Carpal Tunnel Syndrome: Diagnosis & Pathophysiology
advertisement

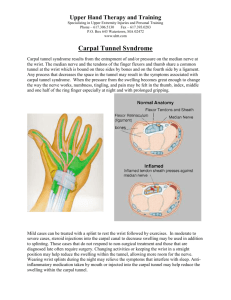
Diagnosis and pathophysiology of carpal tunnel syndrome Aaron M. Freilich and A. Bobby Chhabra Purpose of review Carpal tunnel syndrome is the most commonly reported compression neuropathy. Awareness of the syndrome has increased the frequency of diagnosis. Yet controversy remains regarding the exact mechanism of nerve compression and the existence of a true gold standard for the diagnosis of carpal tunnel syndrome. We examine recent literature regarding pathophysiology and diagnostic criteria. Recent findings Our understanding of changes in the carpal tunnel and median nerve as seen with magnetic resonance imaging and ultrasound continues to improve. These show promise, both as screening tests and to enable understanding of the causes of carpal tunnel syndrome. A clinical diagnostic standard has been proposed, and may allow better comparison among studies. Obesity and age are independent risk factors. Findings and symptoms differ depending on the population examined, with more severe findings in the elderly for given symptoms. Summary Carpal tunnel syndrome is a clinical diagnosis. Electrodiagnostic studies are frequently useful in further evaluation of cases. Alternate imaging methodologies may become useful as screening tools in the future. Causes are related to mechanical strain and increased carpal canal pressures. Noninvasive imaging is assisting in defining the anatomy and dynamic nature of the carpal tunnel with movement. Keywords carpal tunnel syndrome, compression neuropathy, diagnosis, median nerve, nerve conduction study, pathophysiology Curr Opin Orthop 18:347–351. ß 2007 Lippincott Williams & Wilkins. Department of Orthopaedic Surgery, University of Virginia, USA Correspondence to A. Bobby Chhabra, MD, University of Virginia, Department of Orthopaedic Surgery, PO Box 801016, Charlottesville, VA 22908, USA Tel: +1 434 243 0218; e-mail: Ac2h@virginia.edu Current Opinion in Orthopaedics 2007, 18:347–351 Abbreviations CTS EMG MRI NCS carpal tunnel syndrome electromyography magnetic resonance imaging nerve conduction study ß 2007 Lippincott Williams & Wilkins 1041-9918 Introduction Carpal tunnel syndrome (CTS) is the most common peripheral compression neuropathy [1]. Prevalence has been reported anywhere between 0.1–9.2% depending on the population studied, with a prevalence typically reported between 2–3% in the general population [2,3]. Patients typically present with complaints of numbness, tingling or burning in the median nerve distribution. Objective findings include positive provocative maneuvers on examination, such as Tinel’s sign, Phalen’s test, median nerve compression test, or increased latency on nerve conduction testing. These signs and symptoms most commonly result from compression of the median nerve as it travels through the relatively tight confines of the carpal tunnel at the wrist. Despite its prevalence in the population, there is no gold standard for diagnosis. Clinical and electrodiagnostic findings – electromyography (EMG) and nerve conduction study (NCS) – are both used to determine which patients have CTS. It is still up to the individual clinician to decide how to use this information. This is complicated by the fact that a portion of patients have symptoms without concurrent changes on electrodiagnostic testing. This could potentially result in misdiagnosis and poor outcomes after conservative or surgical treatment [4]. Attempts have been made recently to create a consensus clinical and diagnostic picture for accurate diagnosis of CTS. Other testing modalities, including magnetic resonance imaging (MRI) and ultrasound, have also been examined [5,6]. The pathophysiology of CTS is also unclear. While it appears to be a chronic compressive neuropathy, this does not explain symptoms outside of the typical median distribution. Several mechanisms have been proposed for compression, including dynamic changes in carpal tunnel shape or volume, spinal and supratentorial mechanisms, and tenosynovitis [1,7]. Diagnosis of carpal tunnel syndrome CTS is still a primarily clinical diagnosis. The presence of ‘classic’ symptoms of pain or paresthesias in the median nerve distribution, awakening at night, and shaking relieving symptoms, in conjunction with positive signs, such as Phalen’s or Tinel’s, results in a diagnosis of probable or clinically ‘definite’ CTS. These findings are frequently combined with NCS for confirmation. Despite this seemingly simple picture, there remains no gold standard or even true consensus among groups of physicians as to which findings are most important. 