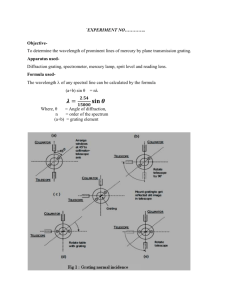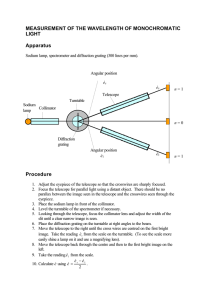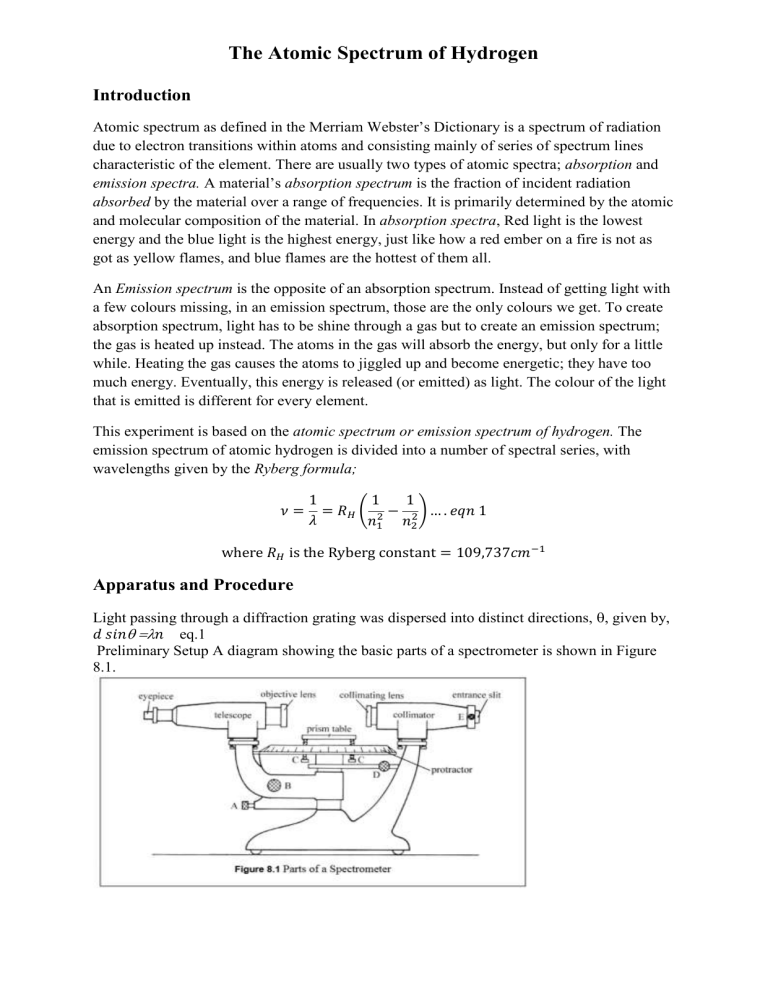
The Atomic Spectrum of Hydrogen Introduction Atomic spectrum as defined in the Merriam Webster’s Dictionary is a spectrum of radiation due to electron transitions within atoms and consisting mainly of series of spectrum lines characteristic of the element. There are usually two types of atomic spectra; absorption and emission spectra. A material’s absorption spectrum is the fraction of incident radiation absorbed by the material over a range of frequencies. It is primarily determined by the atomic and molecular composition of the material. In absorption spectra, Red light is the lowest energy and the blue light is the highest energy, just like how a red ember on a fire is not as got as yellow flames, and blue flames are the hottest of them all. An Emission spectrum is the opposite of an absorption spectrum. Instead of getting light with a few colours missing, in an emission spectrum, those are the only colours we get. To create absorption spectrum, light has to be shine through a gas but to create an emission spectrum; the gas is heated up instead. The atoms in the gas will absorb the energy, but only for a little while. Heating the gas causes the atoms to jiggled up and become energetic; they have too much energy. Eventually, this energy is released (or emitted) as light. The colour of the light that is emitted is different for every element. This experiment is based on the atomic spectrum or emission spectrum of hydrogen. The emission spectrum of atomic hydrogen is divided into a number of spectral series, with wavelengths given by the Ryberg formula; 𝜈= 1 1 1 = 𝑅𝐻 ( 2 − 2 ) … . 𝑒𝑞𝑛 1 𝜆 𝑛1 𝑛2 where 𝑅𝐻 is the Ryberg constant = 109,737𝑐𝑚−1 Apparatus and Procedure Light passing through a diffraction grating was dispersed into distinct directions, , given by, 𝑑 𝑠𝑖𝑛 𝑛 eq.1 Preliminary Setup A diagram showing the basic parts of a spectrometer is shown in Figure 8.1. Light enters the collimator through a slit at the front of the spectrometer. The collimating lens focuses the light into a parallel beam, which then passes through either a diffraction grating or a prism placed on the prism table. After being bent through some angle, the beam of light is then viewed through the telescope, which can be rotated until the image of the slit is centred on the cross hairs. The angle that the light has been bent through can then be read on the protractor using the vernier scale attached to the telescope. As per the labelled parts in the diagram in Figure 8.1, below are the functions and how to use them respectively. A. The knob at A is used to clamp the telescope in place so that it cannot be moved. It should always be loosened when large adjustments to the telescope’s position are made. B. The knob at B is used to finely adjust the position of the telescope. Knob A must be clamped before the fine adjustments can be made. C. The knobs at C can be used to level the prism table with respect to the optical axis. Consult with your lab instructor before attempting this. D. The knob at D is used to clamp the protractor as well as the prism (and grating) table. It must be clamped when angular readings are taken with the combination of the protractor and vernier scales. E. The knob at E is used to adjust the width of the slit at the front of the collimator. One edge of the slit remains fixed on the optical axis, the other edge is adjustable. The initial adjustment of the spectrometer consists of adjustments to the telescope and the collimator. First, adjust the eyepiece of the telescope so that the cross hairs are sharply focused. Next, swing the telescope to one side and point it at some distant object. (Take it out into the hall.) Adjust the telescope lens until the object is as sharply focused as possible and eliminate parallax between the image and the cross hairs. Once these adjustments have been made they should not be touched for the rest of the experiment. Next, place a light in front of the entrance slit of the collimator. View the slit through the telescope. Adjust the collimator lens until the image of the slit is in the plane of the cross hairs. You should use parallax focusing to accomplish this. After these adjustments have been made, the light entering the slit on the front of the col-limator will be focused into a parallel beam by the collimator lens. It will then be focused on the cross hairs of the telescope by the objective lens of the telescope. This image of the slit can then be viewed through the eyepiece. 