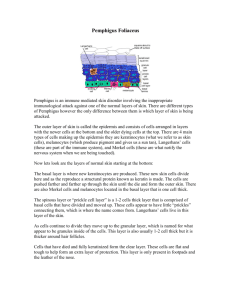
Formalin deposition as artifact in
advertisement

Copyright © 2009 John Wiley & Sons A/S J Cutan Pathol 2009 doi: 10.1111/j.1600-0560.2009.01492.x John Wiley & Sons. Printed in Singapore Formalin deposition as artifact in biopsies from patients affected by a new variant of endemic pemphigus foliaceus in El Bagre, Colombia, South America Background: Most autoimmune diseases occur sporadically; however, endemic pemphigus foliaceus (EPF) is present in specific locales restricted to some geographic rural regions mostly in South America, Central America and in Tunisia (Africa). Its geographic restriction makes it an invaluable natural model for studying how the environment, genetic background and host response contribute to the development of autoimmunity. We described a new variant of EPF in El Bagre, Colombia, (El Bagre-EPF). When we examined the skin biopsies from 10 patients and controls from the endemic area, we detected in a systematic manner several types of pigmentation, sometimes intracellular, and sometimes in the extracellular matrix in most biopsies. Aim: We aim to determine the nature of this pigment in these skin biopsies. Methods: We studied 10 patients and 10 controls matched by sex, age and work activity living in the endemic area by routine hematoxylin and eosin (H&E). Results: We were unable to find any bacteriological or parasitic organism. Specifically, we searched for several tropical disease agents as possible causative agents of this pigment. Iron stains and melanin pigment bleaching techniques failed to determine the etiology of this pigment. We then tried the removal of formalin pigment using picric acid. The pigment was removed after very strong treatment with different acids including picric acid. Conclusions: Formalin pigment shares many properties with hemozoin. In this case, the authors recommend the use of neutral buffered formalin to prevent the formation of formalin pigment especially after long periods of fixation when taking biopsies under extreme temperature and environmental humidity. Abreu Velez AM, Howard MS, Restrepo-Isaza M, Smoller B. Formalin deposition as artifact in biopsies from patients affected by a new variant of endemic pemphigus foliaceus in El Bagre, Colombia, South America. J Cutan Pathol 2009. © 2009 John Wiley & Sons A/S. Ana Maria Abreu Velez1 , Michael S. Howard1 , Marcos Restrepo-Isaza2 and Bruce Smoller3 1 Georgia Dermatopathology Associates, Atlanta, GA, USA, 2 Colombian Institute of Tropical Medicine, Institute of Health Science, CES University, Sabaneta, Antioquia, Colombia, and 3 Department of Pathology, University Arkansas Medical Center, Little Rock, AR, USA Bruce Smoller, Department of Pathology, University Arkansas Medical Center, Little Rock, 4301 West Markham St, Suite 783, Little Rock, AR 72205, USA Tel.: +501 686 5170 Fax: +501 296 1184 e-mail: smollerbrucer@uams.edu Accepted for publication November 13, 2009 1 Abreu Velez et al. Autoimmune diseases can be either organ-specific, such as insulin-dependent diabetes mellitus, or systemic, such as lupus, and their association with other autoimmune diseases in the same individual is not uncommon.1 Because of the chronic nature of this group of diseases, their treatment results in a tremendous cost burden to the Federal government as well as to the health care system, in addition to the serious reduction in the quality of life of the affected individuals. One of the best models to study autoimmunity is the endemic form of pemphigus foliaceus [endemic pemphigus foliaceus EPF].2 – 13 EPF was described in focus in several countries in South America, Central America and in Tunisia (Africa). We discovered a new variant of EPF in El Bagre, Colombia, South America (El Bagre-EPF). This variant of pemphigus resembles Senear–Usher syndrome (SUS), also known as pemphigus erythematosus (PE).14 – 16 PE is defined as an overlap of lupus and pemphigus, with the presence of concomitant antibodies to the basement membrane zone (BMZ) of the skin, as well as with deposition of immunoglobulin and complement concomitant with Intercellular stain between the keratinocytes (ICS). Non-endemic pemphigus foliaceus (PF), or pemphigus vulgaris (PV) lacks BMZ staining. El Bagre-EPF predominantly affects seborrheic areas and also the axilla of patients. Although both EPF variants are endemic, fogo selvagem (FS) is present in some areas of Brazil, affecting both sexes equally, and shows highest incidence of onset at 10–30 years of age.2 – 12 El Bagre-EPF cases resemble SUS because of the presence of lupuslike lesions, with photo-exposed areas of the skin being affected, and the presence of a lupus band detected by direct immunofluorescence (DIF).14 – 16 El Bagre-EPF occurs in a gold-mining municipality in El Bagre, Colombia; completely spares patients in the first and second decades, affects predominantly males of age 40–60 years and a few post-menopausal females.14 – 16 El Bagre-EPF patients are exposed to high levels of mercuric selenides, that act as environmental pollutants, as well to several tropical diseases.14,16 Sun exposure has been shown as the most important exacerbating factor, along with high temperatures and humidity. Figure 1 shows two clinical pictures of a common presentation of this disease and one typical immunofluorescence of the skin of a patient affected by this disease. Methods Samples We examined 10 cases of El Bagre-EPF. All patients selected had the clinical features, lived in the endemic area and showed cell surface 2 staining between keratinocytes by either DIF or indirect immunofluorescence (IIF).9 – 11,14 – 16 The clinical features of patients selected for study included the presence of scaly, crusted erosions on an erythematosus base confined mainly to socalled seborrhoic areas (e.g. face, scalp, upper part of the trunk). Most commonly, the blisters were superficial; however, these flaccid bullae are difficult to find because they are transient and transform into erosions. The erosions can become numerous, showing a tendency to generalize (Fig. 1).16 The patient sera had antibodies to human IgG4 or total IgG against keratynocyte cell junctions in glabrous skin.9 – 11 Other inclusion criteria were that the patient serum immunoprecipitated a ConA affinity purified bovine tryptic 45-kDa fragment of PF antigen or that the serum was positive by immunoblotting for reactivity against desmoglein 1 (Dsg1), as well as for plakin molecules as previously described.14 – 19 Skin biopsies Biopsies from 10 randomly chosen patients and 10 controls matched for sex, age and work activity were studied. Following local anesthesia without epinephrine, lesional skin biopsies were from affected areas on the chest, arms or face. Biopsies were fixed in 10% buffered formalin and processed for hematoxylin and eosin (H&E) staining. Normal skin from the chest was used for the control group. Iron staining In order to evaluate the presence of the heme groups secondary to malaria, we tested for iron in the skin. We performed routine Prussian blue staining.20 Melanin bleach We also considered that the observed pigment could be melanin pigment. Therefore, we performed melanin bleaching by using two identical sections of each specimen, one was bleached and the other was not. We used potassium permanganate 0.15 g, 50.0 ml of 0.3% sulfuric acid and 1% oxalic acid for this procedure as previously described.20 Removal of formalin pigment We examined the sections for the possibility of the presence of ‘formalin pigment’ by dewaxing of sections, rinsing in 100% alcohol, rinsing in 70% alcohol, rinsing in distilled water treating with saturated alcoholic picric acid for 30 min–2 h and washing in running tap water. If yellow staining of the section persisted, we further rinsed in dilute lithium carbonate, and finally rinsed in tap water again.20 Formalin stains in endemic pemphigus Fig. 1. Shows a map of Colombia, with the El Bagre area including some pictures of the El Bagre municipality (urban area in a