Density Lab Worksheet: Experiment & Data Analysis
advertisement

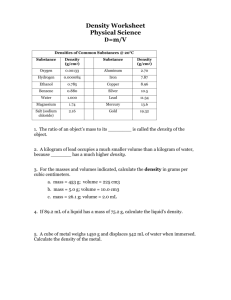
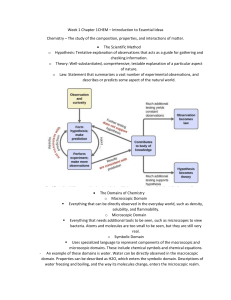
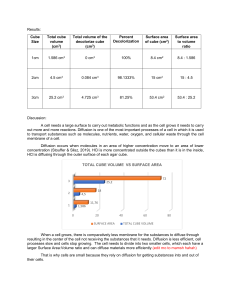
Density Lab Procedures: 1) 2) 3) 4) 5) 6) 7) 8) 9) Obtain a sample block from the set and record your selection in appropriate column. Predict if whether your sample will sink or float in water. Record prediction in data table. Drop cube in cup / beaker of water. Record results in data table. Use a ruler to measure the length, width, and height of each cube to obtain its volume. Record information in data chart. Now, measure the mass of each material by using the balance scale. Calculate the density using the formula D = M / V. Record information in data chart. Give your data table a title. Data Table: _________________________________________ Material Prediction Result Characteristics Length cm Width cm Height cm Volume cm3 Mass g Density g/cm3 Density Lab Analysis: Use the information from the data table to answer the following questions. 1) What rationale did you use to determine your predictions? 2) Water has a density of 1.0 g/cm3. a) How did substances with a density above 1.0 behave in the water? b) How did substances with a density lower than 1.0 behave? 3) What generalizations can you conclude about substances, their respective densities and behaviors in water?