347 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 348 Hand and wrist While a median nerve distribution may be most common, symptoms may be seen in other distribution patterns with clear indication of median nerve involvement on NCS. This is further complicated by a subset of patients with clinical symptoms but normal NCS results [8,9]. Graham et al. [4] looked at criteria for diagnosis among 300 specialists, finding that both intra and intergroup consistency was poor. They suggest that it is difficult to compare outcomes among studies due to this lack of consistency. usefulness of this tool must be evaluated in larger groups of patients before its ability as a diagnostic tool is confirmed. The use of Semmes Weinstein monofilament to test touch thresholds as a method of detecting early changes associated with CTS has also been tested [15]. As an adjunct test to provocative maneuvers, such as hyperflexion, it showed some utility. Another study, however, showed it to be of little use as a screening test when compared to NCS [16]; depending on set testing threshold it had either too low a specificity or too low a sensitivity. Graham et al. [2] attempted to go a step further, and develop and validate diagnostic criteria based on consensus. They created cases and identified the criteria that statistically contributed to the model in diagnosing ‘definite’ CTS. These included numbness/tingling in the median distribution, nocturnal symptoms, weakness or atrophy, positive Tinel’s or Phalen’s signs and loss of two-point discrimination. The authors’ hope is to improve consistency in evaluating treatments or the interpretation of other studies. They did not, however, include EMG or NCS in the model. As they mention, the model has also not been tested clinically. Some promise is seen in using noninvasive imaging modalities to diagnosis CTS, such as ultrasound or MRI. Both are currently most useful in examining the median nerve for compression due to mass lesion, or in patients who have had failed surgical intervention. Diagnosis with imaging techniques relies on an estimation of either nerve swelling, via cross-sectional area or signal change, or size of the carpal tunnel. MRI, while expensive, gives high quality images of the median nerve at the carpal tunnel. Size and flattening of the nerve, and carpal tunnel parameters, can both be measured. Perhaps most useful is increased signal intensity of the nerve on T2 weighted images. This may correspond to nerve injury and increased edema in the pathologic state [17]. There is also an enlargement of the cross sectional area of the nerve just proximal to the carpal tunnel. This corresponds with the intraoperative description of an ‘hourglass’ shape to the nerve. In addition to changes seen in the nerve itself, MRI may be used to estimate palmar bowing of the transverse carpal ligament, which was shown to be associated with CTS [6]. Electrodiagnostic studies are currently used to help confirm the diagnosis of CTS. They may also help in identifying other causes of peripheral neuropathy that may be confused with CTS or contribute to symptoms in patients with the diagnosis, i.e. the ‘double crush’ phenomenon. These tests remain far from perfect, and are painful and somewhat invasive. Abnormalities on NCS result from abnormalities in conduction associated with demyelination. These are frequently later findings and, as mentioned above, clinically apparent CTS can occur in the setting of normal NCS. Identifying sensory nerve deficits may overcome this shortcoming [10]. Differences in sensory nerves result in their being affected earlier than the larger motor nerves. Another study noted a difference in motor axon recruitment of the abductor pollicis brevis in patients with mild CTS [11]. They noted that the sensitivity of EMG/NCS can be increased by looking for these changes, and