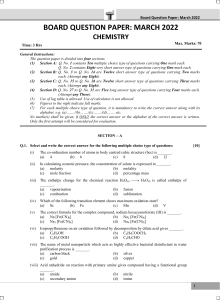
Assignment-2-Thermodynamics-questions Q.1. Look at each process and decide wheather there is greater disorder after the change than before. If so, the entropy increases and the value of ΔS is positive. If the final solution is more ordered, then the value of ΔS is negative. [3 Marks] a). Diffusion of perfume through a room. b). Sublimation of dry ice CO2(s) → CO2(g). c). Ag+ (aq) + Cl-→ AgCl (s) Q.2. Calculate the entropy change in surroundings when 1.00 mol of H2O (l) is formed under standard conditions, ΔfH = -286 kJmol-1 [2 Marks] Q.4. Industrially, methanol is synthesized using the reaction CO(g) + 2 H2(g) ↔ CH3OH(g) Calculate the equilibrium constant at 298 K. ΔGfo CO(g) = −137.2 kJ/mol; ΔGfo H2 (g)= 0 ; ΔGfo CH3OH(g) = -162 kJ/mol [3 marks]