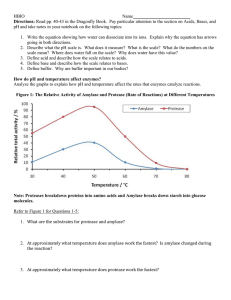
Saranraj87
advertisement

ISSN: 2321-3485 Impact Factor : 1.2305[UIF-2014] Volume - 3 | Issue - 34 | 21th Dec - 2015 Reviews Of Progress COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW M. Munish Kumar1 and P. Saranraj2 1 Department of Biochemistry, Sacred Heart College (Autonomous), Tirupattur, Tamil Nadu, India. 2 Assistant Professor of Microbiology, Department of Biochemistry, Sacred Heart College (Autonomous), Tirupattur, Tamil Nadu, India. ABSTRACT Microbial proteases are among the most important hydrolytic enzymes and have been extensively since the advent of enzymology. They are essential constituents of all forms of life on earth. They can be cultured in large quantities in relatively short time by established fermentation methods and produce an abundant, regulate supply of the desired product. In recent years there has been a phenomenal increase in the use of alkaline protease as industrial catalysts. Proteases are enzymes occurring everywhere in nature be it inside or on the surface of living organisms such as plants, animals and microbes. Proteases are ubiquitous being found in all living organisms and are essential for cell growth and differentiation. The extracellular proteases are of commercial value and find multiple applications in various sectors. The inability of the plant and animal proteases to meet current world demands has led to an increased interest in microbial proteases which account for the total worldwide enzymes sale. Key words: Enzymes, Protease, Bacillus sp., Industrial application. 1. INTRODUCTION: Proteases are the group of enzymes that have been found in several microorganisms like bacteria and fungi which are involved in breakdown of complex protein molecules into simple polypeptide chains (Absida, 1985). The induction of protease requires a substrate like peptone, casein and other proteins. The ammonia as final product of enzymatic reaction of substrate hydrolysis, responses enzyme synthesis by a well known mechanism of catabolite repression. This extracellular protease has also been commercially exploited to assist protein degradation in various industrial processes (Srinubabu et al., 2007). Extracellular protease high commercial value and multiple application in various industrial sectors, such as detergent, food, pharmaceutical, leather, diagnostic, waste management and silver recovery industries (Godfrey and West, 1996). Among proteases, alkaline proteases are defined as enzymes that are active from the Available online at www.lsrj.in 1 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW neutral to the alkaline pH range (Gupta et al., 2002). These enzymes are generally active between pH 9.0 and 11.0 with the exception of a few higher pH values of about 12.0 and 13.0 (Kumar and Takagi, 1999). The microbial protease deals with very large group of enzymes from the complete diversity of microorganisms. Microbial proteases are ubiquitous in all microorganisms where they have a variety of biochemical, physiological, and regulatory functions. Microbial proteases, especially from Bacillus sp. have traditionally held the predominant share of the industrial enzyme market of the worldwide (Beg and Gupta et al., 2003). Bacteria belonging to Bacillus sp. are by far the most important source of several commercial microbial enzymes. They can be cultivated under extreme temperature and pH conditions to give rise to products that are in turn stable in a wide range of harsh environments. Bacillus is a rod shaped, Gram positive, spore forming, aerobic, usually catalase positive, chemoorganotropic bacterium. Alkaliphilic Bacillus sp. can be found mostly in alkaline environments such as soda soils, soda lakes, neutral environments and deep-sea sediments. Animal manure, man-made alkaline environments such as effluents from food, textile, tannery and potato processing units, paper manufacturing units, calcium carbonate kilns and detergent industry are also good sources (Akbalik, 2003; Siva Sakthi et al., 2011; Saranraj et al., 2012 and Geetha et al., 2012). Protease are among the most valuable catalysts used in food, pharmaceutical and detergent industries because they hydrolyze peptide bonds in aqueous environments and synthesize peptide bonds in microaqueous environments (Ogino et al., 1999). Microbial proteases dominate the commercial applications with large market share taken from Bacillus subtilis. For laundry detergent applications, a major requirement for commercial applications is thermal stability because thermal denaturation is a common cause of enzyme inactivation (Kavi Karunya et al., 2011; Senthilkumar et al., 2012; Naidu and Saranraj, 2013). Considering the commercial significance of proteases, there were some attempts to study and maximize protease production and economize them in detergents (Chauhan and Gupta et al., 2004). For the prospective uses of proteases and their high demand, the need exists for the invention of new strains of marine bacteria that produce enzymes with novel properties and the development of