port). Most patients with El Bagre-EPF lived in the rural area. Finally in the lower lane are shown some clinical features of the El Bagre-EPF patients and one typical direct immunofluorescence using anti-human IgG conjugated with fluorescein isothiocyanate (FITC) showing intercellular stain among the keratinocytes (green arrow), base membrane zone staining (blue arrow) and the black arrow shows a positive acrosiringium also detected by these antibodies. Results On routine H&E sections, several bizarre, pigmented figures were seen (Fig. 2). Neither the iron stain nor the melanin bleach identified the pigment (Fig. 2). However, the formalin destaining technique abrogated this pigment from the sample. Table 1 shows a summary of the different characteristics of formalin pigment compared to malaria pigment, with lipofuscin and other parasitic and tropical diseases that prevail in El Bagre and surrounding areas. The most common parasitic diseases include malaria and leishmaniosis. In addition to these, the gastrointestinal parasites including Dirofilaria immitis (mostly in dogs) are also to be considered. Discussion We considered several etiologies for this strange pigmentation seen in almost all biopsies from the patients and controls from the endemic area. Our first consideration was malaria because the El BagreEPF is predominant in a tropical area with all types of tropical diseases prevalent all year-round. Malaria pigment Despite the worldwide public health impact of malaria, neither the mechanism by which the Plasmodium parasite detoxifies and sequesters heme, nor the action of current antimalarial drugs is well understood.21 – 23 The intraerythrocytic malarial parasite uses hemoglobin as a major nutrient source. Digestion of hemoglobin releases heme that the parasite converts into an insoluble microcrystalline material called hemozoin or malaria pigment. Some authors purified hemozoin from the causative agent of malaria in humans, Plasmodium falciparum, and used infrared spectroscopy, x-ray absorption spectroscopy and chemical synthesis to determine its structure. The molecule consists of an unusual polymer of hemes linked between the central ferric ion of one heme and a carboxylate side-group oxygen of another. The hemes are sequestered via this linkage into an insoluble product, providing a unique way for the malarial parasite to avoid the toxicity associated with soluble heme.21,22 The heme groups released from the digestion of the hemoglobin of infected red blood cells are aggregated into an insoluble material called hemozoin or malaria pigment. Synthetic β-hematin (FeIIIprotoporphyrin-IX)2 is chemically, spectroscopically and crystallographically identical to hemozoin.21 – 23 Some authors reported the crystal structure of β-hematin determined using simulated annealing techniques by analyzing powder diffraction data obtained with synchrotron radiation.21 – 23 The authors reported that the molecules were linked into dimers through reciprocal iron–carboxylate bonds to one of the propionic side chains of each porphyrin, and the dimers form chains linked by hydrogen bonds in the crystal.21 – 23 3 Abreu Velez et al. Fig. 2. Shows different staining; some seem to be intracellular, some extracellular, some with bizarre forms and others resembling malaria shistosomiasis. In the upper lane different microscopic pictures of the melanin bleach that unsuccessfully clear the pigment are shown. The fourth lane of pictures shows the iron stain (blue) (red arrows) which does not colocalize with the formalin pigment. We further considered dracunculiasis, onchocerciasis, schistosomiasis, trachoma and hydatidoses based on the bizarre chitin-like structures that may 4 resemble some of these parasitic diseases.24 – 26 As mentioned above, we also took into consideration the diagnosis of schistosomiais as a possible causing Commonly seen in biopsies from tissues containing large hemorrhage or tissues heavily pigmented with blood Yellowish-brownish Location Shape and color Formalin