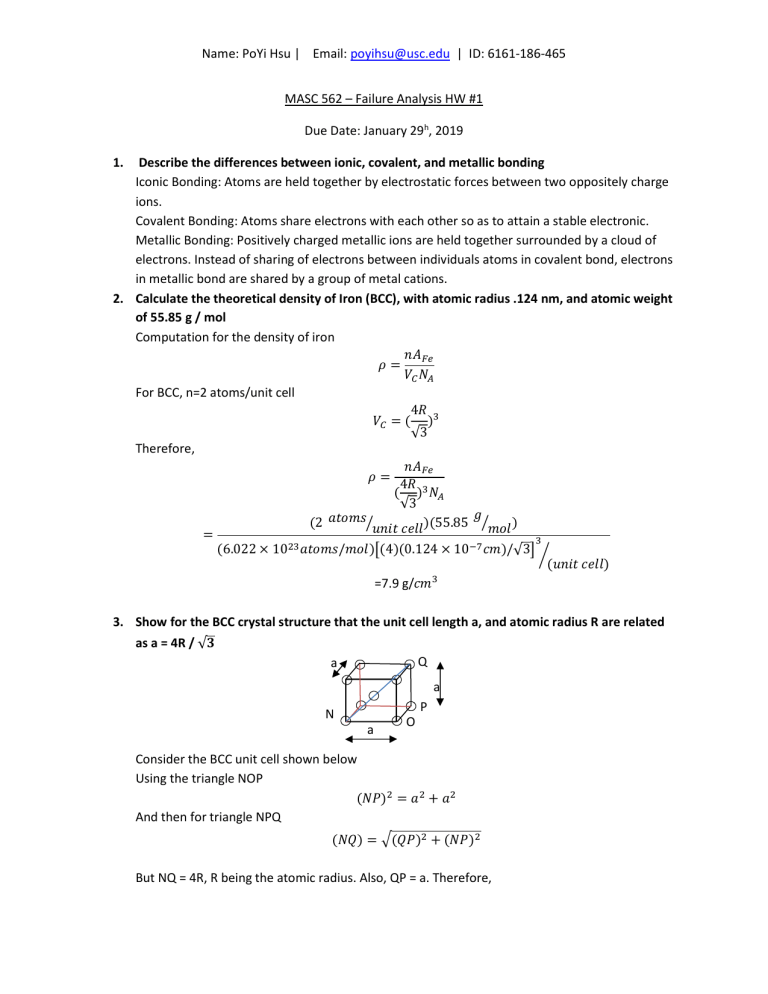
Name: PoYi Hsu | Email: poyihsu@usc.edu | ID: 6161-186-465 MASC 562 – Failure Analysis HW #1 Due Date: January 29h, 2019 1. Describe the differences between ionic, covalent, and metallic bonding Iconic Bonding: Atoms are held together by electrostatic forces between two oppositely charge ions. Covalent Bonding: Atoms share electrons with each other so as to attain a stable electronic. Metallic Bonding: Positively charged metallic ions are held together surrounded by a cloud of electrons. Instead of sharing of electrons between individuals atoms in covalent bond, electrons in metallic bond are shared by a group of metal cations. 2. Calculate the theoretical density of Iron (BCC), with atomic radius .124 nm, and atomic weight of 55.85 g / mol Computation for the density of iron 𝑛𝐴 𝜌= 𝑉𝑁 For BCC, n=2 atoms/unit cell 4𝑅 𝑉 =( ) √3 Therefore, 𝑛𝐴 𝜌= 4𝑅 ( ) 𝑁 √3 𝑔 𝑎𝑡𝑜𝑚𝑠 (2 )(55.85 𝑚𝑜𝑙 ) 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 = (6.022 × 10 𝑎𝑡𝑜𝑚𝑠/𝑚𝑜𝑙) (4)(0.124 × 10 𝑐𝑚)/√3 (𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙) =7.9 g/𝑐𝑚 3. Show for the BCC crystal structure that the unit cell length a, and atomic radius R are related as a = 4R / √𝟑 Q a a N a O P Consider the BCC unit cell shown below Using the triangle NOP (𝑁𝑃) = 𝑎 + 𝑎 And then for triangle NPQ (𝑁𝑄) = (𝑄𝑃) + (𝑁𝑃) But NQ = 4R, R being the atomic radius. Also, QP = a. Therefore, Name: PoYi Hsu | Email: poyihsu@usc.edu | ID: 6161-186-465 (4𝑅) = 𝑎 + 2𝑎 or 𝑎= 4𝑅 √3 4. Why is the grain boundary energy lower for a low angle grain boundary when compared to a high angle grain boundary? The small-angle grain boundary energy is lower than for a high-angle one because more atoms bond across the boundary for the small-angle, and, thus, there are fewer unsatisfied bonds. 5. Why are HCP metals more brittle than FCC and BCC metals? The HCP crystal structure has a lower degree of symmetry than cubic crystal structures; this lower symmetry provides fewer active slip systems and, in general, lower ductility in HCP structures. The BCT structure lacks the symmetry of the BCC and FCC cubic structures and the close-packed arrangement of the FCC structure. We expect the BCT structure to have fewer active slip systems and lower ductility (higher brittleness) compared to BCC and FCC crystals. 6. Describe the main strengthening techniques for metals and alloys (1) Solid solution strengthening: Solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element to the crystalline lattice of another element, forming a solid solution[1]. (2) Work hardening Work hardening is the strengthening of a metal or polymer by plastic deformation. This strengthening occurs because of dislocation movements and dislocation generation within the crystal structure of the material [2]. (3) Precipitation hardening Precipitation hardening is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminum, magnesium, nickel, titanium, and some steels and stainless steels[3]. 7. Why do alloys of eutectic composition form microstructures consisting of alternating layers of solid phases? Upon solidification, an alloy of eutectic composition forms a microstructure consisting of alternating layers of the two solid phases because during the solidification atomic diffusion must occur, and with this layered configuration the diffusion path length for the atoms is a minimum. Name: PoYi Hsu | Email: poyihsu@usc.edu | ID: 6161-186-465 8. What is precipitation hardening? Precipitation hardening, also called age or particle hardening, is a heat treatment process that produces uniformly dispersed particles within a metal's grain structure. These particles hinder dislocation motion and thereby strengthen the metal, particularly those that are malleable. Name: PoYi Hsu | Email: poyihsu@usc.edu | ID: 6161-186-465 Reference [1] https://en.wikipedia.org/wiki/Solid_solution_strengthening [2] https://en.wikipedia.org/wiki/Work_hardening [3] https://en.wikipedia.org/wiki/Precipitation_hardening

