Hydrogen - atomic structure and uses
advertisement

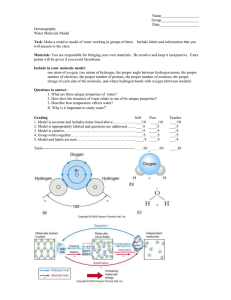
What is hydrogen? Hydrogen is one of the chemical elements that exist in nature. An element has one type of atom, and it cannot be broken down into other substances. Hydrogen exists naturally as a molecule, consisting of two hydrogen atoms. The chemical formula of hydrogen is H₂. With a standard atomic weight of 1.008, hydrogen is the lightest element in the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all mass. Non-remnant stars are mainly composed of hydrogen in the plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons. Since hydrogen has one proton, it has the atomic number 1 and it is in group IA. The Sun also consists of hydrogen for the most part (75%). When two hydrogen atoms combine their nuclei, they turn into helium. This is what heats up the Sun. After 4-5 billion years, the Sun will run out of hydrogen and life on Earth will cease to exist. Hydrogen atoms were the first to be formed after the Big Bang. Out of all atoms in the universe, about 89% are hydrogen. On Earth, the amount of hydrogen is much lower. Most of hydrogen atoms on Earth are in water molecules. In human body, hydrogen is the third most abundant element. About 10% of all body mass is hydrogen. Practice! Which statements are true? ● In a human that weighs 50kg, there is 5kg of hydrogen atoms. ● 89% of all atoms on Earth are hydrogen. ● Hydrogen atom has a simple structure: it consists of only one proton. ● Hydrogen’s atomic nr is 1 and it is the first element in the periodic table. ● Most of the hydrogen atoms on Earth are in water molecules. Hydrogen as pure substance Hydrogen atom is chemically unstable on its own and usually only exists as a molecule or different compounds. Hydrogen as pure substance exists as a diatomic molecule (H2) that’s molecular mass is approximately 2. Compared with air that’s average molecular mass is 29, it is so light that gravity cannot hold it in the atmosphere. Hydrogen will quickly rise higher and gather in the upper layers of the Earth’s atmosphere. Practice! How many times smaller is the molecular mass of hydrogen compared with the molecular mass of air? Properties of hydrogen Hydrogen as a pure substance is colorless, transparent and odorless. Hydrogen as a pure substance is not very stable. It can ignite easily and it burns with a soft blue flame. High amount of heat energy is released when hydrogen is burning, that’s why this reaction is used in welding and cutting metals. Due to the danger of explosion, before igniting hydrogen it is important to check its purity. Often in experiment demonstrations, a mixture of oxygen and hydrogen is ignited and there is an explosion. As a result of this reaction, water is formed. A mixture that contains ⅔ of hydrogen and ⅓ of oxygen is called oxyhydrogen or knallgas ( “bang-gas”). Hydrogen is dangerous even starting from low concentration (5%) so it is very important to be cautious when using it. Even though hydrogen is in IA group with alkali metals, it doesn’t have metallic properties. Alkali metals are metals as pure substances and have metallic properties but hydrogen is a nonmetal and gaseous at its standard state. Practice! Mixture of hydrogen and oxygen is explosive when there is at least _____% of hydrogen in the mixture. Hydrogen is a ____________________ and ___________________ gas that burns with a _______________ flame. What is hydrogen as a pure substance used for? Additional reading - hydrogen cars Most cars burn petrol or diesel in their engines. These chemical reactions make carbon dioxide and water. Carbon dioxide is a greenhouse gas, which means too much of it can promote global warming. Global warming causes climate change, resulting in droughts, floods and extreme weather. Hydrogen cars help to reduce greenhouse gas emissions. They either burn hydrogen in an engine or they react both hydrogen and oxygen together in a fuel cell. Both processes produce electricity which powers an electric motor. Hydrogen cars produce one harmless exhaust gas – water vapour. Key ideas to remember ● ● ● ● Hydrogen is an element; it exists naturally as a molecule. Hydrogen atom consists of one proton and one electron in its elemental form. Each hydrogen molecule is made up of two hydrogen atoms. *In petrol and diesel cars, the burning of fuel produces carbon dioxide and water. Extra carbon dioxide in the air promotes global warming. ● *In hydrogen cars hydrogen reacts with oxygen in a fuel cell, making electricity to run the car. The only waste product of this process is water vapour.