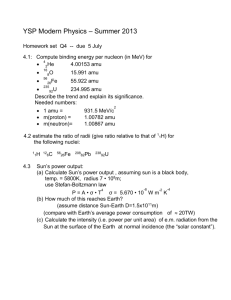
SI Session 1 Handout SI Leader: Whitney Stidham CHEM 1030 DATE: 9/4/2019 1. Find new study buddies! a. Exchange phone numbers with one person around you and then calculate their age in hours. b. Exchange phone numbers with a different person around you and then calculate their height in nanometers. 2. The chemical formula for caffeine is C8H10N4O2. . What is the empirical formula? 3. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon’s reported atomic mass, which isotope exist as the most abundant in nature? Explain. 4. A pool is filled with 375,000,000 mL of water and this water weighs approximately 37,500 kg. Calculate the density of water in English units (lb/ft^3) HINT: 1000L = 1m3 5. A sample of naturally occurring silicon consists Si-28 (amu = 27.9769), Si-29 (amu = 28.9765) and Si-30 (amu = 29.9738). If the atomic mass of silicon is 28.0855 and the natural abundance of Si-29 is 4.67%, what are the natural abundances of Si-28 and Si30?