Report 4
advertisement

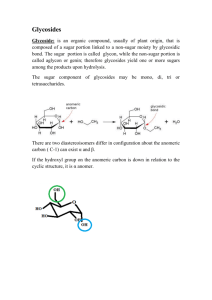
LAB REPORT Alkafeel University/Pharmacy Department Name: Mohannad Fadhal Lab Name: Pharmacognosy Date: 11/11/2018 Group: B1 Title Anthraquinone Glycosides’ Chemical Test Abstract In this experiment, we will evaluate the compound that we extracted from the previous experiment, and make sure that what we extracted is indeed what we want through the usage of the general and specific tests that are in experiment. Then after taking the result from the general and specific test, we will now analyze the compound using TLC, and compare the Rf value between the standard sample and our compound to get what the TLC is generally capable of doing, which is identification and impurities checking. Introduction The anthraquinone glycosides are the ones whose aglycone component is a polyhydroxyanthraquinone derivative. The drugs having these glycosides possess cathartic activity. The polyhydroxyanthraquinone derivatives present in these drugs are chrysophanic acid (1, 8- dihydroxy- 3- methylanthraquinone), aloe emodin (1, 8- dihydroxy-3- methyl anthraquinone), Frangula emodin and rhein (1, 8- dihydroxy anthraquinone -3-carboxylic acid). Glycosides of anthranol and anthrones, reduced derivatives of anthraquinones, also occurs in the plant materials, and they make significant contributions to the therapeutic action of these natural products. The free anthraquinone aglycones exhibit little therapeutic activity. The sugar residue facilitates absorption and translocation of the aglycone to the site of action. The anthraquinone and related glycosides are stimulant cathartics and exert their action by increasing the tone of the smooth muscle in wall of the large intestine. Glycosides of anthranol and anthrones elicit a more drastic action than the corresponding anthraquinone glycosides and a preponderance of the former constituents in the glycosidic mixture can cause discomforting griping action. LAB REPORT Alkafeel University/Pharmacy Department Procedure General test reactions procedures: 1- Schonteten’s Reaction (Borax test): To 2ml of the Senna extract, add 0.1gm of Borax and heat until dissolved. Pour a few drops of the liquid into test tube nearly full of water. 2- Bromine Test: Take 2ml of the Senna extract, add an equal volume or an excess of freshly prepared solution of bromine. Record the color. Specific test reaction procedure: Borntrager's test: Take 1 ml the fraction B (free aglycone) and shake it with 2 ml dilute ammonia (10%). Check the intensity of the color. Chromatography test procedure: 1) Prepare 100ml of mobile phase, and place it in the glass tank. 2) Cover the tank with glass lid and allow standing for 45 minutes before use. 3) Apply the sample spots (fraction A, fraction B& fraction C), and the standard spot on the silica gel plates, on the base line. 4) Put the silica gel plate in the glass tank and allow the mobile phase to rise to about two-third of the plate. 5) Remove the plate from the tank, and allow drying at room temperature, spray first with 25%nitric acid solution and heat for 10 minutes at 110 0C. 6) Allow to cool, and then spray with 5% w/v alcoholic KOH solution. Detect the spot formed and calculate the Rf values. Results 1. 2. 3. 4. Shonteten’s reaction light green color Bromine test clear but turbid solution Borntrager’s test light pink color TLC Rf are nearly matching together with tailing, which proves the existence of impurities LAB REPORT Alkafeel University/Pharmacy Department Discussion In this lab, we will be doing three tests; the first is general, and it is used to prove the existence of glycoside only without specifying the type. The second is specific, and it will help us identify what kind of glycosides we have. The third is TLC, and it will help with both identification and impurities checking. The first test, Shonteten’s test, will be use, and it includes the addition of 1 ml of senna 0.1 g of borax. The benefit of borax here is its usage to make a complex with sugar, which can be benefited from to identify the existence of the glycone part of a glycoside, and if the test gives a positive result the color will change to light green. In the first test, we will be using water bath because the reaction with borax is slow, which will increase borax solubility and hastens the formation of the complex. This general test may give a false positive result because borax react with sugar in general despite it is part of a glycoside or free sugar. So, if we don’t have a previous idea about what type of compound we are testing for; anything with sugar, even pure sugar will be suspected. After the reaction finishes, we will need to add few drops of the complex to a water-containing test tube. We use the water here to dilute the complex because the senna glycoside’s brown color is shadowing the light green color of the complex. So, by diluting the color of the complex will appear proving the existence of a glycone part. The second test will use bromine as an oxidizing agent that helps in also identifying the existence of a glycone part in our glycoside through reacting with it. The reaction positive result will appear as a clear solution that contains turbidity. When we use bromine, we must take our precautions by wearing the labs gadgets (gloves & facemask) because bromine is a dangerous material that even one drop of it could cause a burn. The third test will be done after the first and second tests, and that is after proving the existence of the glycone part of the glycoside. This test, Borntarger’s test, will allow us to prove what kind of glycoside we have in our hands. It includes the addition of ammonia to the aqueous layer portion of the sample which contains the aglycone dianthrone. The color that we will get will be light pink color while if we had monoanthrone, we would get dark pink color. The reason as to why we have dianthrone in the aqueous portion of the previous experiment sample when we should have LAB REPORT Alkafeel University/Pharmacy Department monoanthrones is because dianthrones bounds from the previous experiment didn’t break to form monoanthrones. This happened because we didn't finish the experiment until the end by heating it to about 20 min in order to break the bounds by generating enough activation energy. The compound that will be formed by the color change is a gel-like salt that will precipitate and take the lower layer of the test tube. After we have finished with all the tests; now, we will start running the TLC test. In this test, we will compare between the Rf value of a standard senna glycoside, which is a pill, and the sample glycoside that is supposed to be senna. This TLC test will help us prove that the compound we have is a senna glycoside because even if we proved in the first test that the compound contains glycone part, and the second that it contains aglycone (dianothrone), but that doesn’t prove it is senna anthraquinone glycoside, which is our sample because they are a lot of other compounds, which are anthraquinone glycosides. So, this identification test will help us prove that what we have is senna glycoside by comparing the Rf value of a standard sample and our sample. The result showed that our sample is indeed a senna anthraquinone glycoside with a companying tailing in our sample, which indicates that our sample contains impurities. Conclusion The process partaken in the experiment proves that for us to identify the presence of a glycoside so far in our compounds we need to do three procedures; the first will be general, and it is used to identify if this compound is within the generic group we want. The second will give specificity on which category of the generic group the compound belong too. The third will allow us to specify the exact compound from the category that the compound belongs too. References http://www.yourarticlelibrary.com LAB REPORT Alkafeel University/Pharmacy Department