Either way, you shall obtain very similar performance curves for our refrigerated water bath. QH P2 , T2 2 condenser expansion device Tr 1 P1 , T1 compressor Win water bath 3 P3 , T3 evaporator QL Tw 4 P4 , T4 Figure 1. Schematic of our refrigerated water bath. Arrows: flow direction of refrigerant. Win: power consumed by the compressor. QL and QH: heat absorbed and rejected by the refrigerant, respectively. Tr: room temperature. Tw: water temperature in the bath. P1 – P4: pressures at locations 1 – 4; T1 – T4: temperatures at locations 1 – 4. Pressure gauges and thermocouples are installed at locations 1 – 4 on our machine. In experiment, you will measure P1 – P4 and T1 – T4 each time you reach a steady state. The measurements directly reveal the refrigerant’s state at these locations. Additionally, they allow you to assess the performance of our refrigerant under each steady state. With P1 – P4 and T1 – T4, you can calculate the refrigerant’s enthalpies at these locations. According to figure 1, QL is proportional to the enthalpy change between locations 3 and 4, and Win to that between 4 and 1. Therefore, the refrigerant’s coefficient of performance COPr is COPr = (h4 – h3) ÷ (h1 – h4), where hi is the enthalpy at location i (i = 1, 2, 3, 4). You will compare COPr to the COP you obtained from Win and QL. Then you comment on how they differ and what causes any difference. It should be noted that our machine utilizes R-134a refrigerant. Relevant thermodynamic properties are available from your thermodynamic textbook or miniRefProp.exe (by National Institute of Standards and Technology or NIST, USA). Refer to the appendix of this document for the basics of using the program. Refer to figure 2 for the photos of our refrigerated water bath. The photo to the left is the lower portion of the machine. The compressor is the black cylinder ‘A’. In front of the compressor is our expansion device, a roll of capillary tube ‘B’. The condenser is fan-and-tube, crossflow heat 2 Lab Handout 2019 05 26 Chun-Ti Chang exchanger ‘C’. Finally, the evaporator is the galvanized copper tubing in the water bath, marked ‘D’ in the photo to the right. For this experiment, pressure gauges are connected to locations 1 – 4 via copper tubing. Thermocouples are attached to these locations. It is your job to trace the refrigerant pipeline and figure out which gauge / thermocouple measures which pressure / temperature. Finally, a thermometer will be partially immersed in the water tank to measure Tw, and another placed in front of the condenser for Tr. Figure 2. Refrigerated water bath and its major components. Left figure is the lower part of the water bath in the right figure. A: comporessor. B: capillary tube. C: condenser. D: evaporator. Operational Instructions 1: temperature measurement 1. In this experiment, temperatures are measured with a voltmeter. The voltage V (in mV) and temperature (in ℃) are related by V = aT – b. For each thermocouple, write down its ID (on the wire itself) and find its constants a and b in the following table: ID 1 2 3 4 5 6 7 8 a 0.0558 0.0547 0.0532 0.0537 0.0557 0.0550 0.0532 0.0536 b 1.644 1.610 1.494 1.566 1.643 1.509 1.444 1.536 ID 9 10 11 12 13 14 15 16 a 0.0585 0.0560 0.0531 0.0527 0.0512 0.0538 0.0555 0.0556 b 1.620 1.562 1.434 1.429 1.407 1.489 1.503 1.488 Operational Instructions 2: AC power measurement 1. Turn the knob to “A” for current measurement. 2. Hook the clamp onto different wires of the power cable and identify the one with the highest current. 3. Turn the knob to “1ψ2W” (single-phase, two-wire) for power measurement. 4. Touch the screws of the L1 and N terminals on the motor controller with the voltage probes. 3 Lab Handout 2019 05 26 Chun-Ti Chang 5. Read off and record the true power from the clamp meter. Safety Instructions Refrigerated water bath: You are encouraged to carefully look at what’s underneath the water bath. But NEVER touch anything there without the permission of the TA or the instructor. Power measurement: Be careful with the in- and output terminals of the variac and motor controller: Do not short them when you take any measurement. Heating rod: Keep at least 2/3 of its metal part immersed to avoid any damage to the heaters. Additionally, the heating rod only takes up to 110V AC. For safety, you are only allowed to supply up to 100V AC to the heating rod with the variac. (100V AC is way more than what you need for this experiment. In fact, you shall see Tw keep rising regardless of how fast the compressor spins. With 100V AC, you already supply way more heat than what the machine can remove.) Preparation 1. Identify points 1 – 4 in the refrigerated water bath. Then identify which pressure gauge / thermocouple measures which pressure / temperature. 