AP Coursework
advertisement

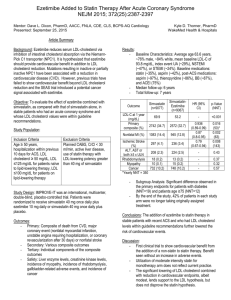
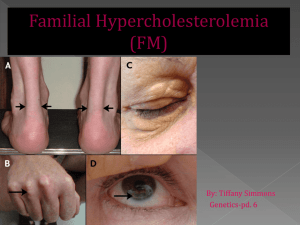
Ezetimibe as an adjunct treatment with simvastatin which she is currently on Evolocumab Colesevelam Evolocumab is a human monoclonal antibody which is a proprotein convertase subtilisin/kexin type 9 (PCSK9) enzyme inhibitor which prevents PCK9 from binding to the low density lipoprotein (LDL) receptors on the liver cell surface and thereby prevent PCSK9mediated LDL receptor degradation (Datapharm, 2018a). This therefore leads to an increase in LDL receptors in the liver and hence reduction in serum LDL. In contrast, colesevelam is a bile acid sequestrant which binds to bile acids to prevent their reabsorption from the gut thereby causing the liver to use up the cholesterol in the blood to normalise the bile acid levels. (Datapharm, 2017). Additionally, ezetimibe differs from evolocumab because ezetimibe inhibits the Niemann-Pick C1-like 1 (NPC1L1) receptor to prevent cholesterol absorption in the small intestine thereby reducing the cholesterol content of the liver (Datapharm, 2018b). These three drugs do not have similarities in terms of mechanism of action but they all serve to reduce LDL. The type of hypercholesterolemia the patient has is heterozygous familial hypercholesterolemia which is a genetic disorder that causes a severe increase in the levels of LDL from birth (Onorato & Sturm, 2016). According to the NICE guidelines (2017), statins are recommended as the initial treatment for all adults with heterozygous familial hypercholesterolemia (FH). The use of a high intensity statin such as atorvastatin, rosuvastatin or simvastatin at the maximum licensed or tolerated dose is suggested with the aim of achieving greater than 50% reduction in LDL concentration from pre-treatment levels (NICE, 2017). Since the patient is on 80mg simvastatin it indicates that the patients is currently on the maximum licensed dose of statin and is on the secondary prevention stage in the NICE pathway (2016a). However, from the scenario although the statin therapy is tolerated by the patient, even at the highest possible licensed dose, the patient’s LDL level is still not controlled therefore other lipid-lowering drugs should be considered. Although statins are recommended as first line, ezetimibe is an alternative option to be considered for treating adults with primary (heterozygous familial or non-familial) hypercholesterolemia. NICE (2017) recommends ezetimibe as a monotherapy for adults when the initial statin therapy is contraindicated or not tolerated by the patient. Ezetimibe is also recommended as a dual therapy with the initial statin therapy for primary hypercholesterolemia in adults who have started statin therapy where the total serum cholesterol or LDL level is not adequately controlled after optimisation of the initial statin therapy or where there is statin intolerance (NICE, 2017). Since the patient is tolerant to the statin therapy which he is currently on, ezetimibe in combination with simvastatin can be a potential treatment alternative. As stated by Williams (2016), ezetimibe used alone reduces LDL by 15–20%, with a small increase in high density lipoprotein (HDL) and a small reduction in triglycerides. However, it was indicated in a double-blind, randomized, 24-month trial (Kastelein et al., 2008) that when the effect of 10mg of ezetimibe used in conjunction with 80mg of simvastatin was compared to the use of only simvastatin, the levels of triglycerides and C-reactive protein were reduced by 25.7% and 6.6% respectively with the two groups demonstrating similar side effect and safety profiles. The greater reduction in LDL concentration demonstrates that the use of simvastatin with ezetimibe as a combination therapy is more efficacious than the use of simvastatin on its own. In another randomized double-blind study (Bays et al., 2004), the combination of simvastatin and ezetimibe was found to result in a greater reduction in LDL compared to ezetimibe used alone or simvastatin used alone. In the study, more patients were able to achieve an LDL concentration of less than 100mg/dL with the combination of ezetimibe and simvastatin compared to simvastatin used alone. The results from all the studies show the efficacy of the use of ezetimibe in conjunction with simvastatin as a dual therapy for the reduction of LDL levels. The NICE guidelines (2017) state that bile acid sequestrants should not be routinely