Candle Lab: Gases and Fuels Worksheet
advertisement
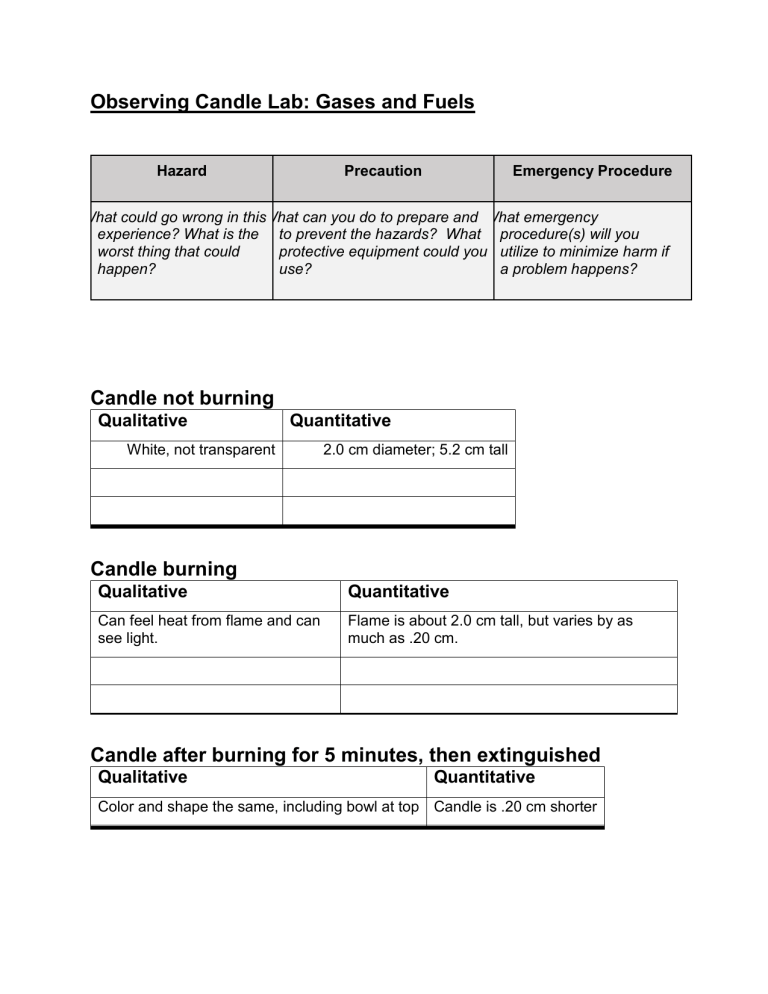
Observing Candle Lab: Gases and Fuels Hazard Precaution Emergency Procedure What could go wrong in thisWhat can you do to prepare and What emergency experience? What is the to prevent the hazards? What procedure(s) will you worst thing that could protective equipment could you utilize to minimize harm if happen? use? a problem happens? Candle not burning Qualitative Quantitative White, not transparent 2.0 cm diameter; 5.2 cm tall Candle burning Qualitative Quantitative Can feel heat from flame and can see light. Flame is about 2.0 cm tall, but varies by as much as .20 cm. Candle after burning for 5 minutes, then extinguished Qualitative Quantitative Color and shape the same, including bowl at top Candle is .20 cm shorter Question Answer to question based on video evidence Does carbon dioxide contain carbon? When magnesium burns with carbon dioxide, _________ is produced. This means ____ ________________. How does gravity affect flames? Does burning a fuel use oxygen or nitrogen from the air? Reflect and Connect 1. Water is produced in the combustion of candle wax. Where do the oxygen atoms in water come from in the reaction? Explain the logic of your answer. Feel free to use molecular sketches instead of sentences. 2. Water vapor is clear and colorless. Carbon dioxide gas is clear and colorless. Why does the candle flame have color? After your answer, list two or three objects from your everyday life that behave in a similar way. 3. Why do you need something like a match to light a candle? 4. Gasoline, biodiesel, methane are common fuels for transportation. In what phase of matter must they be for them to combust in an engine? Conclusion Questions 1. 2. 3. 4. 5. 6. What is the fuel for the combustion reaction? What phase is it? Solid, liquid, or gas? Is one of the combustion products water vapor? Does the amount of oxygen in the air make a difference? Does carbon dioxide contain carbon? How does gravity affect flames? Does burning a fuel use oxygen or nitrogen from the air?