2. On the motor controller’s control box, turn the knob fully counterclockwise. Then turn on the compressor by switching on ‘ST’ and ‘F’. Let the compressor run for 10 minutes. 3. Turn on the valves connected to the pressure gauges. 4. Plug the power cord of the submersible water pump to the power outlet. 5. Start Combivis v.5 software on the computer. Make sure the parameter ru00 is “forward constant” and ru01 “1800 1/min.” Report to TA if either of these doesn’t happen. 6. Talk to your group member and decide whether you want to prescribe the compressor speed or the heating power (voltage). Then follow the experimental procedure A or B accordingly. 7. Record Tw and Tr. Experimental Procedure A: prescribe compressor speed 1. Set your compressor to 1800 rpm. 2. Carefully tune the variac to keep Tw constant. 3. If Tw stays constant for 5 minutes, record the heating power. Calculate COP. 4. Measure the voltages of the thermocouples at points 1 – 4. Calculate T1 – T4. 5. Record the pressures P1 – P4. 6. Measure the true power consumed by the compressor with a clamp meter. 7. Record Tr. 8. Enter T1 – T4 and P1 – P4 into miniRefProp to calculate the enthalpies h1 – h4 and COPr. 4 Lab Handout 2019 05 26 Chun-Ti Chang 9. Repeat 1 – 7 with compressor speed 2400, 3000, 3600, 4200, 4800 and 5400 rpm. Experimental Procedure B: prescribe heating power 1. Set your heating power to 300 W. 2. Carefully tune the compressor speed to keep Tw constant. 3. Follow 3 – 8 in procedure A. 4. Repeat 1 – 3 with heating power 350, 400, 450, 500, 550 and 600W. Data Processing & Discussion 1. Plot QL, COP and COPr against the compressor speed. Identify the optimal operating speed for our machine. 2. For each test you performed, estimate the mass flow rate ṁ = Win ÷ (h1 – h4). Then plot ṁ against compressor speed. 3. Identify the states of the refrigerant at points 1 – 4 for each compressor speed / heating power. Are the states close to what you expected? Anything wrong? Pre-Lab Questions 1. What are the main components in a vapor compression cycle? 2. What are the states of the refrigerant at locations 1 – 4 in figure 1? 3. We looked inside a window air conditioner in the class. Sketch the fans for its (a) evaporator and (b) condenser. 4. Sketch the structure of a rotary compressor and describe how it works. 5. What’s the metal cylinder connected to the inlet of a rotary compressor? Sketch what’s inside and describe how it works. 6. Draw a simple diagram to illustrate how thermocouples work. 7. Describe what apparent, reactive and true powers are. 8. Draw a simple diagram to illustrate how you would measure the true power consumed by an AC motor. 5 Lab Handout 2019 05 26 Chun-Ti Chang Appendix: Note on the Use of miniRefProp This appendix is not meant to be a comprehensive tutorial for the miniRefProp. It is provided to help you quickly pick up the basics of using it. Follow the steps and you shall be able to process your temperature and pressure measurements as you run your experiment. The software is available at https://trc.nist.gov/refprop/MINIREF/MINIREF.HTM. It will be installed on all computers in the lab. 1. The software allows you to pick the units and fluid properties you want to work with: Figure A1 The Option menu of miniRefProp. 2. After picking your favorite units, you might also want to click on Use Gage Pressure below so that you can directly plug P1 – P4 you measured directly into miniRefProp. You will have to convert P1 – P4 into absolute pressure otherwise. 6 Lab Handout 2019 05 26 Chun-Ti Chang Figure A2 The Select Units dialog box in miniRefProp. 3. You don’t need every property available from miniRefProp. Just pick what you need: Figure A3 The property selection dialog box. 4. Make sure you pick the correct fluid to work with: In the Substance menu, select Pure Fluid (Single Compounds). Then pick R134a(1, 1, 1, 2-tetrafluoroethane) in the Select Fluid dialog box that pops up next. Figure A4 The Substance menu in miniRefProp. 7 Lab Handout 2019 05 26 Chun-Ti Chang 5. The easiest way to calculate thermodynamic properties is perhaps to click on Specified State Points in the Calculate menu. Once you’ve done so, a spreadsheet-like table will show up, you will enter any two known properties of a fluid to calculate all others you selected in step 4. Figure A5 The Calculate menu in miniRefProp. 8