that motor nerves may be affected earlier than previously thought. In addition to standard EMG/ NCS data, comparison of wrist-palm motor conduction velocity can improve diagnostic yield, as can comparison of sensory latency between the median and either the radial or ulnar nerve [12]. There is further difficulty in defining the limits of ‘normal’ for patient populations. Studies have shown that both age and obesity independently affect nerve conduction velocities [13]. Another method that has yet to be fully explored is the use of somatosensory evoked potentials (SSEP) as a diagnostic or confirmatory tool. There appear to be changes in patients with mild symptoms, although they did not correlate with NCS findings in the same study [14]. The Ultrasound may similarly be used to directly visualize the median nerve. In addition to its common use in identifying mass lesions, ultrasound can be used to calculate the crosssectional area of the carpal canal [18,19,20]. An increase in area measured at the level of the pisiform was found to correlate with the presence of CTS in one recent study [21]. In a comparison with NCS, they report a sensitivity of 91% and specificity of 84%, using a cutoff of 11 mm2. Sympathetic vasomotor fibers innervate blood vessels in a corresponding sensory territory. There is also evidence that they are affected in CTS [22]. A small study used continuous wave Doppler ultrasound to evaluate changes in response to stimulation of the radial digital artery of the index finger to that of the small finger [23]. The authors found a decrease in responsiveness in patients with CTS, although the usefulness of this in diagnosis has yet to be determined. Ultrasound and MRI imaging currently both serve a confirmatory role in diagnosis for confusing cases and those with recurrence, and have yet to be proved useful as initial screening tools. Pathophysiology While it is clear that carpal tunnel syndrome is a chronic peripheral nerve compression phenomenon, the specific Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Carpal tunnel syndrome Freilich and Chhabra 349 etiology and pathophysiology are less clear. The structural and biochemical mechanisms need to be further explored. Increases in intracanal pressure have been implicated, and sustained pressures of over 20–30 mmHg may cause damage to the median nerve by compromising blood flow [24]. Several studies have confirmed that elevations in canal pressure are present in patients with CTS, and that it correlates with clinical signs [25]. Further evidence is provided by the increase in pressure with flexion or extension of the wrist, and that maximum resting pressures occur in the early morning, corresponding with symptoms [26]. Carpal canal pressures were measured by Ikeda et al. [27] at various points. They found that pressure was highest 10 mm distal to the wrist crease and that there was a ‘slight’ (cc 0.393) relationship between sensory latency and canal pressures, and a ‘moderate’ (cc 0.402) relationship with symptom duration. Their results correlated with others that showed median nerve conduction abnormalities occurring at the same level [28]. Increases in the content of the carpal tunnel, such as tenosynovitis or synovial fibrosis, are one potential cause of an increase in canal pressure. As a mechanism for CTS, this was first proposed by Phalen [29]. One study looked at changes in the subsynovial connective tissue in the tunnel [7]. Changes consistent with chronic fibrosis and scarring from a shearing mechanism were noted in samples taken from patients with CTS. Other studies have shown similar histological changes [30]. Biomechanical studies have demonstrated that loading by palmaris longus can increase canal pressure [31]. A small case–control study by Keese et al. [32] concluded that the presence of a Palmaris longus was an independent risk factor for CTS. Another proposed mechanism is canal stenosis. There is inconsistent literature as to whether this is best measured by width or cross-sectional area, and whether it is statistically different in patients with CTS. The narrowest width was found on anatomical studies to correspond to the level of the hook of the hamate [33]. This correlates with a recent intraoperative study that found the