low cost industrial media formulations (Esakkiraj et al., 2011; Annamalai et al., 2013). Optimization of media components by classical methods which involves the change of single variable optimization strategy has some disadvantages, such as time consuming, requirement of more experimental data sets, and missing the interactions among variables (Cazetta et al., 2007; Li et al., 2008). Owing to these disadvantages, it has been replaced by statistical optimization such as response surface methodology, which is an efficient experimental strategy to seek optimal conditions for the multi- variable system. This method has been successfully applied for the optimization of multiple variables in many fermentation processes and showed satisfactory results (Montgomeryd and Runger, 2002). Enzyme cost is also the most critical factor limiting wide use of alkaline proteases for different applications. A large part of this cost is accounted for the production cost of the enzyme which includes cost of media components as well as downstream processing. In submerged fermentation up to 40 % of the total production cost of enzymes was due to the production on the growth substrate (Enshasy et al., 2010; Siva Sakthi et al., 2012; Saranraj and Stella, 2013). The protease production mainly requires the appropriate substrates. There are many substrates used for protease production, which include skim milk, milk, peptone and casein. Some of the agricultural wastes, animal wastes, and plant wastes are also used as substrates for the production of protease, because they are readily available and economically very cheap and also they have high protein content. Yang et al. (1999) stated that whey is one of the good substrates used for protease Available online at www.lsrj.in 2 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW production due to its high protein content. The experiments showed that the whey produced in dairies constituted a large amount of protein and consequently the study of its utilization by fermentation process could be of greater significance (Romero et al., 1998; Saranraj and Naidu, 2014). The thermostable proteases are advantageous in some applications, due to employing higher processing temperatures, thus yielding faster reaction rates, increasing solubility of nongaseous reactants and products and reducing incidence of microbial contamination by mesophilic organisms. Proteases secreted from thermophilic bacteria are unique and have become increasingly useful in a wide range of commercial applications (Adams and Kelly et al., 1998). The potential use of thermostable enzyme in range of biotechnological applications is widely acknowledged. Thermostable proteases are advantageous in some applications because higher processing temperature can be employed, resulting faster reaction rates due to a decrease in viscosity and increase in diffusion co-efficient of substrates. Furthermore, higher processing temperature will also increase the solubility of nongaseous reactants and products as well as reduce the incidence of microbial contamination by mesophilic organisms (Olajuyigbe and Ajele, 2005). It was expected that the applications will keep increase in the future as will the need for stable biocatalysts capable of withstanding harsh conditions of operation which occurred normally in industry (Beg and Gupta, 2003; Ellaiah et al., 2003; Nascimento and Martins, 2004). 2. ALKALINE PROTEASE Alkaline proteases are one of the most important groups of microbial enzymes that find varied uses in various industrial sectors such as leather, detergents, textile, food and feed etc. Industrially important alkaline proteases from bacterial sources have been studied extensively, of which Bacillus sp. was most reported. Most of the alkaline proteases that play a role in industries are thermostable as their optimal activity lies between 50 °C to 70 °C. The recently used statistical methods have given way to a more rapid optimization process for alkaline protease production. Other than traditional industrial uses, alkaline proteases have promising application in feather degradation and feather meal production for animal feed (Singhal et al., 2012). An effective proteolytic enzyme producing microbial strain has been isolated from marine soil banana tree and evaluated its extracellular protease production properties with respect to different fermentative physiological parameters. The strain has been identified based on biochemical tests according to Bergey’s Manual of systematic Bacteriology as Bacillus sp and designated as SVN12. This strain has potential to hydrolyse Starch, Tributyrin, Gelatin and Casein revealing its industrial potential for production of multi-enzyme complex. Since the isolated strain which is not inhibited by EDTA suggesting the enzyme not belongs to the metalloprotease. But the produced enzyme is inhibited by phenyl methyl sulfonyl fluoride (PMSF) suggesting the enzyme belongs to the serine type of protease. The maximum enzyme production is observed at pH 8.0 and incubated at 37°C under aerated