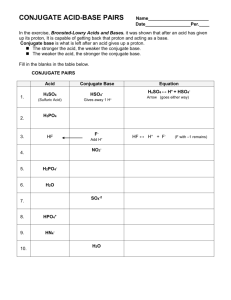
Bronsted/Lowry Theory of Acids and Bases • 1923 Danish chemist Bronsted + English chemist Lowry independently proposed new definitions for an acid and a base • These simply state that: • A base is a proton donor • An acid is a protons aceptor Consider This • When hydrogen chloride is added to water the following reaction occurs HCl Because this donates a protono it is a B/L acid + H2O Because this accepts a proton it is a B/L base H3O+ + Cl- Consider This • When ammonia gas dissolves in water the following reaction occurs NH3 Accepts a proton B/L base + H2O Donates a proton B/L acid NH4 + + OH- • Water can act as either an acid or a base and for this reason is known as amphoteric or amphiprotic Bronsted Lowry theory does not only refer to reactions where water is the solvent • In the following reaction between Hydrogen chloride and Ammonia HCl + NH3 NH4+ + Cl- Advantages of the Bronsted/Lowry Theory over Arrhenius’ Theory 1. Arrhenius’ theory was confined to aqueous solutions 2. Bronsted/Lowry’s theory broadens the range of species that can be defined as acids and bases 3. Substances that are not classified as acids or bases under Arrhenius’ theory are classified as acids or bases in the Bronsted/Lowry theory Note • An acid will only donate a proton when there is something there to accept it • A base will only accept a proton if there is something there to donate it Conjugate acid/base pairs • Certain reactions are reversible and can happen in both directions • We use the symbol to show this Consider This • If the following reaction is written as CH3COOH + H2O CH3COO- + H3O+ Ethanoic acid Water This means the reaction CH3COOH + H2O And the reaction CH3COO- + H3O+ Can take place CH3COO- + H3O+ CH3COOH + H2O Identify the B/L acid in the following Equations • CH3COOH + H2O CH3COO- + H3O+ • CH3COO- + H3O+ CH3COOH + H2O • An acid changes to a conjugate base when it donates a proton • A base changes to a conjugate acid when it accepts a proton • CH3COO- is the conjugate base of CH3COOH • CH3COOH is the conjugate acid of CH3COOEvery base has a conjugate acid and every acid has a conjugate base Since CH3COO- and CH3COOH only differ by a proton we refer to them as a conjugate acid base pair • Why can H2O and H3O+ be called a conjugate acid base pair? • NB Study examples 12.1 and 12.2 on pages 140 and 141 of your text book Neutralisation • When acids and bases react with each other in the right proportions they cancel each other out • When this happens both the acid and the base lose their characteristic properties • The solution formed is neutral and has no effect on litmus paper • This type of reaction is known as neutralisation • Neutralisation is the reaction between an acid and a base to form salt and water Reaction between HCl and NaOH HCl + NaOH H2O + NaCl Acid + Base Water + Salt • The word salt is a general term used to describe the substance formed when the hydrogen in an acid is replaced by a metal or an ammonium ion Understanding what happens • HCl + NaOH H2O + NaCl • In this equation the following ions are all present in solution H+ + Cl- + Na+ + OHNa+ + Cl- + H2O The spectator ions are crossed out as what is important is the reaction where the H+ (Or more correctly Hydronium ions )and OH- ions react to form water H + + OHH2O Everyday examples of Neutralisation 1. Medicine – Hydrochloric acid in stomachs helps digestion. Over eating and stress can produce too much acid. Antacids such as Alka Seltzer, Bisodol and Milk of Magnesia may be used to try to neutralise excess acid 2. Agriculture – If soil is too acidic crop yields can be low, therefore farmers often spread lime (CaO) on soil to neutralise the acidity 3. Environmental Protection- In areas affected by acid rain limestone is often added to lakes to neutralise acidity • Toothpaste is slightly basic to neutralise acids that cause tooth decay • Baking soda may be used to neutralise the acid of bees • Vinegar is used to neutralise the alkaline sting of wasps • Shampoo is slightly basic and can cause scales on hair to stick out, conditioner is slightly acidic so neutralises the base flattening the scales and making hair more shiny and manageable