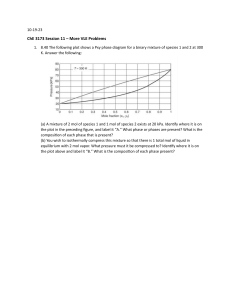
1- Concentrated binary solution containing mostly species 2 (but x= 0.10 bar. 2 ≠ 1) is in equilibrium with a vapor phase containing both species 1 and 2. The pressure of this two-phase system is 1 bar; the temperature is 298.0K. Determine from the following data good estimates of x1 and y1. H1= 200 bar; P2 sat = 0.1 bar. 2- What is the composition of the vapor that is in phase equilibrium at 300 K with a binary, non-ideal liquid mixture for which x1=0.4? At 300 K, the saturation pressure are 0,7 atm for component 1 and 0,2 atm for component 2. Use the single-component Margules equation A=2 to model the non-ideal liquid. 3- A vapor that is 65 mol% benzene and 35 mol% toluene is in equilibrium with a liquid mixture of the same two spiecs. The absolute pressure in the system is 150 mm Hg. Estimate the composition of the liquid and the system temperature? 4- For binary system at 50 C and 1.4 bar, the mole fractions of component 1 in the liquid phase is 0.4, and the vapor phase 0.7. The saturation pressures at 50 C are P1sat=1 bar and P2sat =1.2 bar. What vapor phase composition would be in equilibrium at 50 C with a liquid whose mole fraction of component 1 is 0.8? 5- A binary vapor phase mixture is to be compressed at fixed temperature until the vapor completely condenses. The vapor contains 30 mol% of component 1 and 70 mol% of component 2. The saturation pressures at the temperature of the system are 0.82 bar and 1.93 bar, respectively. If is also known, that the bubble pressure of 50:50 mixture of components 1 and 2 is 1.08 bar. Estimate the pressure required to completely liquefy the 30:70 mixture, assuming that the excess Gibbs free energy of the liquid is modeled by the one-parameter Margules equation. 6- Calculate the bubble temperature for a binary system Acetone(1)/ Methanol(2). The mole fraction of component 1 in the liquid phase is 0.4 and the pressure is 70 kPa. Saturation pressures are according to Antoine, where T in C and P in kPa. The liquid phase is non-ideal and its activity coefficient can be determined by using Margules equation. A=0,708 and B=0,69