Redox Review Released items
advertisement
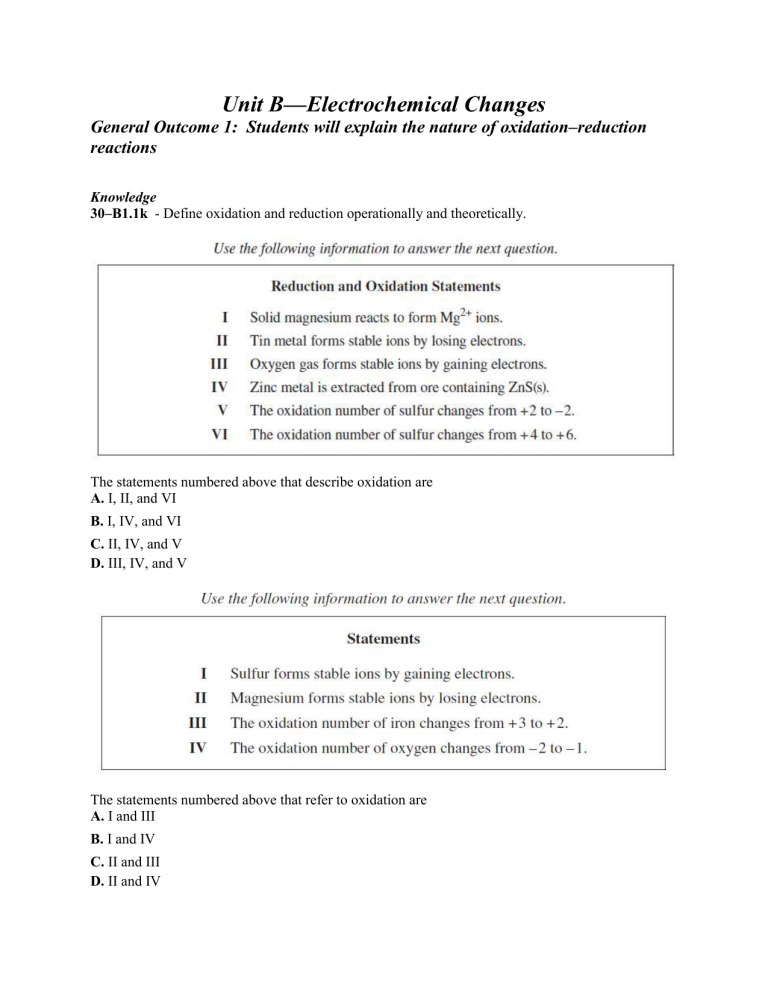
Unit B—Electrochemical Changes General Outcome 1: Students will explain the nature of oxidation–reduction reactions Knowledge 30–B1.1k - Define oxidation and reduction operationally and theoretically. The statements numbered above that describe oxidation are A. I, II, and VI B. I, IV, and VI C. II, IV, and V D. III, IV, and V The statements numbered above that refer to oxidation are A. I and III B. I and IV C. II and III D. II and IV In the reaction represented by the equation above, copper undergoes A. reduction only B. oxidation only C. both oxidation and reduction D. neither oxidation nor reduction Which of the following statements is an operational definition of the metal undergoing reduction? A. Iron metal undergoes a formation reaction with oxygen gas. B. Magnesium metal increases in mass when heated in air. C. Iron(III) hydroxide reacts with oxygen in the air to form ionic compounds. D. Zinc sulfide ore is roasted in the presence of oxygen gas to produce zinc metal. Knowledge 30–B1.2k - Define oxidizing agent, reducing agent, oxidation number, half-reaction, disproportionation. In the reaction represented by the equation above, oxygen acts as the i agent, and the oxidation number of the sulfur atom increases by ii . The statement above is completed by the information in row Row i ii oxidizing 2 A. oxidizing 6 B. C. reducing 2 D. reducing 6 Which of the following equations represents a disproportionation reaction? A. 2Na(s) + I2(s) → 2NaI(s) B. 2F2(g) + O2(g) → 2OF2(g) C. Cl2(aq) + H2O(l) → HOCl(aq) + H+(aq) + Cl–(aq) D. 2NH3(aq) + NaOCl(aq) → N2H4(aq) + NaCl(aq) + H2O(l) In the equation above, the species that undergoes disproportionation is A. Cl2(g) B. OH–(aq) C. OCl–(aq) D. H2O(l) During the reaction represented by equation II, nitrogen monoxide undergoes i and ii electrons. The statement above is completed by the information in row Row i ii disproportionation oxygen gains and A. loses disproportionation nitrogen gains and B. loses oxidation oxygen loses C. oxidation nitrogen loses D. Knowledge 30–B1.3k - Differentiate between redox reactions and other reactions, using half-reactions and/or oxidation numbers. Which of the following equations represents a redox reaction? A. NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l) B. 2AgNO3(aq) + Cu(s) → 2Ag(s) + Cu(NO3)2(aq) C. H2SO4(aq) + 2KOH(aq) → K2SO4(aq) + 2H2O(l) D. CaCl2(aq) + Ba(OH)2(aq) → Ca(OH)2(aq) + BaCl2(aq) Knowledge 30–B1.4k - Identify electron transfer, oxidizing agents and reducing agents in redox reactions that occur in everyday life, in both living systems (e.g., cellular respiration, photosynthesis) and nonliving systems; i.e., corrosion. During cellular respiration, the oxidizing agent is A. O2(g) B. CO2(g) C. H2O(l) D. C6H12O6(s) The metallurgical processes in which the metal loses electrons are A. I and II B. I and III C. II and IV D. III and IV Use the following information to answer the next question. During the operation of a car battery, which of the following observations can be made? A. The amount of Pb(s) increases as PbO2(s) is reduced. B. The amount of PbO2(s) increases as Pb(s) is reduced. C. The amount of PbO2(s) decreases as Pb(s) is oxidized. D. The amount of Pb(s) decreases as PbO2(s) is oxidized. The equations above that represent a reaction in which the metal is being oxidized are A. I and IV only B. II and III only C. I, III, and IV D. II, III, and V During the explosion of ammonium nitrate, hydrogen A. is oxidized B. loses electrons C. is the oxidizing agent D. has no change in oxidation number In this reaction, the