p-Nitroacetanilide Synthesis Lab: University Chemistry
advertisement

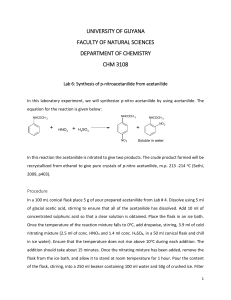
UNIVERSITY OF GUYANA FACULTY OF NATURAL SCIENCES DEPARTMENT OF CHEMISTRY CHM 3108 Lab 6: Synthesis of p-nitroacetanilide from acetanilide In this laboratory experiment, we will synthesize p-nitro acetanilide by using acetanilide. The equation for the reaction is given below: NHCOCH 3 NHCOCH 3 + HNO3 + NHCOCH 3 + H2SO4 NO 2 NO 2 Soluble in water In this reaction the acetanilide is nitrated to give two products. The crude product formed will be recrystallized from ethanol to give pure crystals of p-nitro acetanilide, m.p. 213 -214 oC (Sethi, 2009, p403). Procedure In a 100 mL conical flask place 5 g of your prepared acetanilide from Lab # 4. Dissolve using 5 ml of glacial acetic acid, stirring to ensure that all of the acetanilide has dissolved. Add 10 ml of concentrated sulphuric acid so that a clear solution is obtained. Place the flask in an ice bath. Once the temperature of the reaction mixture falls to 0oC, add dropwise, stirring, 3.9 ml of cold nitrating mixture (2.5 ml of conc. HNO3 and 1.4 ml conc. H2SO4, in a 50 ml conical flask and chill in ice water). Ensure that the temperature does not rise above 10 oC during each addition. The addition should take about 15 minutes. Once the nitrating mixture has been added, remove the flask from the ice bath, and allow it to stand at room temperature for 1 hour. Pour the content of the flask, stirring, into a 250 ml beaker containing 100 ml water and 50g of crushed ice. Filter 1 the crude product, using the Buchner funnel and wash using a small portion of water. Recrystallize from ethanol (30 – 60 ml) and collect the colourless crystals of p-nitro acetanilide. Record the melting point and the yield. Questions 1. State two uses of glacial acetic acid in the experiment. [2marks] 2. Write a reasonable mechanism for the formation of the nitronium ion. [9marks] 3. How is the nitronium ion formed? [2marks] 4. Why is the nitronium ion used in this synthesis? [1mark] 5. In this experiment a dehydrating agent is used, what is this agent? [1mark] 6. Why is the nitrating mixture added in in small portions to the acetanilide solution? [2marks] 7. How could you account for the orientation of the NO2 group in the product? [1mark] ~End of Lab~ 2