Chemistry Solutions Test: High School Level
advertisement
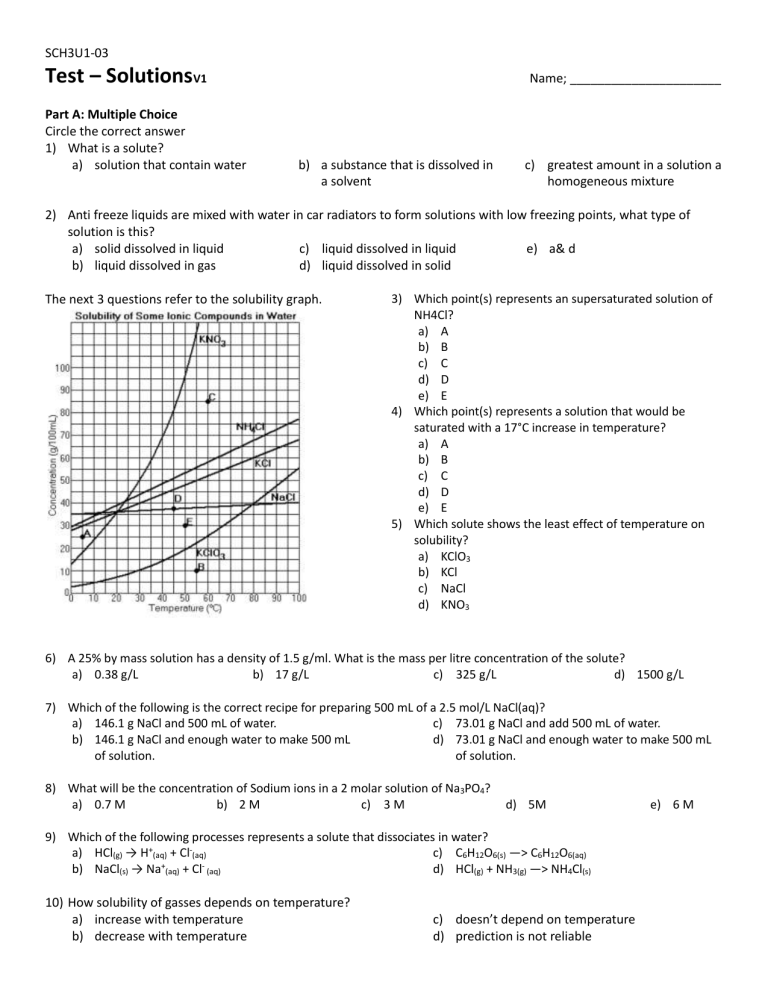
SCH3U1-03 Test – SolutionsV1 Part A: Multiple Choice Circle the correct answer 1) What is a solute? a) solution that contain water Name; ______________________ b) a substance that is dissolved in a solvent c) greatest amount in a solution a homogeneous mixture 2) Anti freeze liquids are mixed with water in car radiators to form solutions with low freezing points, what type of solution is this? a) solid dissolved in liquid c) liquid dissolved in liquid e) a& d b) liquid dissolved in gas d) liquid dissolved in solid The next 3 questions refer to the solubility graph. 3) Which point(s) represents an supersaturated solution of NH4Cl? a) A b) B c) C d) D e) E 4) Which point(s) represents a solution that would be saturated with a 17°C increase in temperature? a) A b) B c) C d) D e) E 5) Which solute shows the least effect of temperature on solubility? a) KClO3 b) KCl c) NaCl d) KNO3 6) A 25% by mass solution has a density of 1.5 g/ml. What is the mass per litre concentration of the solute? a) 0.38 g/L b) 17 g/L c) 325 g/L d) 1500 g/L 7) Which of the following is the correct recipe for preparing 500 mL of a 2.5 mol/L NaCl(aq)? a) 146.1 g NaCl and 500 mL of water. c) 73.01 g NaCl and add 500 mL of water. b) 146.1 g NaCl and enough water to make 500 mL d) 73.01 g NaCl and enough water to make 500 mL of solution. of solution. 8) What will be the concentration of Sodium ions in a 2 molar solution of Na3PO4? a) 0.7 M b) 2 M c) 3 M d) 5M 9) Which of the following processes represents a solute that dissociates in water? a) HCl(g) → H+(aq) + Cl-(aq) c) C6H12O6(s) —> C6H12O6(aq) + b) NaCl(s) → Na (aq) + Cl (aq) d) HCl(g) + NH3(g) —> NH4Cl(s) 10) How solubility of gasses depends on temperature? a) increase with temperature b) decrease with temperature c) doesn’t depend on temperature d) prediction is not reliable e) 6 M SCH3U1-03 Part B: Short Answer Answer in point form only, one sentence per point. Maximum of 4 points per answer. 1) Give three examples of aqueous solutions: one with a solid solute, one with a liquid solute, and one with a gas solute? 2) When you open a can of pop, which is more likely to fizz and spray: a can at room temperature or a cold can from refrigerator? Explain why using your understanding of gas solubility. 3) Explain why oil (non-polar substance) doesn’t dissolve well into water (polar substance) SCH3U1-03 Part C: Problem Solving Show evidence for what each number represents. Box your final answer. 1) Calculate the following: a) What mass of NaC2H3O2 must be used to create 400 mL of a 3.5% (m/v) solution? b) Water is added to 100g of Sodium Nitrate to make 0.7 L of diluted solution. What is the molarity of the new solution? c) 350 mL of a 2.5 M solution of NaCl is mixed with 1.0L of water and 500 mL of a 1.2 M solution of NaCl, what is the new concentration? 2) The task is to obtain silver from of 0.015 M solution of AgNO3. Given is 0.4 g of metal Cu. Would all silver in 1000 ml of 0.015 M solution of AgNO3 precipitate with given amount of Cu. If not, how much silver will remains in solution and how many gram of Cu should we add to obtain all silver from 1L of given solution? SCH3U1-03 3) How many grams of silver chromate will precipitate when 150. mL of 0.500 M silver nitrate are added to 100. mL of 0.400 M potassium chromate?