reducing agent is A. FeCl2(aq) B. HCl(aq) C. H2(g) D. Fe(s) Which of the equations numbered above represents a reaction in which the metal is oxidized? A. I and II only B. I, II, and V C. I, IV, and V D. III and IV In the titration reaction, the reducing agent is i and ii atoms are reduced. The statement above is completed by the information in row Row i ii I2(aq) iodine A. I 2 (aq) sulfur B. S2O32– (aq) iodine C. S2O32– (aq) sulfur D. Compared with solid zinc, solid copper is a i reducing agent, and during the operation of this cell, the zinc electrode ii electrons. The statement above is completed by the information in row Row i ii weaker gains A. weaker loses B. C. stronger gains D. stronger loses Knowledge 30–B1.5k - Compare the relative strengths of oxidizing and reducing agents, using empirical data. When listed in order from strongest to weakest, the oxidizing agents are A. Ra(s), Be(s), V(s), Cd(s) B. Cd(s), V(s), Be(s), Ra(s) C. Ra2+(aq), Be2+(aq), V2+(aq), Cd2+(aq) D. Cd2+(aq), V2+(aq), Be2+(aq), Ra2+(aq) From the student’s data, the strongest reducing agent is A. M2+(aq) B. X–(aq) C. X2(aq) D. M(s) Which of the following substances is the strongest reducing agent? A. Pt(s) B. Rh(s) C. Sm(s) D. Te(s) The strongest oxidizing agent in the equations above is A. Co3+(aq) B. Ce3+(aq) C. Hg+(aq) D. Ce4+(aq) Knowledge 30–B1.6k - Predict the spontaneity of a redox reaction, based on standard reduction potentials, and compare the predictions to experimental results. Which of the following equations represents a spontaneous oxidation–reduction reaction? A. Ac(s) + 3Cs+(aq) → 3Cs(s) + Ac3+(aq) B. Cs(s) + Am4+(aq) → Am3+(aq) + Cs+(aq) C. 2Ac3+(aq) + 3Tl+(aq) → 3Tl3+(aq) + 2Ac(s) D. Tl3+(aq) + 2Am3+(aq) → 2Am4+(aq) + Tl+(aq) Which of the following actions could prevent the corrosion of the pipeline? A. Using a pipeline made of copper B. Using a pipeline made of chromium C. Connecting strips of lead to the pipeline at appropriate intervals D. Connecting strips of zinc to the pipeline at appropriate intervals A 1.0 mol/L solution of Ni(NO3)2(aq) could be stored in a container made of A. tin B. iron C. zinc D. chromium Which of the following equations represents a spontaneous reaction? A. Te+(aq) + Rh(s) → Te(s) + Rh+(aq) B. Sm2+(aq) + Pt(s) → Sm(s) + Pt2+(aq) C. Pt2+(aq) + 2Te(s) → Pt(s) + 2Te+(aq) D. Sm2+(aq) + 2Rh(s) → Sm(s) + 2Rh+(aq) Which of the following equations represents a spontaneous redox reaction? A. Zn2+(aq) + Pb(s) → Zn(s) + Pb2+(aq) B. Sn4+(aq) + Fe(s) → Sn2+(aq) + Fe2+(aq) C. Zn2+(aq) + Co(s) → Zn(s) + Co2+(aq) D. O2(g) + 2H2O(l) + 4Br–(aq) → 2Br2(l) + 4OH–(aq) The reducing agent that can convert 1.0 mol/L Sn4+(aq) ions to Sn2+(aq) but not 1.0 mol/L Sn2+(aq) to Sn(s) is A. Cu(s) B. Pb(s) C. Ni(s) D. Cr(s) Use the following additional information to answer the next question. C6H4O2(aq) + 2H+(aq) + 2e– → C6H4(OH)2(aq) E° = +0.70 V Which of the following substances would oxidize C6H4(OH)2(aq)? A. Ag+(aq) B. Cu2+(aq) C. Ag(s) D. Cu(s) The test tubes in which a spontaneous redox reaction will occur during this experiment are labelled A. I and II only B. I, II, and III C. I, II, IV, and V D. IV and V only Knowledge 30–B1.7k - Write and balance equations for redox reactions in acidic and neutral solutions by using half-reaction equations obtained from a standard reduction potential table developing simple halfreaction equations from information provided about redox changes assigning oxidation numbers, where appropriate, to the species undergoing chemical change The oxidation number for nitrogen in (Record in the NH3(g) is +/– _______ first column) NO(g) is +/– _______ N2O(g) is +/– _______ NO2(g) is +/– _______ (Record in the second column) (Record in the third column) (Record in the fourth column) In the reaction represented by the equation above, the species that loses electrons is i , and the total number of electrons transferred in the reaction is ii . The statement above is completed by the information in row Row i ii I2(s) 30 A. I2(s) 10 B. ClO3–(aq) 30 C. ClO3–(aq) 10 D. When the equation above is balanced using lowest whole-number coefficients, the coefficient of (Record in the first column) SO42–(aq) is ______ + H (aq) is ______ (Record in the second column) e– is ______ (Record in the third column) H2O(l) is ______ (Record in the fourth column) In an acidic medium, the balanced reduction half-reaction for the reaction represented by the equation above is A. I2(s) + 2H+(aq) + 4e– → 2I–(aq) + H2O(l) B. 2H2O(l) + 2e– → H2(g) + 2OH–(aq) C. ClO3–(aq) + 6H+(aq) + 6e– → Cl–(aq) + 3H2O(l) D. ClO3–(aq) + 3H2O(l) + 6e– → Cl–(aq) + 6OH–(aq) When the equation above is balanced under acidic conditions, the whole number coefficient for H+(aq) is i and the amount of electrons transferred is ii . The statement above is completed by the information in row Row i ii 1 1 mol A. 1 2 mol B. C. 2 1 mol D. 2 2 mol The balanced oxidation half-reaction equation is A. Cl2(g) + 2e– → 2Cl–(aq) B. S2O32–(aq) + H2O(l) → SO42–(aq) + 2H+(aq) + 4e– C. S2O32–(aq) + 5H2O(l) → 2SO42–(aq) + 10H+(aq) + 8e– D. S2O32–(aq) + 5H2O(l) + 4e– → 2SO42–(aq) + 10H+(aq) Use the following information to answer the next question. The oxidation number of xenon in XeF2 is +/–__________ (Record in the first column) XeF4 is +/–__________ (Record in the second column) XeF6 is +/–__________ (Record in the third column) XeO3 is +/–__________ (Record in the fourth column) The oxidation number of nitrogen in NO(g) is __________ (Record in the first column) NO2(g) is __________ (Record in the second column) N2O(g) is __________ (Record in the third column) N2O4(g) is __________ (Record in the fourth column) Which of the following equations represents the reduction half-reaction when molten aluminium oxide undergoes electrolysis? 3+ A. Al (l) → Al(l) + 3e– B. Al3+(l) + 3e– → Al(l) C. 2O2–(l) → O2(g) + 4e– D. 2O2–(l) + 4e– → O2(g) Knowledge 30–B1.8k - Perform calculations to determine quantities of substances involved in redox titrations. The concentration of the Na2S2O3(aq) solution is ______mmol/L. (Record your three-digit answer in the numerical-response section on the answer sheet.) The concentration of I2(aq), expressed in scientific notation, is a.bc × 10–d mol/L. The values of a, b, c, and d are _____, _____, _____, and _____. The amount of potassium dichromate solution required to complete this titration is A. 8.33 × 10–4 mol B. 6.45 × 10–3 mol C. 2.50 × 10–3 mol D. 2.15 × 10–3 mol Use your recorded answer from Multiple Choice 47 to answer Numerical Response 48.* The concentration of Sn2+(aq) in the sample used in the titration, expressed in scientific notation, is a.bc × 10–d mol/L. The values of a, b, c, and d are _____, _____, _____, and _____. *You can receive marks for this question even if the previous question was answered incorrectly. General Outcome 2: Students will apply the principles of oxidation–reduction to electrochemical cells Knowledge 30–B2.1k - Define anode, cathode, anion, cation, salt bridge/porous cup, electrolyte, external circuit, power supply, voltaic cell and electrolytic cell. Match the numbers on the electrochemical cell above with the descriptors below. (Record in the first column) Increases in mass ______ Movement of cations ______ (Record in the second column) Movement of electrons ______ (Record in the third