offered to patients unless considered by specialists in adults with FH who are intolerant or contraindicated to statins or ezetimibe to reduce LDL concentration. It is not recommended for patients who are already being treated with primary or secondary prevention of cardiovascular disease and it should not be given in combination with statins for primary or secondary prevention of cardiovascular disease unless otherwise stated by a specialist (NICE, 2017). Additionally, Williams (2016) stated that the reason for the limited use of bile acid sequestrants in clinical practice is due to poor tolerability in patients, drug interactions and potential increase in triglyceride levels. In a 16-week randomized, double blind study (Handelsman et al., 2010) comparing the efficacy of colesevalam with placebo, it was indicated that there was a significantly higher reduction in LDL concentration of the patients using colesevalam compared to the patients using the placebo. About 29% of patients using colesevalam compared to 11% using the placebo achieved the target LDL concentration of less than 100mg/dL. According to (Davidson, 2013), current data demonstrate that the use of colesevelam is efficacious and well tolerated in the treatment of heterozygous familial hypocholesteraemia in adults. Evolocumab is a newer lipid modifying drug which is recommended by NICE (2016b) as a second line option in the management of primary familial hypercholesterolemia in specific circumstances. It is recommended at a dose of 140 mg injection every 2 weeks only when LDL concentrations are not adequately controlled and they are persistently above the specified thresholds despite the use of maximal tolerated lipid-lowering therapy (i.e either the maximum dose has been reached or further dose titration is limited due to intolerance) (NICE, 2016b). These decisions are also based on the fact that evolocumab is very expensive so it should only be recommended if the company provides evolocumab with the discount agreed in the patient access scheme. A multicentre, randomised, double-blind, placebo-controlled trial (Raal et al., 2015) indicated that compared to placebo, 140mg of evolocumab used every 2 weeks led to a 59.2% reduction in mean LDL concentration. Based on a cohort study (McDonagh, Peterson, Holzhammer, & Fazio, 2016), 120mg of Evolocumab administered every 2 weeks resulted in a 32% reduction in LDL concentration in patients with heterozygous or homozygous familial hypercholesterolemia, patients intolerant of statins, and patients not at their target LDL concentration with statin therapy. Additionally, according to the recommended drug formulary for lipid management in familial and non-familial hypercholesterolemia (Gidding et al., 2015), it is suggested that if the LDL concentration is still above the target with a stain therapy then the next step is a dual therapy with statin and ezetimibe. This is in line with the recommendation by NICE. The patient’s history indicates that simvastatin is tolerated by the patient however the LDL is not well controlled with the use of stain so based on the evidence, low dose ezetimibe is recommended to be used in conjunction with statin to control the cholesterol level instead of evolocumab. As discussed above, there is evidence for the use of ezetimibe with statin in a situation whereby the LDL concentration is still not controlled after using the maximum dose possible of a high intensity statin. Although evolocumab is effective as a lipid lowering agent, it is not appropriate to be used as the next line of treatment. According to the evidence, evolocumab is recommended as a therapy for people with heterozygous familial hypercholesteremia when the maximum lipid lowering therapy has been used however based on the patient’s medical history in the scenario, the only lipid lowering agent that has been tried so far is only simvastatin. More so, considering the cost implications, evolocumab is very expensive compared to ezetimibe. Therefore, based on all the evidence gathered, instead of considering evolocumab as the next line of treatment, it is recommended that ezetimibe is used as an adjunct treatment to simvastatin which the patient is currently on. Advice for the doctor (Datapharm, 2018c; Joint Formulary Committee, 2017 & NICE, 2017): The patient should be started on oral doses of 10mg of ezetimibe with the already established 80mg dose of simvastatin once daily. Since simvastatin is intended for evening