environment. Analysis of the pH profile before and after fermentation depicted that irrespective of initial medium pH, it is shifted to pH 9.0 after fermentation suggesting the enzyme produced is alkaline in nature. The strain Bacillus SVN12, showed the maximum growth at 37°C with alkaline protease production of 9900 U/ml in 72h of incubation at pH 8.0 and at rpm 150 with 1.0 % inoculums. Several carbon and nitrogen sources were screened to understand their impact on growth and subsequent production of enzyme (Srinivas et al., 2013). Microorganism was found to be closely related to Bacillus cereus based on 16S ribosomal DNA sequencing. The culture conditions for higher protease production were optimized with respect to carbon and nitrogen sources, metal ions, pH and temperature. Maximum protease production was Available online at www.lsrj.in 3 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW obtained in the medium supplemented with 1 % skim milk, 1 % starch and 0.6 % MgSO4.7H2O, initial pH 8.0 at 35 °C. The best enzyme production was obtained during the stationary phase in which the cell 8 density reached to 1.8 × 10 cells/ml. The level of protease was found to be low in the presence of inorganic nitrogen sources. The protease production was diminished in the presence of sucrose and lactose. The extreme stability towards Triton X-100, Tween 20 and SDS was observed by Bacillus sp. CA15 alkaline protease. The enzyme activity was inhibited by PMSF suggested that presence of serine residues at the active sites (Fikret et al., 2011). Roja et al. (2012) isolated and identified the Bacillus licheniformis and used to examine the changes in alkaline protease production following UV irradiation. Induction of mutation in Bacillus licheniformis strain was carried out by 0, 3, 6, 9, 12, 15, 18 and 20 min with 30-W germicidal lamp that 0 has radiation at 2540 – 2550 A at a distance of 15 cm in dark and irradiated. A total of 17 mutants were selected. They were designated as Bl1 to Bl9 and Bl10 to Bl17. Among these, only three strains viz., Bl2, Bl11, and Bl16 did exhibit high efficiency in production on the basis of relative growth production. Of the seventeen mutants of Bacillus licheniformis, ten were chosen to assay their productivity. Mutants like Bl8, Bl3, Bl16 were the most effective in enzyme production under submerged conditions being 180, 140, 128 U/ml respectively. Results of their research revealed that the alkaline protease activity assay under submerged culture conditions was more accurate than the relative growth production method because there is no correlation between zone diameter and the ability to produce the enzyme in submerged cultures. High level of productivity was increased with Bl8 mutant of Bacillus licheniformis, indicating that the enzyme is to be thermo-alkaliphilic protease. Among the various protease producing isolates, two species namely Bacillus licheniformis and Bacillus coagulans efficiently produced alkaline protease in glucose extract – asparagine (GYA) medium. The protease production efficiency of these organisms was measured with different carbon sources, incubation time, pH and temperature. Enzyme production was better in Bacillus licheniformis than in Bacillus coagulans. From the above investigations, it was concluded that the protease production by these microbes at wide temperature and pH ranges could be explored for varied industrial applications (Asokan and Jayanthi, 2010). The protease enzyme was found to be a thermostable alkaline serine protease with optimal activity at 75 °C and pH 10. The enzyme had a half life of 45 min at 80 °C and 12 hrs at 70 °C. It was stable over the pH range of 5.0 to 11.0. The enzyme was inhibited by phenylmethane - sulfonyl fluoride and EDTA but not by N-Tosyl-L phenanyl alanine chloromethyl, iodoacetamide and o-phenathroline. The 2+ 2+ 2+ 2+ ions Ca and Fe at 0.5 and 2.5 mM concentration were stimulatory, while Mg and Mn had little effect on the enzyme activity. The enzyme produced by bacterium Bacillus sp. was concluded to be an alkaline protease that requires calcium and iron ions for its activity (Parawira and Zvauya, 2012). 3. PRODUCTION OF ALKALINE PROTEASE FROM PROTEOLYTIC Bacillus ISOLATES Proteolytic enzymes can be produced by submerged and solid state fermentation. For the growth of fungi, Solid state fermentation is most appropriate method because it resembles the natural habitat of the fungi. Some characteristics make Sold state fermentation (SSF) more attractive than Submerged fermentation (SMF): simplicity, low cost, high yields and concentrations of the enzymes and the use of inexpensive and widely available agricultural residues as substrates (Chutmanop et al., 2008). Solid state fermentation (SSF) is preferred over Submerged fermentation (SMF) since it exhibit advantages such as; reduced production cost, higher yield and less energy consumption (Pandey, 2003). Proteases are also envisaged as having extensive applications in development of eco-friendly Available online at www.lsrj.in 4 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW technologies as well as in several bio-remediation processes (Bhaskar et al., 2007; Wang et al., 2008). Most of the studies on microbial proteases are confined to characterization of enzymes with relatively fewer reports on optimization of enzyme production (Bajaj and Sharma et al., 2011). Proteases can resist extreme alkaline environments produced by a wide range of alkalophilic microorganisms. Different isolation methods are discussed which enable the screening and selection of promising organisms for industrial production. Further, strain improvement using mutagenesis and recombinant DNA technology can be applied to augment the efficiency of the producer strain to a commercial status. The various nutritional and environmental parameters affecting the production of alkaline proteases are delineated. The purification and properties of these proteases was also discussed by various researchers, and the use of alkaline proteases in diverse industrial applications was highlighted (Ganesh and Hiroshi, 1999). Li et al. (2008) isolated a 41 Bacillus subtilis from a raw milk sample. Forty-one isolates with a clear zone surrounding a colony were primary selected and identified by using staining techniques, biochemical characteristics and growth of bacteria at 50 °C. Ten out of 41 isolates showing a clear zone diameter of more than 10 mm were selected and evaluated for the presence of protease activity. The BA26 and BA27 gave high levels of protease activity with 12 U/ml protein towards 1.5% casein at 50 °C for 10 min. Based on the biochemical and physiological characteristics, BA26 and BA27 were classified as Brevibacillus non reactive. However, their 16S rRNA gene sequence showed 99 % identity to that of Bacillus subtilis. The enzymes were more specific to 1 % casein than 1 % gelatine. Moreover, the selected bacteria selected extracellular protease upon incubation at 50 °C and 121 °C. This confirmed that the enzyme proteases produced by Bacillus sp. are thermotolerant proteases. Randa et al. (2009) isolated thermostable organic solvent-tolerant protease producer and identified as Bacillus subtilis strain, based on the morphological characteristics, biochemical properties and 16S rRNA analysis. The production of the thermostable organic solvent tolerant protease was optimized by varying various physical culture conditions. Inoculation with 5.0 % (v/v) of inoculum size, in a culture medium (pH 7.0) and incubated for 24 hrs at 37 °C with 200 rpm shaking, was the best culture condition which resulted in the maximum growth and production of protease (444.7 U/ml; 4042.4 U/mg). The protease was not only stable in the presence of organic solvents, but it also exhibited a higher activity than in the absence of organic solvent, except for pyridine which inhibited the protease activity. The enzyme retained 100 %, 99 % and 80 % of its initial activity, after the heat treatment for 30 min at 50 °C, 55 °C, and 60 °C respectively. Debananda et al. (2010) analyzed the biochemical, physiological characterization and acid production from various carbohydrates by API 50 CHB tests led to its identification as Bacillus subtilis and it was designated as Bacillus subtilis strain. Corn starch (1 %) and peptone (0.2 %) was as optimal C and N sources for protease production. The enzyme was active over a wide range of temperatures and pH with optima at 500 °C and pH 8. It was inhibited by PMSF as well as EDTA and seems to be a metalactivated serine protease or a mixture of enzymes. SH1, interestingly, was stimulated by FeSO4. Geethanjali and Anitha (2011) screened the best protease producing Bacillus subtilis. Then, production medium for Bacillus subtilis were optimized by using different pH, temperature, carbon and nitrogen sources for 48 hours fermentation period. The findings of their study revealed that the protease production can be optimized at pH – 9.0, temperature 40 °C by utilizing carbon as glucose and nitrogen source as peptone. Sharma and Aruna (2011) carried out the primary screening for protease production by observing the zone of clearance on Skim milk agar, GYEA milk agar and Gelatin plates. Different parameters like temperature, pH, incubation time and aeration studies were initially done to get Available online at www.lsrj.in 5 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW maximum protease production. A temperature of 55 ºC and pH 9 gave maximum production in 24 hours under shaker conditions. Different carbon and nitrogen source in the form of fine powder of organic and inorganic meals were studied to select a suitable substrate for protease production. The highest level of protease was obtained to be the best inducer while inorganic source in the form of ammonium salts repressed the enzyme activity. Media components at 0.2 % MgSO4, 0.05 % KCl was found to give maximum enzyme activity. The substrates with highest water absorption index and more heterogeneous granulometric distribution have positively influenced on protease production. Some cultivation parameters were studied by Ruann