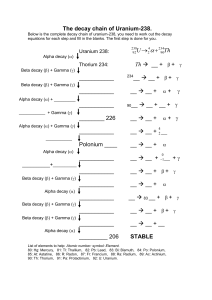
Name _______________________________ Date _____________________ Nuclear Equations Worksheet 1. Using your notes, fill out the following table for the 3 types of decay/emission: Type of Decay Consists of 2 protons and 2 neutrons Electron High-energy electromagnetic radiation α (He) β γ Mass (amu) Symbol 2. Thorium-232, named after the Norse god of thunder, is radioactive and undergoes alpha decay. What is the resulting product? A 232 4 90Th → Z ? + 2He *Note: A represents unknown mass number, and Z represents unknown atomic number Mass Number: ____ = ____ + ____ Atomic Number: ____ = ____ + ____ Product: 3. Some isotopes of iodine are used in positron emission tomography (PET) scans as a diagnostic tool for the human body. When Iodine-135 undergoes beta decay, what is the resulting product? Mass Number: Atomic Number: Product: 135 53I → A Z? + 0 −1𝛽𝛽 4. Americium-241, an alpha emitter, is used in smoke detectors. When smoke enters the ionization chamber, it absorbs the alpha particles emitted by Americium-241 and detects a change in current, setting off the alarm. Write the equation for the alpha decay of Americium241. 5. Carbon-14 is a naturally-occurring radioisotope of carbon. Radiocarbon dating is a scientific process in which the age of a deceased organism can be determined by measuring the amount of Carbon-14 present. Write the equation for the beta decay of Carbon-14. 6. Uranium-238, the most common isotope of uranium, is non-fissile, meaning it cannot be used in nuclear reactors and bombs. However, it undergoes simultaneous (at the same time) alpha and gamma emission (decay), emitting 2 different energies of gamma radiation and forming Thorium-234. Write the equation for the alpha and gamma emission of Uranium-238. 7. Fill in the missing particles or isotopes in the following equations, and then choose 2 of the equations. Write a description, like in the previous problems, of what is occurring. a. b. 129 53I → ____ + 216 86Rn 0 −1𝛽𝛽 → ____ + 42𝛼𝛼 c. ____ → 239 97Bk d. ____ → 52 23V + + 42𝛼𝛼 0 −1𝛽𝛽 e. f. g. h. 255 104Rf 85 35Br 257 101Md i.e. For a, Iodine-129 is undergoing beta decay to form ___________ . Description 2: Problem letter ___ 0 → ____ + 85 36Kr + 0𝛾𝛾 ____ → Write 2 descriptions of the above equations: Description 1: Problem letter ___ → ____ + 42He 32 16S + 0 −1𝛽𝛽 + 00𝛾𝛾 → ____ + 42𝛼𝛼 + 42𝛼𝛼