pigment Malaria pigment formed by the breakdown of hemoglobin of RBC; usually located in the hypertrophy Kupffer cells containing parasitized in the liver Finely granular yellowish-brown pigment granules, around the nucleus Group of lipid pigments residues of lysosomal digestion found in cardiac, smooth muscle, macrophage sweat glands, liver, kidney, adrenals, nerve cells and ganglion cells Malaria pigment It is brownish-black pigment and yellowish-orange in polarized light Lipofuscin or lipochrome granules May be present in areas of old hemorrhage or are deposited in tissues with iron overload Small yellowish-brown granules Melanins are pale brown to dark brown and even black pigments that are found intracellular in the cytoplasm; melanins are found in hair, skin, retina and the substantia nigra of the brain The shape of melanosomes considerably varies Melanin pigment Iron (or hemosiderin pigment) Table 1. Summary of histopathologic characteristics of formalin pigment compared to malaria pigment and other pigmented structures The heartworm is a type of filaria, a small thread-like worm After infection, the heartworms deposit under the skin; then they migrate to the muscles of the chest and abdomen and into the bloodstream to the heart to reside in the right ventricle and in the pulmonary artery. Sometimes seen in eye, brain, or an artery in the leg Dirofilaria immitis Promastigotes that reach the puncture wound are phagocytized by macrophages and transform into amastigotes; amastigotes multiply in infected cells and affect different tissues, depending in part on which Leishmania species is involved. Usually found on skin, liver or spleen Flagellated extracellular promastigotes in the sandfly vector and as aflagellar obligate intracellular amastigotes within mononuclear phagocytes Leishmania Formalin stains in endemic pemphigus 5 6 Not available 100% alcohol, rinsing in 70% alcohol, then potassium permanganate 0.15 g, 50.0 ml of 0.3% sulfuric acid, and 1% oxalic acid Bleaching Formalin pigment Special stains or other annotations Table 1. Continued Fontana–Masson, DOPA-oxidase Strong oxidizing agent such as potassium permanganate or hydrogen peroxide Sudan black B, long Ziehl–Neelson acid fast and Schmorl’s methods Not available Ammonium hydroxide–alcohol for 5–10 min, and with 0.1% nuclear fast red for 5 min Melanin pigment Romanowsky, Fields, Giemsa stains Malaria pigment Lipofuscin or lipochrome granules Leishmania Visualization of the amastigote(Leishman– Donovan bodies); buffy-coat preparations of peripheral blood or aspirates from marrow, spleen, lymph nodes or skin lesions should be spread on a slide and stained with Leishman’s or Giemsa’s stain Not available Dirofilaria immitis Acid phosphatase histochemistry. Not available Perl’s iron stain, the Prussian blue reaction Pappenhein stain Bleached by 10% H2 O2 Iron (or hemosiderin pigment) Abreu Velez et al. Formalin stains in endemic pemphigus agent of this pigment. We considered this entity, based on the fact that we recently discovered several autoantibodies to nerves and mechanoreceptors on the skin of many El Bagre-EPF cases (Abreu et al., submitted for publication). It is known that schistosomiais is probably to be responsible for a significant proportion of cases of myelopathy occurring in endemic areas. Spinal cord schistosomiasis is the most severe ectopic form of schistosomal infection with the presence of lumbar spinal cord infection in patients with transverse myelitis, produced by Schistosoma japonicum. Several of the El Bagre-EPF patients also describe lower limb weakness associated with lumbar and/or lower limb pain, urinary retention and intestinal dysfunction. These symptoms are similar to those described when spinal cord schistosomiasis is present.27 However, we also considered that pigment was seen in biopsies taken from the palms of the patients affected by El Bagre-EPF. The cercaria could be introduced through the skin, but it is extremely rare that the schistosoma parasite could be located