most constricted part of the median nerve was at 2.5 cm from the distal wrist crease, at the level of the hook of the hamate [34]. A separate study measured the smallest cross-sectional area by MRI at 4 mm distal to the distal wrist crease [35]. In yet another MRI study [6], measurement showed the smallest cross-sectional area at the level of the hook of the hamate, but also an increase in canal area, as well as transverse carpal ligament bowing in patients with CTS. Bower et al. [36] demonstrated smaller carpal tunnel volumes with the wrist in extension via MRI, but the smallest area at the distal level of the tunnel in flexion. Similarly vexing is the question of extramedian symptoms in isolated CTS. While typical symptoms usually present in a classic distribution pattern, a significant minority will present with symptoms ranging from those in the ulnar distribution to whole hand glove patterns. In some series, this minority accounts for almost 40% [37,1]. Concomitant ulnar neuropathy in patients with NCS evidence of isolated CTS is unlikely as an explanation for these findings. Crossover fibers in the forearm are not an adequate explanation either, as they are more unusual than motor crossover and frequently absent on NCS [38]. Zanette et al. [1] evaluated 103 patients, and found that objective findings, i.e. thenar atrophy, were more common with typical median nerve patterns. Subjective complaints were more severe in whole-hand distributions. They hypothesize that this extramedian spread results from a combination of spinal and supraspinal mechanisms. Neuroplastic changes in the discharge of the dorsal root ganglion, may contribute, as well as changes in the thalamus or somatosensory cortex [39,40]. The contribution to CTS of obesity, repetitive labor, and age is becoming clearer. One series looked at differences in presentation of elderly patients (over 65 years of age) [41]. Older adults exhibit more severe muscle weakness and wasting when compared to a younger population, despite similar levels of symptoms. Findings on EMG/ NCS were also more marked, including prolonged distal latencies, slower conduction velocities, and reduced response amplitudes. They concluded that more attention should be paid to objective rather than subjective findings in evaluating elderly patients. Other studies have also suggested that CTS progresses more rapidly in elderly patients [42]. Obesity is another established risk factor for CTS. Bland [13] looked at two groups of patients, one with electrophysiological evidence of CTS and one without. This author found that obesity was an independent risk factor for CTS, but only in patients less than 63 years old. The study did not look at clinical symptoms, however, only electrodiagnostic evidence. Other studies have found a correlation with increased body mass index and slower median nerve conduction velocities in patients without symptoms [43]. The relationship between repetitive work activities and CTS remains controversial. A primate model tested the effects of repetitive pinch tasks on sensory nerve conduction velocity [44]. Declines of up to 30% were seen in the working hands of the primates, with values returning to within 87% of normal within weeks of the end of the task. MRI showed concurrent enlargement of the nerve in the carpal tunnel. Symptoms are of course impossible to elicit or measure in such a model. The importance of this model as it relates to humans in jobs with repetitive tasks is unclear. Conclusion CTS remains an area of active inquiry. While consensus is that it is a compression neuropathy, the exact mechanism and cause of the pathology remains illusive. In diagnosing Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 350 Hand and wrist the condition, a gold standard must be agreed upon. This will allow better comparison between studies. CTS is mainly a clinical diagnosis, with the role of EMG/NCS particularly useful for confirming the diagnosis in unclear or mild cases. Alternative noninvasive imaging modalities, such as ultrasound or MRI, may become useful as screening tests, but these are currently reserved for difficult or recurrent cases. They may also serve a useful role in better defining the dynamic and anatomic pathophysiology of CTS. References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 418–419). Zanette G, Marani S, Tamburin S. Extra-median spread of sensory symptoms in carpal tunnel syndrome suggest the presence of pain-related mechanisms. Pain 2006; 122:264–270. The cause of symptoms in CTS that do not follow the typical median distribution is discussed. This may result from spinal mediated as well as supratentorial mechanism. Subjective complaints are more common in extramedian distribution, while objective findings are more common with the typical pattern. 