column) Site where oxidation occurs ______ (Record in the fourth column) The electrochemical cell descriptions that apply to this operating, standard copper–silver cell are numbered _____,_____ ,_____ , and_____ . (Record all four digits of your answer in any order in the numerical-response section on the answer sheet.) The anode of an electrochemical cell is the site at which A. oxidation occurs B. cations gain electrons C. cations are attracted to the electrode D. electrons are attracted to the electrode During the production of aluminium metal in the electrolytic cell, anions travel toward the i and electrons travel through the ii . The statement above is completed by the information in row Row i ii cathode electrolyte to A. the anode cathode wire to the B. cathode anode electrolyte to C. the anode anode wire to the D. cathode Match the numbers in the diagram above with their appropriate labels given below. (Record in the first column) Anode _____ Cathode _____ (Record in the second column) Anion movement _____ (Record in the third column) Electron movement _____ (Record in the fourth column) Which of the following rows identifies the type of electrochemical cell in the diagram above and describes what happens during its operation? Row Type of Cell What Happens Voltaic Electrons move A. toward the cathode Voltaic I–(aq) moves toward B. the cathode Electrolytic Electrons move C. toward the cathode Electrolytic I–(aq) moves toward D. the cathode Match four of the numbers in the diagram of the electrochemical cell used in the refining of coppper with their descriptions given below. Site where reduction occurs __________ (Record in the first column) Direction of anion movement __________ (Record in the second column) Direction of electron movement __________ (Record in the third column) Electrode that increases in mass __________ (Record in the fourth column) Knowledge 30–B2.2k - Identify the similarities and differences between the operation of a voltaic cell and that of an electrolytic cell. An electrolytic cell differs from a voltaic cell in that the electrolytic cell A. is spontaneous B. consumes electricity C. has a positive E°cell value D. has an anode and a cathode The statements above that correctly describe an electrolytic cell are A. I, III, and V B. I, IV, and VI C. II, III, and VI D. II, IV, and V The statements above that correctly describe both an electrolytic cell and a voltaic cell are A. I and III B. III and VI C. IV and V D. IV and VI The statements numbered above that apply to both electrolytic cells and voltaic cells are _____, ______, _____, and ______. If a student were to build a voltaic cell using solid zinc and hydrochloric acid, which of the following equipment would also be needed? A. An inert electrode for the cathode and a salt bridge B. An inert electrode for the cathode and a power source C. An inert electrode for the anode and a salt bridge D. An inert electrode for the anode and a power source Which of the following rows gives the composition of the bubbles and the process through which they were formed? Row Composition Process of Formation Cl2(g) oxidation of A. chloride ions H2(g) reduction of B. hydrogen ions H 2 (g) reduction of C. water O2(g) oxidation of D. water The electrochemical cell used in the refining of copper is i , and the reaction is ii . The statement above is completed by the information in row Row i ii voltaic spontaneous A. voltaic nonspontaneous B. electrolytic spontaneous C. electrolytic nonspontaneous D. Which of the following statements applies to the operation of the electrochemical cell? A. A precipitate forms on the Zn(s) electrode. B. The concentration of Zn2+(aq) ions decreases. C. Electrons move through the connecting wires toward the Zn(s) electrode. D. The NO3–(aq) ions move through the salt bridge toward the Zn(s) electrode. The statements above that apply to both a voltaic cell and an electrolytic cell are numbered ______,______ _____ , and _____. 