on the hands. Usually, it is present in the vascular plexus of the peritoneum and peri-vesical areas. The eggs can pass through different organs, including the liver, the intestine, and are discharged via urine or feces. If the eggs are trapped in tissues, the formation of granulomas is almost expected. Finally, El Bagre and the surrounding area are not endemic for schistosoma, which was the parasite most similar in appearance to what we found in the biopsies. Finally, we considered formalin pigment. Formalin pigment may precipitate into the tissue, particularly when the biopsies have been fixed in a formalincontaining fixative with an acid pH.20 Formalin pigment artifacts are commonly seen in biopsies from tissues containing abundant hemorrhage or tissues heavily pigmented with blood.20 Other authors reported encountering similar problems with placental biopsies from patients from Tanzania where the environmental temperatures and humidity are also very high.28 The authors tested the accuracy of standard and polarization microscopy in the evaluation of 500 placental specimens from an area of high malarial endemicity in Tanzania.28 They reported the use of polarization microscopy to increase the sensitivity of detection of pigment and parasites because of the marked birefringence of hemozoin present in mature stage parasites which accumulate in the placenta.28 The authors noticed that in placental specimens with long periods of fixation with formalin, formalin pigment formation could be a problem, based on the fact that this reagent shares many properties with hemozoin. The authors recommended avoiding these problems by using neutral buffered formalin to prevent the formation of formalin pigment in placentas.28 Our samples were fixed for longer periods and were under extremely high temperature and humidity in the jungle. This may be the problem, because we used buffered formalin containers. Perhaps under such extreme conditions the prevention of formalin pigment formation maybe even more difficult. This study brings to light a problem that many researchers and clinicians may face when working in extreme tropical conditions of temperature and humidity. We highly recommend the use of fresh formalin at pH 7, with the tissue fixed for a maximum of 24 h to avoid these false positive artifacts that can mislead the investigations, resulting in confusion with parasitic and tropical diseases. Acknowledgments We acknowledge Drs Patricia Adem, MD, Wun-Ju Shie, MD, PhD, MPH and Sherif R. Zaki, MD, PhD of the Centers for Disease Control and prevention, National Center for Zoonotic, Vector-Borne and Enteric Diseases, Division of Viral and Rickettsial Diseases, Infectious Diseases Pathology Branch, for their excellent second opinion on the biopsies. We also acknowledge Ms Kimberly Hall at University Arkansas Medical Center and Mr Jonathan Jones, for their excellent technical work. This work was performed with funds from Georgia Dermatopathology Associates (GDA). References 1. Theofilopoulos AN. The basis of autoimmunity: Part I: mechanisms of aberrant self-recognition. Immunol Today 1995; 16: 90. 2. Diaz LA, Sampaio SAP, Rivitti EA, et al. Endemic pemphigus foliaceus (fogo selvagem). II. Current and historical, epidemiologic studies. J Invest Dermatol 1989; 92: 4. 3. Squiquera HL, Diaz LA, Sampaio SAP, et al., and The Cooperative Group for Fogo Selvagem Research. Serological abnormalities in patients with endemic pemphigus foliaceus (fogo selvagem), their relatives and normal donors from endemic areas and non-endemic areas. J Invest Dermatol 1988; 91: 189. 4. Stanley JR, Kiaus-Kovtun V, Sampaio SAP. Antigenic specificity of fogo selvagem autoantibodies is H similar to North American pemphigus foliaceus and distinct form pemphigus vulgaris. J Invest Dermatol 1986; 87: 197. 5. Vieira JP. Novas contribucioes ao estudo do penfigo foliaceo (fogo-selvagem) no estado de Sao Paulo. Sao Paulo, Brazil: Empresa Grafica da Revista dos Tribunais, 1940. 