Intermolecular Forces & Boiling Points Worksheet
advertisement
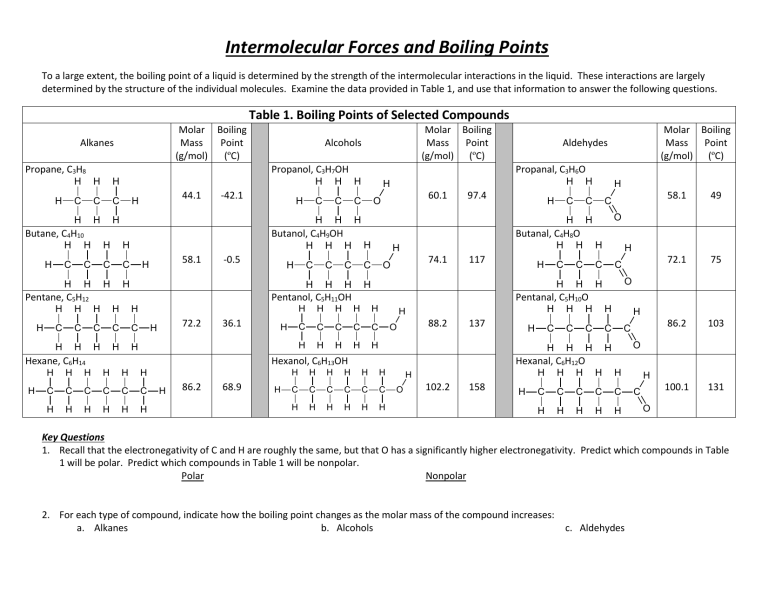
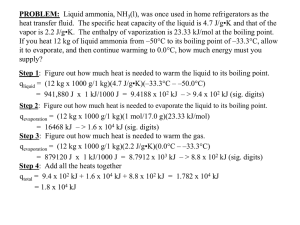
Intermolecular Forces and Boiling Points To a large extent, the boiling point of a liquid is determined by the strength of the intermolecular interactions in the liquid. These interactions are largely determined by the structure of the individual molecules. Examine the data provided in Table 1, and use that information to answer the following questions. Table 1. Boiling Points of Selected Compounds Molar Boiling Mass Point (g/mol) (ºC) Alkanes Propane, C3H8 H H Propanol, C3H7OH H H C C C H H H H 44.1 H -42.1 Butane, C4H10 H H H H C C C H H H H 58.1 H -0.5 H H C C C C H H H H H H H H H H H 60.1 O 97.4 C C C H H H H 72.2 H 36.1 H H H 74.1 O 117 H H H H H C C C C C H H H H H C C C C C C H H H H H H H 86.2 68.9 H H H H C C H H H C 58.1 49 72.1 75 86.2 103 100.1 131 O H H H H H C C C H H H H C O Pentanal, C5H10O H H 88.2 O 137 Hexanol, C6H13OH H H H Butanal, C4H8O H H C H H C H C H Pentanol, C5H11OH H Hexane, C6H14 H C H Molar Boiling Mass Point (g/mol) (ºC) Aldehydes Propanal, C3H6O H C H Pentane, C5H12 H H H Butanol, C4H9OH H C H Molar Boiling Mass Point (g/mol) (ºC) Alcohols H H H H H C C C C H H H H H C O Hexanal, C6H12O H H C C C C C C H H H H H H H O 102.2 158 H H H H H H C C C C C H H H H H H C O Key Questions 1. Recall that the electronegativity of C and H are roughly the same, but that O has a significantly higher electronegativity. Predict which compounds in Table 1 will be polar. Predict which compounds in Table 1 will be nonpolar. Polar Nonpolar 2. For each type of compound, indicate how the boiling point changes as the molar mass of the compound increases: a. Alkanes b. Alcohols c. Aldehydes 3. What happens to number of electrons in the molecule as molecular mass increases? ______________________________________ Based on your answers to Key Question #2, how do the intermolecular forces change as the size of the electron cloud increases? 6. Repeat Key Question #5 with at least two more sets of compounds. Using grammatically correct English sentences, describe any general pattern that you can identify about the relative boiling points of alkanes, alcohols, and aldehydes of roughly the same molar mass. 4. Find an alkane and an alcohol with roughly the same molar mass (within 5 g/mol). How do their boiling points compare? 7. Using the data in Table 1, does the presence of a molecular dipole tend to increase or decrease the strength of intermolecular attractions? Explain your reasoning. 5. Find an alcohol and an aldehyde with roughly the same molar mass (again within 5 g/mol). How do their boiling points compare? Applications 1. Using your knowledge of interparticle forces, which would need more energy to separate: water molecules or methane molecules? Explain. 4. For each grouping below, rank each of the substances in order of increasing boiling point (1=lowest, 4=highest), and explain your reasoning. Start by drawing a Lewis dot structure for each molecule. a. _____ CH4 _____ SnH4 2. Based on your answer to #1, which would have a higher boiling point: water or methane? Justify your answer using the concepts of energy, particle motion, and interparticle forces. 3. Based on the data in Table 1, estimate approximate boiling points for _____ GeH4 _____ SiH4 b. _____ NH3 _____ He a. Heptane, C7H16 ____________ _____ CH3F b. Ethanol, C2H5OH ____________ _____ CH4