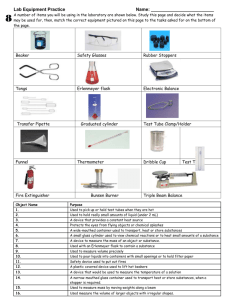
Pan- American School Chemistry Ms. Castillo Experiment 1: Fundamental Operations in the Laboratory. 1. Mass determination: The scales are the instruments intended to determine the mass of a body or a substance. They are characterized by their accuracy, precision and sensitivity. Accuracy refers to the property that any physical instrument possesses to supply the result of a measurement with a value that most closely matches the true one; This implies that the error is as small as possible. The term accuracy is often taken as equivalent to precision, however, precision is the similarity between a series of measurements made in the same way by the analyst. Sensitivity is determined by the ability to accurately determine results of very small values, and can be expressed as the difference between extreme values of several measures of the same magnitude. In general, in all chemical analysis methods it is necessary to determine the exact mass (weighing) at some stage, and for this purpose an analytical balance of precision of at least 0.1 mg is used, that is, they deliver the result with a minimum of Four decimals On other occasions it is not necessary to know the mass in such a precise way, and then granatary scales that are more resistant and of less precision are used, give the result for example with one or two decimal places. When a mass or weighing determination is to be made, the following care must be taken to avoid damaging the balance: • The substances should not be weighed directly on the balance plate. • A clean, dry container should be used to weigh the substance, such as a weighing paper, a watch glass, a beaker or a container as small as possible. • The container and the substance to be weighed must be at room temperature. You should never weigh hot items. • Place the weighing material in the center of the balance plate. • Once the material is weighed, the load must be removed from the balance plate. Never leave the balance loaded. • The balance must never be overloaded, that is, a weight greater than its weighing capacity must never be placed on it. The scales always have a maximum weighing capacity, which is always noted on the body of the same. The scales have a very important function that is the tare , this function what it does is set the balance to zero when you have a container on the balance plate, so that you can eliminate the weight of the container and therefore directly grants the weight of the object or the substance that is placed in said container. 2. Volume determination: One of the most commonly used materials in the Laboratory is volumetric equipment, which in addition to measuring a volume can be used to contain or pour a given volume. The equipment to contain are those where you can store a liquid for a certain time such as erlenmeyer, volumetric ball, among others. Pouring equipment is what is used to measure a volume such as burette, test piece, among others. Beakers and erlenmeyers may have printed graduation marks, but these containers only give a "raw" appreciation, that is, just an idea, of the volume and should never be used when measurement accuracy is required. The beaker is a laboratory material that is used to contain liquids or substances, in order to dissolve, heat, cool, etc. Its main objective is to contain various liquids or chemical substances of different types, to be able to pour them into other equipment. It allows to obtain precipitates from the reaction of other substances, it is normally used to transport liquids to other containers, but it can also be used to heat, dissolve, or prepare chemical reactions. Although the beaker contains a volume scale, it does not serve to measure approximate volumes, much less accurate , it only gives a slight idea of how much volume it contains since the error contributed by said scale is too large for this value to be useful in an analysis. The Erlenmeyer is a glass container with a narrower mouth than the bottom. It is used to mix solutions because its shape allows stirring so that they mix or react more quickly without any loss of liquid because the shape it has reduces the danger of spilling its contents. It usually has a volume scale in milliliters to give an orientation of what the contained volume may be, but as with the beaker, the error contributed by that scale is too large for this value to be useful in an analysis. The graduated cylinders or cylinders, allow to obtain the reading of an approximate volume, the pipettes and the burettes as well as the graduated balloons allow to obtain an exact volume measurement. The graduated pipettes and the graduated balloons give us only a volume value, which is determined by their capacity up to the capacity mark and is indicated on the same device. The graduated pipettes, like the burettes, allow us to determine, by means of the scale, the volume that has been exactly delivered by the instrument. To make a correct reading of a volume using a test tube, burette or pipette, the analyst's eyes are required to be at the same height as the meniscus of the liquid. as shown in figure 1, since if it is placed above or below the line of sight, the reading will be wrong. 