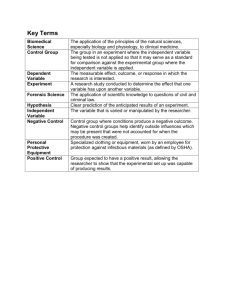
Please adjust the informed consent form here according to your study. Complete the sections in brackets (<___>) with the necessary information regarding your study. REMOVE ANY UNNECESSARY INFORMATION. <Title of Study> Description of the project The aim of this study is to find out/examine/explore <detail what your study is about here>. To participate in this study, you have to be an undergraduate student enrolled in the PSY106 Qualitative Research module at HELP University, May 2019 semester. What will be done If you agree to participate in this study, you will be required to <participate in the interview/ understanding of the portion/ this interview will take 1 hour/ audio recording, you can stop at anytime/ rest of group members will act as observer/explain steps involved in your qualitative study here including any extra procedures; remember to mention audio recording>. Risks or discomfort <minimal risks> will be anticipated in this study as a result of <discussing of personal emotional experience/ abortion/steps taken in this study>. However, you may decide to pull out anytime and you will not face any consequences. If you experience any discomfort in participating in this study, you may terminate your participation. If you’ve decided that you would not want to have your results included in this study, you may inform the researcher and your results will be excluded. CONTACTS Benefits of this study There will not be any direct benefit to you in participating in this study, but you will help the researcher to learn more about <your specific area of research for this assignment>. Confidentiality Any information collected from you during your participation in this study will be anonymous. You will not be identified as no names are required for participating in this research, and all identifying information will be de-identified in the transcription process. The data collected will only be accessed by the researchers and the supervisor of the researcher. Decision to quit at any time Participation in this study is on a voluntary basis. Even if you decided to participate in this study, you may withdraw at any time. No adverse action will be taken against you for withdrawing your participation. If you wish to terminate participation please inform the researcher immediately, so that your responses can be separated and destroyed right at that point. Rights and complains This study has been reviewed and approved by the Ethics Review Board, Department of Psychology, Faculty of Behavioral Sciences at HELP University. For any research related problems or questions regarding participants’ rights, please contact the chairperson of the Ethics Review Board. I understand that this research study has been reviewed and approved by the Ethics Review Board, Department of Psychology, Faculty of Behavioral Science at HELP University. For research related problems or questions regarding participants’ rights, I can contact: Chairperson Ethics Review Board Department of Psychology Faculty of Behavioral Science Level 3, Block B HELP University Subang 2 Persiaran Cakerawala Seksyen U4 40150 Shah Alam Selangor Malaysia Phone: 03-78493000 For any enquiries of this particular study, please feel free to contact the researcher via the email address as stated below: Researcher <List your name and contact details here, including your email> Researcher’s Supervisor Karuna Thomas karuna.s@help.edu.my Thank you. I have read and understand the explanation provided to me. I have had all my questions answered to my satisfaction, and I voluntarily agree to participate in this study. ________________________ Signature of Participant ______________________ Date