2. Grating Spectrometer Mount the diffraction grating holder on the prism/grating table and put the diffraction grating in place. Loosen the knob D and rotate the table so that the grating is perpendicular (judged by eye) to the optical axis of the collimator. Tighten knob D to clamp the table in place. Place the sodium lamp in front of the entrance slit of the collimator. Rotate the telescope so that it is in line with the collimator axis and view the slit through the telescope. Vary the slit width while observing it through the telescope. Notice that only one side of the slit moves. When determining line positions, you should align the cross hairs with the fixed side of the slit. Start with the slit fairly wide open; you can make the slit wider for faint spectral lines to make them easier to see. If you have a problem seeing the cross hairs in the telescope, illuminate them with a light near the telescope. You will have to experiment with the positioning of the desk lamp so that it is bright enough to illuminate the cross hairs, but not so bright as to obliterate the spectral lines. Align the telescope cross hairs with the fixed side of the slit and note the protractor and vernier scale3 readings. Rotate the telescope to the left of the collimator axis and observe the lines in the sodium spectrum. Choose one easily visible line and align the cross hairs with the fixed side of the slit. Read the protractor and vernier scales. Move the telescope to the other side of the collimator axis and align the cross hairs on the same spectral line (check to make sure you are using the fixed side of the slit). Note the scale readings. The left and right deviation angles (relative to the centre reading) should agree with within 0.1º. If they do not, the grating is not perpendicular to the axis of the collimator. Correct the orientation of the grating by half of the difference and re-peat until agreement at the 0.1º level is obtained. For all the first and second order spectral lines that can be seen on both sides of the col-limator axis, record the angular positions in a table similar to the one shown. You may have to adjust the slit width for brighter and/or fainter spectral lines. The deviation angle θ is calculated by dividing the difference between the left and right scale readings by two. B. Balmers series 1. Replace the sodium lamp with the hydrogen lamp. Make sure that the center of the hydrogen bulb aligns well with the spectrometer slit. This will maximize the intensity of the spectral lines. You will need to protect your line of sight from all stray light to clearly see and measure the violet lines. It may be helpful to carefully drape black cloth around your hydrogen lamp or over your spectrometer to prevent any unneeded light from entering the spectrometer or from interfering with your ability to view the spectrum. 2. Record the angles at which each spectral line appears. You should measure both first and second orders on both the left and right sides of the zero angle of the red, violet and indigo lines, for a total of 12 measurements. Record your data in the table provided. After the experiment was conducted, the results were collected and presented as per below in the results section. Results and Discussion Data: Sodium as Standard Reference Source Order Colour Wavelength Intensity 1st Order Yellow 589nm Strong Ө1(origin) Ө2(meas) V1 281.5 304.6 23.1 = 11.55 2 V2 281.1 297.5 14.8 = 7.4 2 V3 282.0 296.8 14.8 = 7.4 2 11.55 + 7.4 + 7.4 = 8.783 3 Average Diffraction spacing (𝑑 = Ө (Ө2- Ө1)/2 𝑛ʎ ) 2 sin Ө Calculations for the Values in the Table Type equation here. Hydrogen Analysis (The visible part of the Hydrogen spectrum is called the Balmer series.) Color Ө(origin) Ө(meas) Red 281.5 304.6 Blue 281.1 297.5 Violet 282.0 296.8 Calculations for the table devӨ 23.1 2 = 11.55 14.8 2 = 7.4 14.8 2 = 7.4 ʎ n2 3 4 5 ѵ RH Discussion In order to calculate the Rydberg Constant for Hydrogen, measurements of the three Balmer Lines were obtained as per the results. The