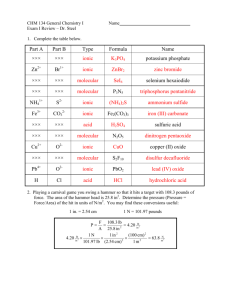
ChemWorks #1 Chapters 1-4, and 19.2 Chemistry 101 S. Bakshandeh 1. A 14-karat gold ring contains 58.30% gold by mass and weighs 12.41g. If gold sells $1232.00 per ounce, what is the value of gold in this ring? a. $464 b. $540 c. $924 d. $235.6 e. $314.4 f. None of these. The correct answer is $____________ 2. Which of the following occupies the most volume? (Look up any information you may need to solve for this problem, and state your source) a. 200 mL of methanol b. 1.36 kg of mercury c. 5.07 fluid ounce of water d. 4.50 lb of gold 3. An atom has 28 neutrons. Its cation with a +3 charge has 21 electrons. What is the symbol for this ion? 49 3+ 3+ 3+ 3+ 3+ a. 25 b. 28 c. 28 d. 52 e. 21 𝑆𝑐 28𝑁𝑖 21𝑆𝑐 24𝐶𝑟 24𝐶𝑟 4. An element has 5 stable isotopes. The mass and percentage of each are: 89.9043 51.46% 90.9053 11.23% 91.9046 17.11% 93.9061 17.40% 95.9082 2.80% The element is which of the following? a. Nb b. Y c. Zr d. Sr e. Rb 5. The name of which compound ends in “ite”? a. HIO3 b. Na2SO3 c. H2SO3 d. Na2CO3 e. CaH2 6. The name of which compound ends in “ate”? a. HBrO3 b. H2SO3 c. Na2SeO3 d. Na3AlO3 e. Na2S 7. The name of which compound does NOT end in ‘ide”? a. HCl b. HCN c. Fe(CN)2 d. NaSCN e. NF3 8. When a sample of 6.853 mg of a hormone was burned in a combustion analysis, 19.73 mg CO2 and 6.391 mg of H2O were obtained. The compound was found to contain only C, H, and N, hand have a formula mass of 290. What is the empirical formula? What is the molecular formula? a. C9H21O/ C18H42O2 b. C10H16O/ C20H18O2 c. C19H30O2 / C19H30O2) d. C9H21O/ C18H42O2 e. Need more information 9. Nitroglycerin, can be prepared by carefully controlled reaction of glycerol with nitric acid, according to the equation, C3H8O3 + 3 HNO3 → C3H5N3O9 + 3 H2O What mass of nitric acid is required for the production of 2.8 g of nitroglycerin, if the process has a 67% yield? a. 1.9 g b. 3.4 g c. 4.2 g d. 4.5 g e. 10. g f. 1.0 g 10. Potassium permanganate reacts with potassium iodide in presence of sulfuric acid to produce elemental iodine. The unbalanced net ionic equation (NIE) for this reaction is; MnO4— + I— →Mn2+ + I2 Write the balanced net ionic equation for this reaction. Use half-reaction method and show both oxidation and reduction half-reactions. 11. Chromium in its +6 oxidation state (such as dichromate ion) is a hazardous carcinogen, while chromium (II) is harmless. One way to destruct chromium (VI) is to reduce it to chromium (II) by reacting it with a metal like zinc in presence of sulfuric acid. If 1.0 mol of potassium dichromate is mixed with 1.0 mol Zn and 500.0 mL of 2.0 M sulfuric acid, what is the limiting reagent, and what is the theoretical yield of chromium (II) sulfate? a. Zn, 0.50 mol b. K2Cr2O7, 2.0 mol c. H2O, 1.0 mol d. H2SO4, 0.29 mol e. No limiting reagent, 1.0 mol 12. Atomic mass of magnesium is 24.3050 amu and has 3 stable isotopes with masses of 23.98504, 24.98584, and 25.98259 amu, respectively. The natural abundance of Mg—25 is found to be 10.13%. What are the percentages of the other two isotopes? a. 73.70% and 16.17 % b. 78.93% and 10.94% c. 32.24% and 57.63% d. 78.70% and 21.30% e. 44.94% and 44.93% f. 83.63% and 15.36% 13. 4.573 g of a compound of carbon, hydrogen, oxygen, and silver was burned in air, yielding 6.152 g of CO2, 0.8996 g H2O, and a mixture of silver and silver oxide. Because the production of this mixture yielded an indeterminate value for amount of silver, another sample of the compound weighing 8.125 g was reacted with a solution of NaCl, yielding 5.085 g of AgCl. What is the empirical formula of the compound? a. C5H5OAg b. C7H5O2Ag c. C7H7O2Ag d. C8H5OAg e. C6H6OAg