Janser Soares and Helia Harumi (2013) and the results showed that the optimum fermentation medium was composed of wheat bran, 2.0 % (w/w) peptone and 2.0 % (w/w) yeast extract, and the conditions for maximum protease production were an initial moisture content of 50.0 %, an inoculums level of 107 spores g-1 and an incubation at 23 1C. The biochemical characterization using experimental design showed that the enzyme was most active over the pH range 5.0 – 5.5 and was stable from pH 4.5 to 6.0, indicative of an acid protease. The optimum temperature range for activity was 55 – 60°C and the enzyme was stable at 35 – 45°C. The results showed that wheat bran have great potential as support matrix for protease production by Aspergillus oryzae in Solid State Fermentation (SSF). Mrunmaya et al. (2013) tested the ability of the bacterium to tolerate high temperatures and identified as Bacillus amyloliquefaciens by morphology, biochemistry and sequencing of its 16S rRNA gene. BLAST search analysis of the sequence showed maximum identity with Bacillus amyloliquefaciens. The identified strain exhibited considerable protease activity. Phylogenetic analysis of the isolate revealed close affiliation with thermophilic Bacillus species. The G + C content were found to be 54.7 %. Marcela et al. (2013) isolated hundred and fifty six isolates and type strains Bacillus subtilis and Bacillus amyloliquefaciens were classified according to phenotypic and molecular characteristics. Only differences in growth temperature could be used to distinguish isolates among the phenotypic traits tested and these distinctions were supported by molecular analysis. Randomly amplified polymorphic DNA analysis (RAPD) analysis was shown to be a friendly, technically simple and accurate method for rapid screening and identification of Bacillus subtilis and Bacillus amyloliquefaciens. Further analysis of 16S rRNA, rpoB and gyrA gene sequences of the isolates was done to confirm species identification. Sequences from the isolates and type strains showed between 96.5 – 100 % (16S rRNA), 94.8 – 100 % (rpoB) and 80.6 - 99.6 % (gyrA) similarity, thus allowing for more refined distinction using the rpoB and gyrA genes. In addition, gyrA gene sequences had greater discrimination potential in having higher divergence between species (18.2 ± 0.7 %) than did rpoB sequences (4.9 ± 0.3 %). BOX PCR fingerprinting was shown to have the potential for analysis of genotypic diversity of these species at the strain level. Lakshmi and Prasad (2013) examined the changes in alkaline protease production by Bacillus licheniformis following UV irradiation. Induction of mutation in Bacillus licheniformis strain was carried out by 0, 3, 6, 9, 12, 15, 18 and 20 min with 30-W germicidal lamp that has radiation at 2540 – 2550 A0 at a distance of 15 cm in dark and irradiated and then total of 17 mutants were selected. They were designated as Bl 1 to Bl 9 and Bl 10 to Bl 17. Among these Bacillus licheniformis isolates, only three strains viz., Bl2, Bl11 and Bl16 did exhibit high efficiency in production on the basis of relative growth production (C/G). Of the seventeen mutants of Bacillus licheniformis, ten were chosen to assay their productivity. Mutants no Bl8, Bl3, Bl16 were the most effective in enzyme production under submerged conditions being 180, 140, 128 U/ml respectively. Results of their study revealed that the alkaline Available online at www.lsrj.in 6 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW protease activity assay under submerged culture conditions was more accurate than the relative growth production (C/G) method because there was no correlation between zone diameter and the ability to produce the enzyme in submerged cultures. High level of productivity increased with Bl8 mutant of Bacillus licheniformis, indicating that the enzyme is to be thermo-alkaliphilic proteae. 4. FACTORS INFLUENCING PROTEASE PRODUCTION BY Bacillus SPECIES Microbes which produces alkaline protease needs to be screened and should be optimized to produce substantial amount of protease by adapting favourable conditions like optimal pH, temperature and favourable media should be demonstrated to increase its yield. Alkaline protease from extreme organisms should be produced commercially in high yield at a low-cost method (Rajesh et al., 2005). Although, there are many microbial sources available for producing proteases, only a few are recognized as commercial producers. A large proportion of the proteases are derived from Bacillus strains (Wang et al., 2006). All microorganisms have their optimal conditions for their growth, reproduction and other physiological activities. Depending upon the nutritional factors such as carbon and nitrogen sources, environment factors like incubation temperature and cultural conditions like pH their growth, reproduction and physiological activities showed significant different in growth and enzyme production. Valerie et al. (2009) quantitatively assessed and showed that the strains of Bacillus subtilis, the Bacillus cereus group, Paenibacillus