3. Decantation, Filtration The formation of a precipitate is the most common manifestation that a chemical reaction has been performed. When two perfectly clear solutions are mixed, sometimes a precipitate is obtained, which can vary from a slight cloudiness to a solid in appreciable quantity. In this practice we will learn two techniques used to separate precipitates from the liquid where they are found, which is commonly called mother liquor. These techniques are decantation and filtration. Decanting is one of the methods of separation of mixtures used to separate solids from liquids, as well as non-miscible liquids. In the first case the solid settles (due to its greater weight), then the container is tilted and the liquid is allowed to drain carefully into another container, so that only the sedimented solid remains in the first container. When dealing with immiscible liquids, the separation is carried out by means of a special device known as the separation funnel, in which the liquids are separated by their density difference and are extracted by the key that the device has in the part lower, being able to extract first the one of greater density and later the one of lower density. It is called filtration process for separating solid particles from a liquid using a porous filter material called. The technique consists in pouring the solid-liquid mixture that is to be treated on a filter that allows the passage of the liquid but that retains the solid particles, that is to say that the size of the pores of the must be smaller than that of the minimum size of the particles of the substance to be filtered. Normally, this filter is a paper, but other materials such as cotton, semipermeable membranes, asbestos, among others can be used. Said paper is pure cellulose, unloaded and subjected to special processes according to the use to which it is intended. Thus, for example, there are those with ashes treated to carry out quantitative analyzes, resistant to acids, to bases, to filter gelatinous, thick, fine precipitates, etc. It is very important that the filter has the ability to resist the chemical action of the filter solution. Given this, as well as the pore size, you have to choose conveniently the filter paper to filter the different precipitates, since there are them with very fine grain (as is the case with barium sulfate) and with coarse grain that is the one that is commonly used in laboratory practices. Strong acids and alkalis are better filtered through glass wool or asbestos, and special membranes will be used to filter colloids. The liquid that passes through the filter is called filtering , in some cases this will be the substance of interest and in other cases, the solid that is retained in the filter is what matters. The filtration technique as such consists in pouring the solid-liquid mixture that you want to separate on a filter, for this you first place the filter paper inside the funnel and it is placed on the collection container, supported by the metal ring . The filter can be wetted with the same kind of solvent that contains the suspension. The suspension is then slowly poured onto the filter with the help of a glass rod (stirrer), so that the contents do not spill. Finally, the solid particles retained in the filter can be washed with small portions of solvent (the same one that contains the filtered liquid), if the solid is of interest. 3. Bunsen burner The lighter is the most commonly used device in the chemical laboratory to supply heat in laboratory experiments. The lighters are devices that consist of a metal tube with a regulated air inlet at the base and a mouthpiece of various designs in its upper part. The tufts generate heat energy by burning gas. The gases most commonly used for this are normally propane and butane. The so-called Bunsen burners are used in the laboratory, whereby the highest thermal efficiency in combustion is achieved by the gas. When the gas stream is opened and the lighter is ignited, a jet is formed inside the base of the lighter that draws air from the outside, which penetrates through the ventilation holes located above the base of the lighter. The amount of air can be regulated by rotating a ring that fits outside the lighter tube. When the holes in the ring coincide with those in the tube, the air intake is maximum. The flame of the lighter can have several temperatures, this will depend on whether the combustion of the gas is complete (reaction 1) or incomplete (reactions 2 and 3), this can be regulated by varying the air intake of the lighter. MATERIALS AND EQUIPMENT: • Sand • Water • Filter paper • Balance • Support • Bunsen burner • 250 mL Beaker • 250 mL Erlenmeyer • 100 mL test tube • Short spike funnel • Universal support with ring • Glass rod PROCESS: A- MASS MEASUREMENT: 1. Turn on the balance and wait for the mass value displayed on the scale display to stabilize at 0.00 g. If the reading is not zero, press the 0 key, so that the reading reaches this value. 