Metallic Bonding Sheet
advertisement
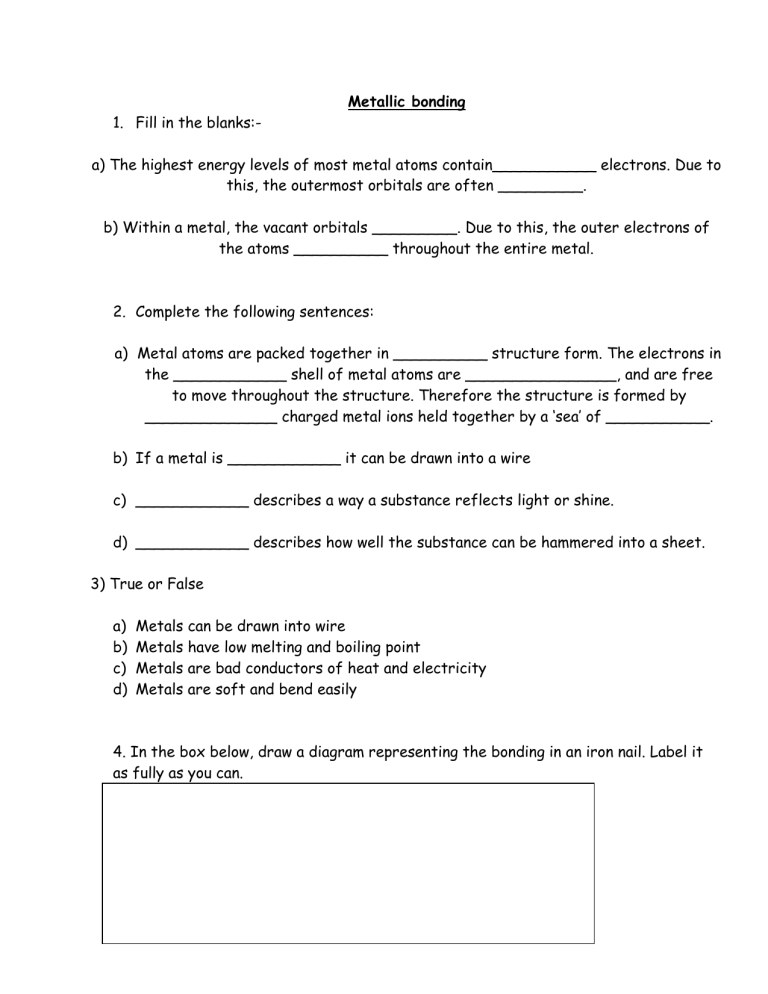
Metallic bonding 1. Fill in the blanks:a) The highest energy levels of most metal atoms contain___________ electrons. Due to this, the outermost orbitals are often _________. b) Within a metal, the vacant orbitals _________. Due to this, the outer electrons of the atoms __________ throughout the entire metal. 2. Complete the following sentences: a) Metal atoms are packed together in __________ structure form. The electrons in the ____________ shell of metal atoms are ________________, and are free to move throughout the structure. Therefore the structure is formed by ______________ charged metal ions held together by a ‘sea’ of ___________. b) If a metal is ____________ it can be drawn into a wire c) ____________ describes a way a substance reflects light or shine. d) ____________ describes how well the substance can be hammered into a sheet. 3) True or False a) b) c) d) Metals Metals Metals Metals can be drawn into wire have low melting and boiling point are bad conductors of heat and electricity are soft and bend easily 4. In the box below, draw a diagram representing the bonding in an iron nail. Label it as fully as you can. _____1- Chemical bonding in metals is a. the same as ionic bonding. b. the same as covalent bonding. c. a combination of ionic and covalent bonding. d. different from ionic or covalent bonding. _____ 2. The valence electrons in a metallic bond a. move freely throughout the network of metal atoms. b. are held tightly by the most positively charged atom. c. are shared equally between two metal atoms. d. continuously move from one energy level to another. _____ 3. Within a metal, the vacant orbitals in the atoms’ outer energy levels a. repel valence electrons. b. attract other metal atoms. c. overlap. d. diffract light. _____ 4. Which of the following properties is not explained by metallic bonding? a. electrical conductivity b. thermal conductivity c. brittleness d. ductility _____ 5. Which of the following is the result of visible light absorbed by a metal? a. Electrons move to higher energy levels and remain there. b. Light at a frequency similar to the absorbed frequency is emitted. c. Electrons fill the vacant orbitals. d. Light is given off as a line spectrum. _____ 6. Metals are malleable because when struck, one plane of metal atoms a. can slide past another plane without breaking bonds. b. cannot easily move out of the way. c. moves in a way that maximizes the repulsive forces within the metal. d. bonds to the plane directly beneath it. _____ 7. Which is a measure of metallic bond strength? a. electron affinity b. electronegativity c. specific heat capacity d. enthalpy of vaporization _____ 8. In general, as you move from right to left across any row of the periodic table, the strength of a metallic bond a. increases. b. decreases. c. stays the same. d. shows no trend. _____ 9. Which of these is responsible for the good electrical conductivity of metals? a. the arrangement of metal atoms in separate layers b. the high density of metals atoms in the crystal lattice c. the ability of electrons to move freely about the crystal structure d. the fact that metal atoms contain many orbitals separated by very small energy _____ 10. The arrangement of valence electrons in a metallic bond is best described as a. fixed positions in a lattice. b. a sea of free-moving electrons. c. concentrated electron density around specific atoms. d. electron pairs existing in multiple bonds. 1. _____ In metals, the valence electrons are considered to be (a) attached to particular positive ions. (c) immobile. (b) shared by all surrounding atoms. (d) involved in covalent bonds. 2. _____ The fact that metals are malleable and ionic crystals are brittle is best explained in terms of their (a) chemical bonds. (c) enthalpies of vaporization. (b) London forces. (d) polarity. 3. _____ As light strikes the surface of a metal, the electrons in the electron sea (a) allow the light to pass through. (b) become attached to particular positive ions. (c) fall to lower energy levels. (d) absorb and re-emit the light. 4. _____ Mobile electrons in the metallic bond are responsible for (a) luster. (c) electrical conductivity. (b) thermal conductivity. (d) All of the above. 5. _____ In general, the strength of the metallic bond _____ moving from left to right on any row of the periodic table. (a) increases (c) remains the same (b) decreases (d) varies 6. _____ When a metal is drawn into a wire, the metallic bonds (a) break easily. (c) do not break. (b) break with difficulty. (d) become ionic bonds. 7. Use the concept of electron configurations to explain why the number of valence electrons in metals tends to be less than the number in most nonmetals. _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 8. How does the behavior of electrons in metals contribute to the metal’s ability to conduct electricity and heat? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 9. What is the relationship between the enthalpy of vaporization of a metal and the strength of the bonds that hold the metal together? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 10. Draw two diagrams of a metallic bond. In the first diagram, draw a weak metallic bond; in the second, show a metallic bond that would be stronger. Be sure to include nuclear charge and number of electrons in your illustrations. a. b. Note: In the strong bond, the charge on the nucleus and the number of electrons must be greater than in the weak bond.