polymyxa and Bacillus amyloliquefaciens are strongly proteolytic, along with Bacillus licheniformis, Bacillus pumilus and Lysinibacillus fusiformis to a lesser extent. Lipolytic activity could be demonstrated in strains of Bacillus subtilis, Bacillus pumilus and Bacillus amyloliquefaciens. Qualitative screening for lecithinase activity was also revealed that Paenibacillus polymyxa strains produce this enzyme besides the Bacillus cereus group that was well known for causing a ‘bitty cream’ defect in pasteurized milk due to lecithinase activity. They found a strain of Paenibacillus polymyxa were able to reduce nitrate. A heat-stable cytotoxic component other than the emetic toxin was produced by strains identified as Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus pumilus and the Bacillus cereus group. Variations in expression levels between strains from the same species were noticed for all tests. The importance of aerobic spore forming bacteria in raw milk as the species that are able to produce toxins and spoilage enzymes are all abundantly present in raw milk. Moreover, some strains are capable of growing at room temperature and staying stable at refrigeration temperature. Nisa et al. (2010) optimized the protease production by bacterial strain, seven fermentation variable were screened using a Placket-Burman design, and were then further optimized via Response surface methodology (RSM) based on a Central composite design (CCD). Three significant variables, i.e., soy flour, skimmed milk and shaker speed were selected for their study. The optimal values were 2.0 % soy flour, 0.1 % skimmed milk and a shaker speed of 280 rpm. The experimental result (1537 units/ml) in a medium optimized for protease production was in good agreement with the predicted value of a quadratic model (1576 units/ml), thus confirming its validity. In addition, the adequacy of the model was supported by a coefficient of determination (R2) of 0.912. protease production in the optimized medium (1537 units/ml) in the shaken flask culture, when the experiment was scaled up in a stirred tank reactor, 1891 units/ml protease activity was achieved at 27 hrs of cultivation, which was an overall 2.6 fold increase over the basal medium. Gitishree and Prasad (2010) identified the Bacillus subtilis and the isolated bacterial were positive on Skim milk agar (1 %) and selected as protease producing strain. The Bacillus subtilis were Available online at www.lsrj.in 7 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW tested for various biochemical tests, which lead to the production of Bacillus subtilis producing protease enzyme. These Bacillus subtilis could group up to 40 ºC and pH range 6 - 9 with optimal growth temperature and pH at 37 ºC and 8.0 respectively. It was also optimized for carbon test and nitrogen test with optimal growth in dextrose and peptone respectively. Enzyme production was carried in 1 litre of optimized media in the fermented at 37 ºC for 48 hours at pH 8.0. Harvested protease product was purified by salt precipitation method. The enzyme protease was purified by Column chromatography. The protein was characterized using SDS-PAGE. The results of their study showed that the Bacillus subtilis was a good producer of extra cellular protease, which can be beneficial for industries. Ozgur and Nilufer (2011) detected the protease production from 15 bacteria isolated from soil samples and the one showed the highest protease activity was selected. The strain was identified and determined as Bacillus cereus by 16S rRNA phylogenetic analysis. After optimization of protease production from the novel medium, the Michaelis-Menten kinetics was also studied. Temperature, pH and, time parameter of protease incubation was determined and maximum temperature was detected at 50 °C as 5.15 IU/ml. The optimum pH range of the enzyme was in between pH 7-9. The crude enzyme was approximately 2-fold purified by dialysis. Ibrahim Noor and Yusoff (2013) isolated the bacteria and identified as Bacillus subtilis and Bacillus licheniformis on the basis of the 16S rRNA gene sequencing. The effect of temperature, pH and inhibitors on enzymes activity and stability were investigated. The crude proteases for both isolates displayed maximal activity at 70 °C and showed characteristic pH optima at pH 9.0. Enzymes activities were totally inhibited by phenyl methyl sulphonyl fluoride (PMSF) suggested that the protease from Bacillus subtilis and Bacillus licheniformis belongs to the family of serine protease. The thermostability profile exhibited the protease from Bacillus subtilis was very stable at 50 °C (maintain 100 % relative activity) and the protease activity retained 89 % of its original activity after heat treatment at 60 °C for 30 min. Meanwhile, protease activity for Bacillus licheniformis retained 96 and 72 % of the original activity after heat treatment at 50 and 60 °C, respectively. Considering their promising properties, Bacillus subtilis and Bacillus licheniformis could be a potential source of enzymes for industrial applications. 