7. rheology dr mahmoud (AutoRecovered)
advertisement

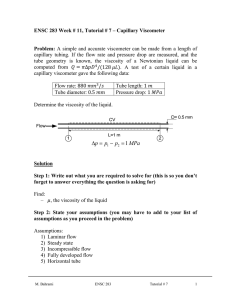
Rheology Rheology: • It is a branch of physics that deals with the flow of matter. Viscosity: • It is the resistance of a fluid to flow. • The higher the viscosity, the greater is the resistance. The flow of liquids in a tube: There are two types of forces (adhesion and cohesion forces): • Adhesion forces; are the forces which act between different molecules e.g. liquid molecules and tube wall. They increase beside the wall and decrease in the center of tube. • Cohesion forces; are the forces which act between same molecules e.g. molecules of liquid. They decrease beside the wall and increase in the center of tube. The velocity of liquid increases in the center of tube due to: • Decreased adhesive forces. • Increased cohesive forces. The unit of viscosity ‘dynamic viscosity’: • Poise or dyne.sec.cm-2. • Poise: is the shearing force required to produce a velocity of 1 cm/sec between two parallel planes of liquid each 1 cm2 in area and separated by a distance of 1 cm. • Centipoise (cp) = 0.01 Poise. Kinematic Viscosity (S): • The absolute viscosity divided by the density. S = η/ρ • The units of kinematic viscosity are the stoke (s) and the centistoke (cs). The fluidity ɸ: • The reciprocal of viscosity. ɸ = 1/η Classification of materials according to types of flow: I. II. 1) 2) 3) 4) Newtonians. Non-Newtonians: Plastic flow. Pseudo-plastic flow. Dilatant flow. Thixotropy. 1 I. Newtonian or simple flow • The flow of material conforms to Newtonian basic equation. • This type of flow is also called simple flow as it is concerned with the flow of simple liquids. Newton law of flow: • Shearing rate (G) is directly proportional to shearing force (F). 𝐹∝𝐺 𝐹 = 𝜂𝐺 𝐹 𝜂= 𝐺 N.B: • The Rheogram is a straight line passing through the origin. Examples of materials that have Newtonian flow: • Water • Alcohol • Benzene II. • Glycerin Non-Newtonian Systems 1. Plastic flow: • These systems are also called Bingham bodies. • The flow of these materials occurs only after application of a sufficient force called yield value. The curve does not pass through the origen. • Yield value: is the force that must be exceeded to overcome the attraction force between particles before the material begins to flow. Plastic viscosity (U) can be calculated as follows: 𝑈= 𝐹−𝑓 𝐺 2 The slope of the rheogram of plastic bodies is termed mobility. It equals = 1/U. Examples of materials that have plastic flow: • Suspensions e.g. ZnO in mineral oil. • Certain paints. • Most ointments. • Jelly. • Toothpaste. 2. Pseudoplastic Flow: 𝜂𝑝𝑝 𝐹𝑁 = 𝐺 • ηpp: viscosity of pseudoplastic flow. • N: constant depends on the material & must be more than 1. • The curve begins at the origin. • Pseudoplastic flow is called shear thinning system; as the viscosity decreases when rate of shear increases. Examples of materials that have pseudoplastic flow: Natural and synthetic gums such as: • Tragacanth. • Carboxymethyl cellulose. • Methyl cellulose. • Sodium alginates. 3. Dilatant Flow: 𝐹𝑁 𝜂𝐷 = 𝐺 • ηD: viscosity of dilatant flow. • N: constant depends on the material & must be less than 1. 3 • The curve begins at the origin. • Dilatant flow is called shear thickening system; as the viscosity increases when rate of shear increases. Examples of materials that have dilatant flow: • Suspensions containing high percentage of dispersed solids e.g. 50% potassium silicate. Explanation of dilatancy: 4. Thixotropy: • Reversible or isothermal gel-sol-gel transformation. • By increasing shearing rate, the viscosity decreases. • Then by removing the stress, the viscosity returns to its original state. Types of Thixotropy: Plastic flow and thixotropy Pseudoplastic flow and thixotropy e.g. petrolatum and bentonite. e.g. methyl cellulose and CMC. • Thixotropy is applied only on shear-thinning systems (plastic & pseudoplastic). Effect of temperature on viscosity of a liquid: ➢ As the temperature of the liquid increases, its viscosity decreases because the contact between molecules is reduced. ➢ For each 1 ⁰C increase in temperature, the viscosity decreases by 1 – 10 %. ➢ But in some polymers (such as MC), increasing temperature will increase the viscosity. Arrhenius equation: 𝐸𝑣 log 𝜂 = log 𝐴 + 2.303 𝑅𝑇 Where: 4 • • • • • η: viscosity. A: constant that depends on the molecular weight of liquid. Ev: activation energy; energy required to initiate the flow between molecules. R: gas constant (1.987 cal/mole.deg) T: the absolute temperature. Determination of viscosity: A. Capillary Viscometers (Ostwald Viscometer = U-tube Viscometer): Principle: The time, for the fluid to flow by gravity from one mark in a vertical capillary tube to the second mark, is measured. Uses: Measurement of viscosity of Newtonian fluids such as: • Water, Glycerin and Chloroform. Procedure: 1) Liquid of known viscosity (standard liquid e.g. water) is introduced into the viscometer through the V-arm. 2) Liquid is sucked through the W-arm till liquid becomes above E-mark. 3) Calculate the time (t) for falling liquid from Emark to F-mark. 4) Repeat the experiment with the liquid of unknown viscosity and calculate the time for its falling through the two marks. 𝜂1 𝜌1 𝑡1 = 𝜂2 𝜌2 𝑡2 B. Falling Sphere viscometer: Uses: measuring the viscosity of Newtonian and very viscous liquids. N.B: • Water jacket: used to fix temperature. • Sphere (ball) may be made of steel, plastic or glass. 5 Procedure: 1) The ball rolls down through a vertical glass tube containing the test liquid at a constant temperature. 2) We calculate the time required for the ball to fall between the two marks. 𝜂 = 𝐾 𝑡 (𝜌𝑏𝑎𝑙𝑙 − 𝜌𝑙𝑖𝑞𝑢𝑖𝑑 ) C. Rotational viscometer (concentric cylinder viscometer): Procedure: 1) Liquid under test is put between outer and inner cylinder. 2) Inner cylinder is suspended by torsion wire. 3) Rotation of outer cylinder causes the movement of liquid and this is indicated by the pointer on the scale (C0). 4ℎ𝜔𝜂 𝐶0 = 1 1 . 𝑟12 𝑟22 Where: • C0: torsion constant of wire. • h: height of inner cylinder. • ω: angular velocity of outer cylinder. • η: viscosity of material under test. It does not matter how s l o w l y • r1: radius of inner cylinder. you go as long as you do not stop. • r2: radius of outer cylinder. 6