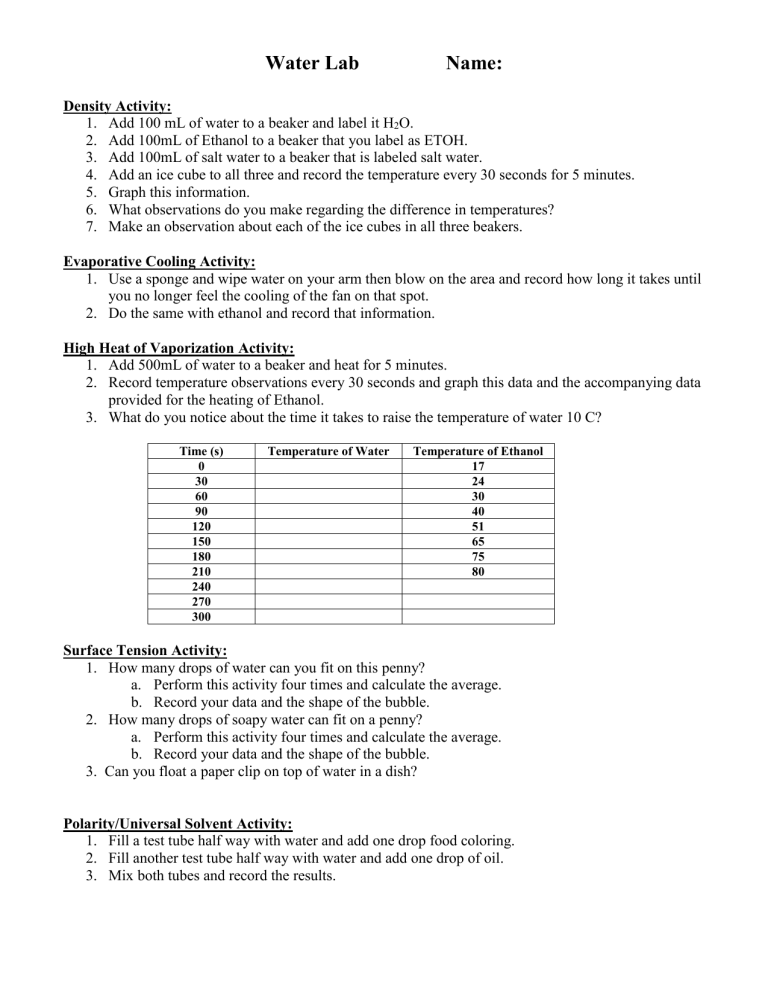
Water Lab Name: Density Activity: 1. Add 100 mL of water to a beaker and label it H2O. 2. Add 100mL of Ethanol to a beaker that you label as ETOH. 3. Add 100mL of salt water to a beaker that is labeled salt water. 4. Add an ice cube to all three and record the temperature every 30 seconds for 5 minutes. 5. Graph this information. 6. What observations do you make regarding the difference in temperatures? 7. Make an observation about each of the ice cubes in all three beakers. Evaporative Cooling Activity: 1. Use a sponge and wipe water on your arm then blow on the area and record how long it takes until you no longer feel the cooling of the fan on that spot. 2. Do the same with ethanol and record that information. High Heat of Vaporization Activity: 1. Add 500mL of water to a beaker and heat for 5 minutes. 2. Record temperature observations every 30 seconds and graph this data and the accompanying data provided for the heating of Ethanol. 3. What do you notice about the time it takes to raise the temperature of water 10 C? Time (s) 0 30 60 90 120 150 180 210 240 270 300 Temperature of Water Temperature of Ethanol 17 24 30 40 51 65 75 80 Surface Tension Activity: 1. How many drops of water can you fit on this penny? a. Perform this activity four times and calculate the average. b. Record your data and the shape of the bubble. 2. How many drops of soapy water can fit on a penny? a. Perform this activity four times and calculate the average. b. Record your data and the shape of the bubble. 3. Can you float a paper clip on top of water in a dish? Polarity/Universal Solvent Activity: 1. Fill a test tube half way with water and add one drop food coloring. 2. Fill another test tube half way with water and add one drop of oil. 3. Mix both tubes and record the results. Name ___________________________ Water Lab Results Density Activity: 1. Temperature observations: Water 2. Graph 3. Ice cube observations: Water Ethanol Salt Water Ethanol Salt Water 4. Density: Based on the observations, what can be concluded in terms of density in each of the three beakers? Evaporative Cooling Activity: 1. Does the water or the ethanol produce a longer cooling sensation? 2. What can be concluded about the evaporative cooling of water? High Heat of Vaporization Activity: 1. Results: Time (s) 0 30 60 90 120 150 180 210 240 270 300 2. Graph. Temperature of Water Temperature of Ethanol 17 24 30 40 51 65 75 80 3. What do you notice about the time it takes to raise the temperature of water 10 C? Why does this happen? 4. What happens to the ethanol? Surface Tension Activity: How many drops of water can you fit on this penny? 1st Trial 2nd Trial 3rd Trial 4th Trial Average How many drops of soapy water can fit on a penny? 1st Trial 2nd Trial 3rd Trial 4th Trial Average Diagrams of bubbles: Water Soapy Water Polarity/Universal Solvent Activity: 1. Does the food coloring mix with the water? 2. Does the oil mix with the water? 3. Water is the universal solvent, so explain your results. Why does one dissolve or mix and the other one does not? **Were you able to float the paper clip on the water? What property of water does this exhibit? Testing pH: Solution pH level