4.1. Effect of different carbon and nitrogen source on the Alkaline protease production Protease production was enhanced 2.3 fold by optimizing the culture conditions. The nutritional factors such as carbon and nitrogen sources and also physical factors like pH, temperature, agitation speed, inoculums level and incubation period were optimized for the maximum yield of protease. Studies on the effect of different carbon and nitrogen sources revealed that lactose and combination of yeast extract and soya bean meal enhances the enzyme production. The bacterium Bacillus stratophericus produced the maximum amount of enzyme when allowed to grow for 48 hrs at 35 °C and pH 10 (Raga et al., 2013). Substantial level of protease enzyme activity for Bacillus sp. AGT isolate was achieved at 40°C, pH 9.0 during 18 hours incubation in our production medium containing maltose as carbon source and 0.5% gelatine as nitrogen source (Ashok et al., 2012). Glucose has been reported to be the best carbon source for protease production by Bacillus subtilis (Gomma et al., 1990), though high levels of glucose are also found to repress protease synthesis in some cases (Battaglino et al., 1991; Sen and Satyanarayana, 1993). Similarly, starch has been reported as a good source of alkaline production by Bacillus licheniformis (Sinhan and Satyanarayana, 1991). Among the various nitrogen compounds tested in early research, 0.5 % (w/w) urea was found to be the best one followed by Tryptone, Yeast extract, Organic nitrogen, Ammonium nitrate, Ammonium sulphate and Potassium nitrate for protease production by Bacillus sp. Among the tested carbon Available online at www.lsrj.in 8 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW compounds, 0.5% (w/w) lactose was observed as the best followed by fructose and glucose for protease production by Bacillus sp. While growth and protease production was optimum at 5% (w/v) NaCl, only marginal growth without enzyme production was evident in the absent of salt. The protease had to highest activity at pH 8.0 and 35°C for 48 hours incubation and inoculum level played a vital role in a protease production was found to be associated with the growth of the bacterial culture (Kuberan et al., 2010). Reddy et al. (2007) selected four significant variables (corn starch, yeast extract, corn steep liquor and inoculum size) for the optimization studies. The statistical model was constructed via central composite design (CCD) using three screened variables (corn starch, corn steep liquor, and inoculum size). An overall 2.3 fold increase in protease production was achieved in the optimized medium as compared with the unoptimized basal medium. Enzyme activity increased significantly with optimized medium (939 U ml-1) when compared with unoptimized medium (417 U ml-1). The maximum alkaline protease activity was 6.376 U/ml in medium M 6 using casein as substrate. Temperature of 60 °C was found to be optimum for enzyme production in medium M 3. Similarly, maximum protease activity was found at pH 10 in production medium. Among the different sources, glucose was found to be best carbon source for production of alkaline protease and gelatin was found to be the optimum nitrogen source for protease enzyme production by Bacillus subtilis (Verma et al., 2011). The highest protease production was attained with casein, peptone and mung seedlings as nitrogen sources. The extracellular protease production and mycelial growth were influenced by the concentration of casein. Other protein sources (yeast extract) supported growth but did not induce such excellent protease synthesis and ammonia as end product repressed it, indicating catabolite repression in this microorganism. Optimal protease production was obtained at final pH 5.3 (Arun Kumar et al., 2011). Nihan and Elif Demirkan (2011) estimated the production of protease and the effects of major medium ingredients such as carbon, nitrogen sources and metal ions on the production of the enzyme were investigated. Among the carbon sources used, fructose showed the highest potential for the production. The best organic nitrogen source was skim milk. Inorganic nitrogen sources were not as effective as organic sources. Addition of combine metal ions minimized the enzyme production. Combinations of Ca2+ and Mg2+ in medium were the best. Both ions were not effective alone. Increased production (51 %) of the enzyme was obtained by manipulating the medium composition. The optimum pH and temperature for the purified enzyme activity were 7.0 and 55 °C, respectively. On their research, stability showed that the enzyme was stable in the alkaline pH range 6.0 - 9.0 and at temperatures between 40 and 70 °C. The enzyme was also thermostable (77 % at 55 °C for 3 hrs). The 2+ 2+ enzyme activity was stimulated by Mn and Ca . The best source found was glucose for Bacillus thuringiensis. Effect of glucose concentration and initial pH on cell and alkaline production was studied by Sugumaran et al. (2012). Based on the optimum condition, alkaline protease production was investigated in