6. do Valle Chiossi MP, Roselino AM. Endemic pemphigus (‘‘fogo selvagem’’): a series from the northeastern region of the state of Sao Paulo, Brazil, 1973–1998. Rev Inst Med Trop Sao Paulo 2001; 34: 59. 7. Heimgartener E, De Heimmagartner V. Experience with endemic dermatological diseases in the Peruvian wilderness: ‘‘Mucocutaneous leishmaniasis and Brazilian foliaceus pemphigus.’’ Med Cutan Ibero Lat Am 1976; 4: 1. 8. Galarza C, Ronceros D, Mendoza G, et al. Penfigo foliaceo en Uyucali Peru, reporte de 16 casos. Dermatol Per 2001; 11: 82. 9. Aldama A, Correa J, Rivelli V, Mendoza G. Tipos y variantes de pénfigo en el Hospital Nacional de Paraguay. Revisión de 70 casos. Med Cut ILA 2000; 28: 242. 10. Ortega Loayza AG, Ramos W, Elgart G, et al. Antibodies against desmoglein 1 in healthy subjects in endemic and 7 Abreu Velez et al. 11. 12. 13. 14. 15. 16. 17. 18. 19. 8 nonendemic areas of pemphigus foliaceus (fogo selvagem) in Peru. Int J Dermatol 2006; 45: 538. Lever WF. Pemphigus and pemphigoid, 1st ed. Springfield: Charles C Thomas, 1965; 15. Bastuji-Garin S, Souissi R, Blum L, et al. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol 1995; 104: 302. Robledo MA, Prada SC, Jaramillo D, Leon W. SouthAmerican pemphigus foliaceus: study of an epidemic in El Bagre and Nechi, Colombia 1982 to 1986. Br J Dermatol 1988; 118: 737. Abreu-Velez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol 2003; 49: 609. Abreu-Velez AM, Maldonado JG, Jaramillo A, et al. Immunological characterization of a unique focus of endemic pemphigus foliaceus in the rural area of El Bagre, Colombia. J Invest Dermatol 1998; 110: 516a. Abreu-Velez AM, Hashimoto T, Bollag WB. A unique form of endemic pemphigus in northern Colombia. J Am Acad Dermatol 2003; 49: 599. Figueira MV, Ferreira de Souzas L. Estudo sobre cobre sėrico. [Tese apresentada ao Departamento de Patologia de Apoio clı́nico da Universidad Federal Fluminense, Rio de Janeiro, para habilitaca̧o a docencia livre de Bioquimica clinica].(Study of seric levels of cooper, thesis for the Departartament of Clinical pathology, Federal University, Rio de Janeiro), 1976. Gamarski J, Auad T. D-Penicilamina no penfigo foliaceo SulAmerricano. (D-penicillamine and South American pemphigus foliaceus). Rev Inst Med Trop Sao Paulo 1977; 9: 138. Abreu Velez AM, Warfvinge G, Herrera WL, et al. Detection of mercury and other undetermined materials in skin biopsies of 20. 21. 22. 23. 24. 25. 26. 27. 28. endemic pemphigus foliaceus. Am J Dermatopathol 2003; 25: 384. Burns T, Breathnach S, Cox N, Griffiths C, eds. Histology of the skin. Rook’s textbook of dermatology, 7th ed, chapter 7. Blackwell Science, Oxford, published online: 4 February 2008; 7.0. Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment β-haematin. Nature 2000; 404: 307. Fitch CD, Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J Biol Chem 1987; 262: 15552. Bohle DS, Conklin BT, Cox D, et al. Structural and spectroscopic studies of β-hematin the heme coordination polymer in malaria pigment. In Wisian-Neilson P, Allcock HR, Wynne KJ, eds. Chapter in inorganic and organometallic polymers II, ACS symposium series 572. ACS, 1994; 497–515. Hopkins DR, Richards FO, Ruiz-Tiben E, Emerson P, Withers PC. Dracunculiasis, onchocerciasis, schistosomiasis, and trachoma. Ann N Y Acad Sci 2008; 1136: 45. Kanamura HY, Dias L, de souza C, et al. A comparative epidemiologic study of specific antibodies (IgM AND IgA) and parasitological findings in an endemic area of low transmission of Schistosoma mansoni. Rev Inst Med Trop Sao Paulo 1998; 40: 85. Giboda M, Zdárská Z. Alkaline phosphatase as marker of Schistosoma mansoni egg viability. Folia Parasitol (Praha) 1994; 41: 55. Nobre V, Silva LCS, Ribas JG, et al. Schistosomal myeloradiculopathy due to Schistosoma mansoni: report on 23 cases. Mem Inst Oswaldo Cruz 2001; 96: 137. Romagosa C, Menendez C, Ismail MR, et al. Polarisation microscopy increases the sensitivity of hemozoin and Plasmodium detection in the histological assessment of placental malaria. Acta Trop 2004; 90: 277.