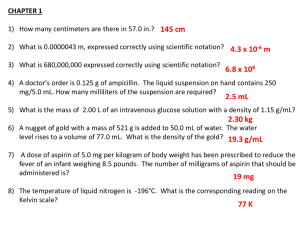
Name: Eliza Hanson Lab Partners: Janae Peterson and Jessica Szuba Date: March, April Title: Differential Scanning Calorimetry Purpose: To calibrate and standardize measurements on a differential scanning calorimeter, and use that to precisely measure melting point and specific heat capacity of a sample of aspirin Background: DSC is a type of highly precise thermal analysis, which can be used to quantitatively and qualitatively understand and characterize a chemical sample. It is the most widely used thermal analysis technique with polymers and organic materials, as well as being useful for a variety of inorganic substances as well (such as indium metal or sapphire). (Source: TA Instruments Modulated DSC Compendium) Theory: Specific Heat – 𝐸 𝛥𝐻 𝐶𝑝 = 60 𝑥 𝐻𝑟 𝑚 Cp=specific heat [J/g℃] E = cell calibration coefficient Hr = Heating rate [℃/min] ΔH = difference in y-axis deflection between sample and blank curves at temperature of interest [mW] m = sample mass Equipment list: Differential Scanning Calorimeter (2010 DSC V4.4E) TA Universal Analysis software Excel Standardized aluminum pans Analytical balance Petri dish for measurement/ sample protection Flash drive to store data Chem wipes Pellet press Sapphire standard Indium Standard Aspirin Reagents: Compound Molecular Formula Sapphire Al2O3 Indium Indium metal Aspirin C9H8O4 Molecule Structure Molecular Weight MP or BP Density (g/cm3) MSDS Indium metal 101.961 MP: 2030-2045℃ 114.818 180.157 156.6℃ 7.31 g/cm3 Dust may irritate eyes, flammable 136-140℃ 1.3 ± 0.1 g/cm3 Dust may cause irritation; Irritate skin and excessive ingestion harmful eyes, harmful if swallowed in excess All chemical data taken from chemspider.com, owned by the Royal Society of Chemistry Cp of sapphire at 330 K: 102.4290 + 38.749t – 15.912t2 + 2.628t3 − Taken from NIST webbook. 3.01 𝑡2 Procedure: Overall Objective: To perform a temperature calibration, a baseline test, a cell constant test, and then to obtain the specific heat and melting point of aspirin. Baseline: 1. Remove pans from cell and replace lids/glass bell cover 2. Check that purge has is connected and on. Choose the desired flow rate based on environmental conditions. Here, N2 flow rate was 97. 3. Select the wizard and run test under the following conditions: Begin at 50℃, heat at 10℃/min until 210℃ Calibration run/baseline Sample size: 0 mg Pan type: aluminum 4. Click apply to save parameters 5. Save file to known location 6. Select start (green arrow “play” symbol) 7. Wait for test to conclude, and save data 8. Use TA universal analysis to plot curve of line. 9. Save data and analysis. Temperature Calibration: 1. Prepare a sample pan containing approx. 10 mg of sample. For first run, choose a material with a known melting point, such as Indium. 2. Place a reference pan (blank) on the back pan platform 3. Replace DSC lids and glass bell 4. Ensure that air flow is on 5. Set instrument for calibration mode, and choose temperature. Use a ramp method 6. Use the following settings: Indium pan/ sample pan Sample size: see results Calibration/temperature calibration Temperature range: 50-210℃ Heating rate: 10℃ /min Cell constant: 1. Prepare a sample as in previous runs. 2. Settings: Calibration/cell constant Same temperature range and heating rate Indium sample/ aspirin sample Heat Capacity: Note: for aspirin, this test must be run before melting point because aspirin will decompose. 1. Create baseline profile using empty sample and reference pans 2. Settings: Conventional DSC/Ramp 50-210℃ Heating rate: 10℃ /min 3. Create a baseline run and hold starting temp for 5 minutes 4. Run a baseline run with a sample of known heat capacity 5. Super impose baseline graph 1 with sapphire graph, and measure change in y-axis heat flow values. Melting Point: 1. Run with the same parameters as the temperature calibration, this time with sample. Results, experimental details, and data: Trial 1: Baseline Trial 2: Temperature Calibration Trial 3: Cell Constant Trial 4: Indium Heat Capacity Trial 5: Aspirin Melting Point Trial 6: Sapphire Specific Heat Trial 7: Aspirin Specific Heat Data transferred from DSC run, graphs generated in excel for ease of measurement and analysis Calculations: Melting point of Indium: 156.21℃ Percent error of Indium MP: 𝑒𝑥𝑝𝑒𝑐𝑡𝑒𝑑 − 𝑚𝑒𝑎𝑠𝑢𝑟𝑒 % 𝐸𝑟𝑟𝑜𝑟 = | | 𝑒𝑥𝑝𝑒𝑐𝑡𝑒𝑑 156.6 − 156.21 | | = 0.24% 156.6 Melting point of Aspirin: 136.13 ℃ Percent error of Aspirin MP: 140 − 136.13 | | = 2.76% 140 Specific Heat of sapphire: 𝐸 𝛥𝐻 𝐶𝑝 = 60 𝑥 𝐻𝑟 𝑚 3.01 102.4290 + 38.749t – 15.911t2 + 2.628t3 − 𝑡 2 Cp taken from 57℃ measurement Cp(330K) = 92722617.7 J/mol = 9.272 * 10^7 𝐸 (3.19𝑚𝑊) 9.272 ∗ 107 𝐽/𝑚𝑜𝑙 = 60 𝑥 ℃ 22.9 𝑚𝑔 (10 ) 𝑚𝑖𝑛 𝐶𝑝 = 60 𝐸 𝛥𝐻 𝑥 𝐻𝑟 𝑚 𝐸= (9.272 ∗ 107 𝐸= 𝐶𝑝 𝑚𝐻𝑟 60∆𝐻 𝐽 1 𝑚𝑜𝑙 𝐾 ) (0.0229 𝑔 ∗ 101.961 𝑔) (0.1667 𝑠 ) 𝑚𝑜𝑙 𝐽 60(0.00319 𝑠) E= 18137 Specific Heat of Aspirin: 𝐸 𝛥𝐻 𝑥 𝐻𝑟 𝑚 𝐽 0.001665 18137 𝑆 𝐶𝑝 = 60 𝑥 𝐾 𝑚𝑜𝑙 0.1667 𝑠 0.0435 𝑔 ∗ 180.157 𝑔 𝐶𝑝 = 60 Cp = 4.5*10^6J/mol Discussion: While there were errors in the DSC lab (someone lost the first indium standard, someone misplaced our own blank, our aspirin was decomposed in the melting point test and thus we had to obtain specific heat twice), we were ultimately able to find the data we were searching for. In addition to finding specific heat of aspirin, we were able to find the melting point within 4 degrees of a common literature value. With % error values below 5%, this lab was fairly precise. Conclusion: DSC is a highly useful analytical method to determine thermodynamic properties of different compounds. The melting point determination was highly accurate, and it afforded the ability to measure the specific heat capacity of aspirin. In the future, it would be an improvement to run multiple tests on each compound, and it would be better to run the tests close together to ensure that other parties didn’t change the DSC settings or move experiment materials. In addition, a working knowledge of windows 98 would have helped immensely to curb the learning curve on the analysis portion of the lab.