30–B2.3k - Predict and write the half-reaction equation that occurs at each electrode in an electrochemical cell. During the electrolysis of a sodium chloride solution, the reduction reaction is A. 2H2O(l) + 2e– ⇌ H2(g) + 2OH–(aq) B. 2H2O(l) ⇌ O2(g) + 4H+(aq) + 4e– C. Cl2(g) + 2e– ⇌ 2Cl–(aq) D. 2Cl–(aq) ⇌ Cl2(g) + 2e– The equation that represents the half-reaction that occurs at the cathode of the fuel cell is A. O2(g) + 4H+(aq) + 4e– → 2H2O(l) B. 2H2O(l) → O2(aq) + 4H+(aq) + 4e– C. HCOOH(l) → CO2(aq) + 2H+(aq) + 2e– D. CO2(aq) + 2H+(aq) + 2e– → HCOOH(l) Which of the following equations represents the net reaction that occurs in the electrochemical cell? A. 2Ag(s) + Sn2+(aq) → 2Ag+(aq) + Sn(s) B. 2Ag(s) + Sn(s) → 2Ag+(aq) + Sn2+(aq) C. 2Ag+(aq) + Sn2+(aq) → 2Ag(s) + Sn(s) D. 2Ag+(aq) + Sn(s) → 2Ag(s) + Sn2+(aq) The reduction half-reaction that occurs during the operation of the electrochemical cell represented in the diagram above is i , and this reaction occurs at the ii . The statement above is completed by the information in row Row i ii Cu2+(aq) + 2e– → anode A. Cu(s) Cu2+(aq) + 2e– → cathode B. Cu(s) Zn2+(aq) + 2e– → anode C. Zn(s) Zn2+(aq) + 2e– → cathode D. Zn(s) The half-reaction that occurs at the anode during the discharge of the nickel–cadmium cell is A. Cd(s) + 2OH–(aq) → Cd(OH)2(s) + 2e– B. Cd(s) + 2OH–(aq) + 2e– → Cd(OH)2(s) C. NiO2(s) + 2H2O(l) + 2e– → Ni(OH)2(s) + 2OH–(aq) D. NiO2(s) + 2H2O(l) → Ni(OH)2(s) + 2OH–(aq) + 2e– Knowledge 30–B2.4k - Recognize that predicted reactions do not always occur; e.g., the production of chlorine gas from the electrolysis of brine. Knowledge 30–B2.5k - Explain that the values of standard reduction potential are all relative to 0 volts, as set for the hydrogen electrode at standard conditions. For the standard reference half-cell, the reduction half-reaction equation and electrical potential are A. H2(g) → 2H+(aq) + 2e– E° = 0.00 V B. 2H+(aq) + 2e– → H2(g) E° = 0.00 V C. 2H2O(l) + 2e– → H2(g) + 2OH–(aq) E° = –0.83 V D. H2(g) + 2OH–(aq) → 2H2O(l) + 2e– E° = +0.83 V If the standard iodine half-cell is chosen as the reference half-cell instead of the hydrogen half cell, then the cell potential for a silver–nickel cell is +/–__________ V. If the Ni2+(aq) + 2e– → Ni(s) half-reaction is designated as the reference half-reaction with an electrode potential of 0.00 V, then the electrical potential for the Fe3+(aq) + e– → Fe2+(aq) half-reaction is A. +1.03 V B. +0.51 V C. –0.51 V D. –1.03 V Knowledge 30–B2.6k – Calculate the standard cell potential for electrochemical cells. If the standard lead reduction half-reaction had been chosen as the reference half-reaction instead of the hydrogen reduction half-reaction, then the electrical potential for this cell would be A. +1.14 V B. +0.93 V C. +0.67 V D. +0.46 V If the standard silver reduction half-reaction had been chosen as the reference half-reaction instead of the hydrogen reduction half-reaction, then the electrical potential of the cell represented by the diagram above would be +/– ______V. The E°net for the forward reaction is A. +2.20 V B. +1.36 V C. +0.55 V D. +0.52 V The E°cell for the electrochemical cell above is A. +1.10 V B. +0.42 V C. –0.42 V D. –1.10 V Use the following information to answer the next question. 