1 Graham B, Regehr G, Naglie G, Wright JG. Development and validation of diagnostic criteria for carpal tunnel syndrome. J Hand Surg 2006; 31:919– 924. A consensus method was used to establish a model of clinical diagnostic criteria for CTS, in order to attempt to define a true gold standard. 2 3 Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999; 282:153–158. 4 Graham B, Dvali L, Regehr G, Wright JG. Variations in diagnostic criteria for carpal tunnel among Ontario specialists. Am J Ind Med 2006; 49:8–13. This report demonstrates that there is no consensus for diagnostic criteria both between and within groups of specialists. No true gold standard exists. 5 6 Horch RE, Allmann KH, Laubenberger J, et al. Median nerve compression can be detected by magnetic resonance imaging of the carpal tunnel. Neurosurgery 1997; 41:76–82. Uchiyama S, Itsubo T, Yasutomi T, et al. Quantitative MRI of the wrist and nerve conduction studies in patients with idiopathic carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 2005; 76:1103–1108. Ettema AM, Amadio PC, Zhao C, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg 2006; 118:1413–1422. Changes in the tenosynovium of patients with CTS include fibrosis, consistent with a mechanical shear mechanism. 7 8 Braun RM, Jackson WJ. Electrical studies as a prognostic factor in the surgical treatment of carpal tunnel syndrome. J Hand Surg 1994; 19: 893–900. 9 Witt JC, Hentz JG, Stevens JC. Carpal tunnel syndrome with normal nerve conduction studies. Muscle Nerve 2004; 29:515–522. 10 Jackson DA, Clifford JC. Electrodiagnosis of mild carpal tunnel syndrome. Arch Phys Med Rehabil 1989; 69:499–501. 11 Ginanneschi F, Mondelli M, Dominici F, Rossi A. Changes in motor axon recruitment in the median nerve in mild carpal tunnel syndrome. Clin Neurophysiol 2006; 117:2467–2472. This study finds that motor fibers are affected even when conventional testing shows normal conduction. 12 Chang MH, Liu LH, Lee YC, et al. Comparison of sensitivity of transcarpal median motor conduction velocity and conventional conduction techniques in electrodiagnosis of carpal tunnel syndrome. Clin Neurophysiol 2006; 117:984–991. Improvements can be made in the diagnostic yield in testing for carpal tunnel. A flow chart is suggested in proceeding with electrophysiological testing. 13 Bland JD. The relationship of obesity, age and carpal tunnel syndrome: more complex than was thought? Muscle Nerve 2005; 32:527–532. 14 Ilkhani M, Jahanbakhsh SM, Eghtesadi-Araghi P, Moayyeri A. Accuracy of somatosensory evoked potentials in diagnosis of mild idiopathic carpal tunnel syndrome. Clin Neurol Neurosurg 2005; 108:40–44. 15 Koris M, Gelberman RH, Duncan K, et al. Carpal tunnel syndrome. Evaluation of a quantitative prevocational diagnostic test. Clin Orthop Relat Res 1990; 251:157–161. 16 Pagel KJ, Kaul MP, Dryden JD. Lack of utility of Semmes-Weinstein monofilament testing in suspected carpal tunnel syndrome. Am J Phys Med Rehabil 2002; 81:597–600. 17 Jarvik JG, Yuen E, Eliot M, et al. Diagnosis of carpal tunnel syndrome: electrodiagnostic and MR imaging evaluation. Neuroimaging Clin N Am 2004; 14:93–102. 18 El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology (Oxford) 2004; 43:887–895. 19 Ziswiler H-R, Reichenbach S, Vogelin E, et al. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome. Arthritis and Rheumatism 2005; 52:304–311. 20 Mallouhi A, Pulzl P, Trieb T, et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roengenol 2006; 186:1240–1245. Retrospective study used gray-scale and color ultrasound to predict CTS by median nerve swelling and nerve hypervascularity. 21 Wiesler ER, Chloros GD, Cartwright MS, et al. