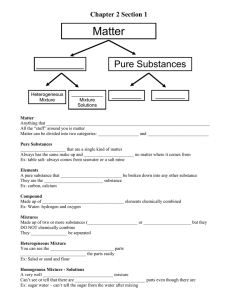
Name________________________ Period ___________ Date__________ Elements, Compounds and Mixtures 1. Classify each of the following as elements (E), compounds (C), Heterogeneous mixture (HM) or homogenous mixture (S). Write the letter X if it is none of these. __Diamond (C) __Water (H2O) __Dry Ice (CO2) __Sugar (C6H12O6) __Alcohol (CH3OH) __Baking Soda (NaHCO3) __Milk __Pail of Garbage __Titanium (Ti) __Air _Ammonia (NH3) __Iron (Fe) __Sulfuric Acid (H2SO4) __Salt (NaCl) __Popcorn and seeds __Gasoline __Noodle Soup __Gold (Au) __Krypton (K) __Wood __Book __Bismuth (Bi) __Salt Water __A dog __Uranium (U) __Ink __Concrete 2. Match each diagram with its correct description. Diagrams will be used once. A B C D _C_ Pure Element – only one type of atom present. __ Mixture of two elements – two types of uncombined atoms present. __ Pure compound – only one type of compound present. __ Mixture of two compounds – two types of compounds present. __ Mixture of a compound and an element. E 3. Read each description and determine whether it is a pure substance or mixture. Then further classify the matter (element, compound, homogeneous mixture, heterogeneous mixture) Description 1. Chocolate syrup is added to milk and stirred 2. Copper metal (used to make wires) 3. Sand is added to water 4. Distilled water 5. Tap water Pure Substance or Mixture? Mixture Pure substance Mixture Pure substance Mixture Classification? Homogenous mixture (solution) Element Heterogeneous Mixture Compound Heterogeneous mixture 6. Diamond Pure substance Element 7. Table sugar Pure substance Compound 8. Table sugar added to a cup of coffee and stirred Mixture Homogeneous mixture (solution) 9. Kool-aid is added to water Mixture Homogeneous mixture (solution) 10. Coca-cola Mixture Homogeneous mixture 11. Helium gas (used to inflate a balloon) Pure substance Element 12. Mercury metal (used in old thermometers) Pure substance Element 13. Hydrogen gas (an explosive gas) Pure substance Element 14. Trail mix (peanuts, pretzels and m&m's) 15. The air we breathe Mixture Heterogeneous mixture Mixture Homogeneous mixture (solution) 4. Define each separation technique. Then come up with 3 everyday examples for each. a. Filtration is____________________________________________________ _____________________________________________________________ i. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ iii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ b. Crystallization is____________________________________________________ _____________________________________________________________ i. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ iii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ c. Distillation is____________________________________________________ _____________________________________________________________ i. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ iii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ d. Chromatography is__________________________________________________ _____________________________________________________________ i. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ iii. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 5. Classify the following changes as Chemical or Physical Changes, and provide a reason for your answer: Change Chemical or Physical Reason You cut your hair Physical It’s still hair Mixing sugar and water Physical Even if sugar is dissolved in water, it’s still the same substance. No new substance is created. Making a peanut, pretzel and cereal mixture Physical Still peanuts, pretzels and cereal Baking soda reacts with vinegar and forms a gas Chemical Difficult to reverse, gas is formed A piece of metal is bent in half Physical Able to bend it back to its original form Methanol is burned and leaves a residue Chemical Unable to reverse New substance is formed An aspirin is crushed into fine powder Physical Changing from solid into powder but still aspirin Copper turns green when exposed to the environment Chemical Colour change, new substance is formed Two clear liquids are mixed and a yellow color forms Chemical Colour change Baking cookies Chemical Hard to reverse Diamonds are used to scratch glass Physical Can fix the glass, still glass A tree burns to form ashes Chemical Can’t reverse this, new substance is formed (soot) Water freezes to form ice Physical Can reverse by melting Glass Breaking Physical Can fix the glass, still glass Water evaporates into steam Physical Reverse by condensation, still water 6. It wouldn’t make much sense to measure a teardrop by the kiloliter or Mr. Cirello by the mile. Using the metric system come up with something you would measure that would be appropriate considering the prefix. Kilo Base unit milli 7. How many/much of a decade(s) are in ONE second? Must use dimensional analysis (hint: which is the given and which is the unknown?) 8. Rewrite each of the following in proper scientific notation, including only the significant figures. a. b. c. d. 60 212 000 000 : ____________________________ 0.0012305000 : _____________________________ 27185 : _____________________________ 0.0021530 : ______________________________ 9. A golden-colored cube is handed to you. The person wants you to buy it for $100, saying that is a gold nugget. You pull out your old geology text and look up gold in the mineral table, and read that its density is 19.3 g/cm3. You measure the cube and find that it is 2 cm on each side, and weighs 40 g. What is its density? Is it gold? Should you buy it?