2Ag+(aq) + Zn(s) → 2Ag(s) + Zn2+(aq) The cell potential for the redox reaction represented by the equation above is A. +0.04 V B. +0.84 V C. +1.56 V D. +2.36 V The net cell potential for this electrochemical cell is +/– __________ V. (Record your three-digit answer in the numerical-response section on the answer sheet.) The cell potential for the electrochemical cell in the diagram above is __________ V. Knowledge 30–B2.7k - Predict the spontaneity or nonspontaneity of redox reactions, based on standard cell potential, and the relative positions of half-reaction equations on a standard reduction potential table. In order to prevent corrosion, a sacrificial anode is connected to an underground propane tank that is made of iron metal. Which of the following metals could not function as the sacrificial anode? A. Copper B. Chromium C. Aluminium D. Magnesium Which of the following statements describes what occurs in each cell? A. In both cells a power source is needed. B. In both cells a spontaneous reaction occurs and Pb(s) is produced. C. In the first cell Ni(s) is produced, and in the second cell a power source is needed. D. In the first cell the reaction is spontaneous, and in the second cell the reaction is non-spontaneous. If the electrochemical cell in the diagram above produces a flow of electrons in the direction indicated, then M(s) and M2+(aq) could be A. Fe(s) and Fe2+(aq) B. Pb(s) and Pb2+(aq) C. Ni(s) and Ni2+(aq) D. Cu(s) and Cu2+(aq) Knowledge 30–B2.8k - Calculate mass, amounts, current and time in single voltaic and electrolytic cells by applying Faraday’s law and stoichiometry. If a current of 0.850 A is applied to the electrochemical cell above for 60.0 min, then the mass of copper produced is A. 0.016 8 g B. 1.01 g C. 2.02 g D. 4.03 g Use the following information to answer the next question. Use the following additional information to answer the next question. Every car battery is given a CCA (cold cranking amps) rating. A CCA rating of 600 means that the battery is capable of generating 600 A of current for a 30.0 s period at 0 °C. Which of the following values indicates how many coulombs a battery with a CCA rating of 600 produces during 30.0 s of operation? A. 20.0 C B. 600 C C. 1.80 × 104 C D. 1.74 × 109 C The reduction half-reaction for a Hall–Héroult electrolytic cell is represented by the following equation. Al3+(l) + 3e– → Al(l) If a current of 10.0 A is applied for 5.00 h to the Hall–Héroult electrolytic cell, then the amount of electrons transferred is A. 5.60 mol B. 1.87 mol C. 6.22 × 10–1 mol D. 5.18 × 10–4 mol Use the following information to answer the next question. A Hall-Héroult electrolytic cell is used to produce molten aluminium from molten aluminium oxide, as represented by the following simplified equation. 2Al2O3(l) → 4Al(l) + 3O2(g) In the Hall-Héroult electrolytic cell, the time required for the cell to operate at 5.55 × 103 A to produce 20.0 kg of aluminium is __________ h. Science, Technology, and Society 30–B2.2sts - describe science and technology applications that have developed in response to human and environmental needs investigate the use of technology, such as galvanism, metallurgy, magnesium coupling, painting, cathodic protection, to solve practical problems related to corrosion From an ecological perspective, a reason why hydrogen–oxygen fuel cells should not be used to power automobiles is that A. hydrogen fuel can be produced through the electrolysis of seawater by using the energy produced from burning fossil fuels B. cars powered by a hydrogen–oxygen fuel cell would be up to 30% more efficient than cars powered by gasoline C. water vapor is the primary by-product of the cell D. oxygen is readily available from the atmosphere