administration while ezetimibe can be taken at any time, both drugs should be prescribed to be taken together at night or to increase patient compliance, inegy tablets which contains simvastatin and ezetimibe can be prescribed. There is increased risk of myopathy and rhabdomyolysis with ezetimibe used with simvastatin Inegy should be avoided in moderate and severe hepatic impairment. If serum transaminases are abnormally high, inegy should be stopped. If myopathy is suspected based on muscle symptoms or is confirmed by abnormally high creatine kinase levels, inegy should be stopped immediately. Inegy should be temporarily stopped few days before elective major surgery and when any major medical or surgical condition supervenes. The patient should be provided information about their specific level of cardiovascular risk, its implications for them and their families Advice for the patient (Datapharm, 2018c; Joint Formulary Committee, 2017 & NICE, 2017): Ezetimibe is a new medication to be used in combination with the already established dose of simvastatin One inegy (10mg/80mg) tablet should be taken in the evening Exercise regularly. Perform at least 30 minutes of physical activity a day. Maintain a healthy balanced diet. NHS live well can be used as a guide for healthy eating. Smoking cessation Do not drink more than 14 units of alcohol a week on a regular basis. Side effects such as muscle pain, fatigue, headache, abdominal pain, diarrhoea, etc may be experienced due to the medication Report any unexplained muscle pain, tenderness or weakness immediately to the pharmacist or GP It is essential to keep taking the medication even after the cholesterol levels are lower for maintenance because if the medication is stopped it can lead to lipids returning back to pre-treatment levels within 4 weeks Grapefruit juice should be avoided when taking inegy as it might increase the plasma concentration of simvastatin and hence increase risk of muscle weakness Monitoring (Datapharm, 2018c; Joint Formulary Committee, 2017 & NICE, 2017): Liver function tests should be performed at initiation of therapy to check for abnormal increase in serum transaminases levels, within 3 months of starting treatment and again at 12 months. Serum creatinine kinase should be measured if the patient experiences unexplained muscle pain, tenderness or weakness to confirm rhabdomyolysis. A regular review is recommended at least annually to check medication adherence, possible side-effects, fasting lipid levels and blood pressure. Bays, H. E., Ose, L., Fraser, N., Tribble, D. L., Quinto, K., Reyes, R., . . . Donahue, S. R. (2004). A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clinical Therapeutics, 26(11), 17581773. doi:10.1016/j.clinthera.2004.11.016 Davidson, M. (2013). The Efficacy of Colesevelam HCl in the Treatment of Heterozygous Familial Hypercholesterolemia in Pediatric and Adult Patients. Clinical Therapeutics, 35(8), 1247-1252. doi:10.1016/j.clinthera.2013.06.014 Gidding, S. S., Ann Champagne, M., de Ferranti, S. D., Defesche, J., Ito, M. K., Knowles, J. W., . . . Wierzbicki, A. S. (2015). The Agenda for Familial Hypercholesterolemia. A Scientific Statement From the American Heart Association, 132(22), 2167-2192. doi:10.1161/cir.0000000000000297 Handelsman, Y., Goldberg, R., Garvey, W., Fonseca, V., Rosenstock, J., Jones, M., . . . Abby, S. (2010). Colesevelam Hydrochloride to Treat Hypercholesterolemia and Improve Glycemia in Prediabetes: A Randomized, Prospective Study. Endocrine Practice, 16(4), 617-628. doi:10.4158/ep10129.or Kastelein , J. J. P., Akdim , F., Stroes , E. S. G., Zwinderman , A. H., Bots , M. L., Stalenhoef , A. F. H., . . . de Groot , E. (2008). Simvastatin with or without Ezetimibe in Familial Hypercholesterolemia. New England Journal of Medicine, 358(14), 1431-1443. doi:10.1056/NEJMoa0800742 McDonagh, M., Peterson, K., Holzhammer, B., & Fazio, S. (2016). A Systematic Review of PCSK9 Inhibitors Alirocumab and Evolocumab. Journal of Managed Care & Specialty Pharmacy, 22(6), 641-653q. doi:10.18553/jmcp.2016.22.6.641 Onorato, A., & Sturm, A. C. (2016). Heterozygous Familial Hypercholesterolemia. Circulation, 133(14), e587-e589. doi:10.1161/circulationaha.115.020701 Raal, F. J., Stein, E. A., Dufour, R., Turner, T., Civeira, F., Burgess, L., . . . Gaudet, D. (2015). PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebocontrolled trial. The Lancet, 385(9965), 331-340. doi:https://doi.org/10.1016/S01406736(14)61399-4