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg [Am] 2006; 31:726–732. Study comparing ultrasound to NCS in diagnosing carpal tunnel syndrome. They found good correlation between increased nerve size as measured and more severe NCS findings. 22 Aminoff MJ. Involvement of peripheral vasomotor fibres in carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 1979; 42:649–655. 23 Galea LA, Mercieca A, Sciberras C, et al. Evaluation of sympathetic vasomotor fibres in carpal tunnel syndrome using continuous wave Doppler ultrasonography. J Hand Surg [Br] 2006; 31:306–310. 24 Michelsen H, Posner MA. Medical history of carpal tunnel syndrome. Hand Clin 2002; 18:257–268. 25 Hamanaka I, Okutsu I, Shimizu K, et al. Evaluation of carpal canal pressure in carpal tunnel syndrome. J Hand Surg [Am] 1995; 20:848–854. 26 Luchetti R, Schoenhuber R, Alfarano M, et al. Serial overnight recordings of intracarpal canal pressure in carpal tunnel syndrome patients with and without wrist splinting. J Hand Surg [Br] 1994; 23:598–602. 27 Ikeda K, Osamura N, Tomita K. Segmental carpal canal pressure in patients with carpal tunnel syndrome. J Hand Surg [Am] 2006; 31:925–929. Intraoperative measurement of carpal canal pressures at various points. Increased pressures related to NCS latency and symptom duration. 28 Kimura J. The carpal tunnel syndrome: localization of the conduction abnormalities within the distal segment of the median nerve. Brain 1979; 102:619– 635. 29 Phalen GS. The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am 1966; 48:211–228. 30 Schuind F, Ventura M, Pasteels JL. Idiopathic carpal tunnel syndrome: histologic study of flexor tendon synovium. J Hand Surg [Am] 1990; 15:497–503. 31 Keir PJ, Wells RP, Ranney DA, Lavery W. The effects of tendon load and posture on carpal tunnel pressure. J Hand Surg [Am] 1997; 22:628–634. 32 Keese GR, Wongworawat MD, Frykman G. The clinical significance of palmaris longus tendon in the pathophysiology of carpal tunnel syndrome. J Hand Surg [Br] 2006; 31:657–660. 33 Cobb TK, Dalley BK, Posteraro RH, Lewis RC. Anatomy of the flexor retinaculum. J Hand Surg [Am] 1993; 18:91–99. 34 Al-Qattan MM. The anatomical site of constriction of the median nerve in patients with severe idiopathic carpal tunnel syndrome. J Hand Surg [Br] 2006; 31B:608–610. 35 Morimoto KW, Budoff JE, Haddad J, Gabel GT. Cross-sectional area of the carpal canal proximal and distal to the wrist flexion crease. J Hand Surg [Am] 2005; 30:487–492. 36 Bower JA, Stanisz GJ, Keir PJ. An MRI evaluation of carpal tunnel dimensions in healthy wrists: Implications for carpal tunnel syndrome. Clin Biomech (Bristol, Avon) 2006; 21:816–825. Carpal tunnel area and volume were calculated using MRI in various wrist positions. Out-of-plane calculations were standardized by using a correction factor. 37 Gupta SK, Benstead TJ. Symptoms experienced by patients with carpal tunnel syndrome. Can J Neurol Sci 1997; 24:338–342. 38 Kimura J. Electrodiagnosis in diseases of nerve and muscle. 3rd ed. New York: Oxford University Press. 2001. pp. 187–190. 39 Bridges D, Thompson SWN, Rice ASC. Mechanisms of neuropathic pain. Br J Anaesth 2001; 87:12–26. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Carpal tunnel syndrome Freilich and Chhabra 351 40 Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288:1765–1769. 41 Blumenthal S, Herskovitz S, Verghese J. Carpal tunnel syndrome in older adults. Muscle Nerve 2006; 34:78–83. Elderly patients have more severe muscle weakness and electrodiagnostic findings than younger patients with similar level of complaints at presentation. 42 Bland JDP, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991–2001. J Neurol Neurosurg Psychiatry 2003; 74:1674–1679. 43 Letz R, Gerr F. Covariates of human peripheral nerve function: I. Nerve conduction velocity and amplitude. Neurotoxicol Teratol 1994; 16:95– 104. 44 Sommerich CM, Lavender SA, Buford JA, et al. Towards development of a nonhuman primate model of carpal tunnel syndrome: Performance of a voluntary, repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J Orthop Res 2007; [Epub ahead of print]. Repetitive pinch task in primates demonstrated changes in NCV with return to baseline after ceasing the activity. MRI also showed changes in the median nerve. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.