2. Place a dry 250 mL beaker on the balance plate. Determine and write down your mass. 3. Turn on the balance and wait for the mass value displayed on the scale display to stabilize at 0.00 g. If the reading is not zero, press the 0 key, so that the reading reaches this value. 4. Place a dry 250 mL beaker on the balance plate. Determine and write down your mass. 5. Place a wooden cylinder inside the beaker and write down the mass value obtained. The difference between these two measures is the mass of the cylinder. Weight of the beaker with the cylinder (g) Weight of empty beaker (g) Weight of the cylinder (g) _______________________ _______________________ _______________________ 6. Place the wooden cylinder directly on the scale plate and write down the mass value obtained. 7. Replace the empty beaker and wait for the dough value to stabilize. Press the tare button (T) on the balance and observe the new value of the mass obtained. Write down what happens in your notebook. 8. Place a wooden cylinder inside the beaker and write down the mass value obtained. 9. Compare the values obtained for the mass of the cylinder at points 3,4 and 6. B- VOLUME MEASUREMENT: 1. Take a 250 mL Erlenmeyer and add water to the first division you see on the scale, usually 50 mL, write down this volume measurement. 2. With great care, transfer the water to a 100 mL test tube, which must be dry, and write down the volume obtained in it. 3. Take a 250 mL beaker and add water to the first division of the scale, usually 50 mL, write down this volume measurement. 4. With great care, transfer the water to a 100 mL test tube, which should be dry and write down the volume obtained in it. 5. Calculate the uncertainty of the Erlenmeyer, the beaker and the specimen, for this, look at the value of the minimum division (not the first division). The uncertainty corresponds to half of the minimum division and is a measure of the confidence that can be associated with a measured value. 6. Calculate the difference between the volume value obtained with the Erlenmeyer and that obtained for this same amount of liquid in the test tube. Repeat the calculation but now with the values derived from the beaker. 7. The difference obtained for the erlenmeyer is within the range given by the uncertainty? And with respect to the beaker, is the difference within the range given by the uncertainty? C- DECANTATION AND FILTRATION: 1. DECANTATION: 1. Weigh 5 g of sand in a 150 mL dry beaker and add 30 mL of distilled water, measured with a test tube. 2. Mix using a glass rod and let the sand settle. 3. Place a clean and very careful beaker, with the help of the glass rod or stirrer, transfer the liquid to the other beaker without allowing the solid to pass, it must remain in the first beaker. Do not discard decanted liquid. FILTRATION: 1. Weigh 5 g of sand in a 150 mL dry beaker and add 30 mL of distilled water, measured with a test tube. 2. Mix using a glass rod and let the sand settle. 3. Place a glass or plastic funnel in a ring holder, and a 150 mL beaker at the bottom. 4. Take a filter paper and fold it in a simple style, according to the indications given in the introduction of the practice. 5. Moisten the filter paper with distilled water using a t-shirt and press it against the funnel walls. 6. The paper must be completely adhered to the inner surface of the funnel without air bubbles. 7. Take another 150 ml beaker and place it on the bottom of the funnel. 8. Shake the beaker containing sand, using a glass rod. Pour the contents into the funnel, being careful to place the rod in the beaker's beak. See the figure. 9. Compare the liquid obtained by decantation with the one you just obtained, which one is cleaner? D- BUNSEN Burner: 1. In accordance with the instructions given by your laboratory professor, proceed to light the lighter as indicated below. 2. Verify that the gas key that leads directly to the lighter is closed, as is the lighter key. 3. Proceed to verify that the gas tap of the Laboratory, as well as the table, are open. 4. Light a match and place it on the cigarette lighter nozzle. 5. Open the gas tap on the cigarette lighter hose. 6. Carefully open the lighter key until it burns. 7. Verify that the flame size is not very large. You should never leave the screen. The flame must have a bluish color. 8. Turn off the lighter, first closing the wrench and then the lighter hose. 9. Finally, the table and laboratory key must be closed, but to do this, verify that no partner is using the gas. Data Collection and Tables