submerged batch fermentation process. The crude enzyme obtained from fermentation was subjected to acetone precipitation. Then, partially purified enzyme was collected. Effect of temperature, pH and substrate concentration on alkaline protease activity was studied under various conditions. The enzyme showed maximum activity at 50 ºC and at pH 10. Krishnan et al. (2012) analyzed the microbiological, biochemical characterization and 16S rRNA phylogenetic analysis of the isolated bacterium was Bacillus subtilis with an optimum alkaline protease producing temperature, 37 °C and pH 9.0. The maximum alkaline protease production was achieved at Available online at www.lsrj.in 9 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW 24 hrs of incubation period. Among various nitrogen (organic and inorganic) sources, beef extract was found to be the best inducer for alkaline protease in the concentration of 1.5 % as was reported for the maximum alkaline protease production. Effect of carbon sources for example xylose, on protease production proved high protease production than the other tested carbon sources and subsequently 2 % concentration registered an optimum to enhance the protease production. The halotolerancy of Bacillus subtilis for alkaline protease production indicated that 3 % of sodium chloride was optimum to yield maximum protease activity. During production, agitation rate was 250 rpm at air flow rate of 1 VVM. Maximum protease activity of 42.7556 U/ml was observed at the end of 24 hrs cell free supernatant of fermentation broth. Crude alkaline protease was most active at 55 °C, pH 9 with casein as substrate. The produced enzyme could be effectively used to remove hair from goat and sheep hide indicating its potential application in leather processing industry. Prabhavathy et al. (2013) isolated and identified the Bacillus subtilis by the sequencing of 16S rRNA gene and BLAST. In their study, protease production was optimized with wheat bran substrate, glucose (carbon source) and peptone (nitrogen source) with optimum pH 7.0, temperature of 45°C and incubation time 96 hrs. The activity of the enzyme was checked by the DNS method. Medium components and culture conditions for alkaline protease production were optimized using statistical optimization. Plackett – Burman design was employed to find out the optimal medium constituents and culture conditions to enhance protease production. Central composite design revealed that four independent variables, such as NaCl (60.53 g/l), beef extract (14.73 g/l), CuSO4 (4.73 g/l) and pH (10.7) significantly influenced the protease production. Protease production obtained experimentally coincident with the predicted value and the model was proven to bead equate. The enhancement of protease from 298.34 U/ ml to 982.68 U/ml was achieved with the optimization procedure (Annamalai et al., 2013). Mohamad et al. (2013) isolated and identified two bacterial isolates viz., Bacillus amyloliquefaciens and Bacillus subtilis based on morphological, biochemical characteristics and 16S rRNA gene sequencing. Bacillus amyloliquefaciens and Bacillus subtilis produced alkaline keratinolytic serine protease when cultivated in Mineral medium containing 1 % of wool straight off sheep as sole carbon and nitrogen source. The two strains were observed to degrade wool completely to powder at pH 7 and 37 °C within 5 days. Under these conditions the maximum activity of proteases produced by Bacillus amyloliquefaciens and Bacillus subtilis was 922 U/ml and 814 U/ml respectively. The proteases exhibited optimum temperature and pH at 60 °C and 9 respectively. However, the keratinolytic proteases were stable in broad range of temperature and pH values towards casein Hammerstein. Furthermore, the protease inhibitor studies indicated that the produced proteases belong to serine protease because of their sensitivity to PMSF while they were inhibited partially in presence of EDTA. The two proteases are stable in most of the used organic solvents and enhanced by metals suggesting their potential use in biotechnological applications such as wool industry. 4.2. Effect of pH on the Alkaline protease production The enzyme Alkaline protease was stable in the alkaline pH range (8.0 - 12.0), with the optimum temperature and pH range of the proteases being 70 ºC and 6.0 - 12.0, respectively. All three proteases were also highly stable at 70 ºC. After 60 min of incubation at 70 ºC, the enzymes retained 100 % of their original activities. Enzymes were mostly inhibited by Phenyl methyl sulfonyl fluoride (PMSF), however 80 – 90 % enzyme activities were retained in presence of 2-mercaptoethanol and iodoacetate. Addition of SDS and ethylene diamine tetra acetic acid (EDTA) also marginally influenced protease activities, but 2+ addition of Ca to the proteases did not bring about any change (Li et al., 2008). Available online at www.lsrj.in 10 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW The optimum protease activity at pH 9 was 34 Unit/ml at 70 °C for Geobacillus sp. and 46 Unit/ml at 60 °C for Bacillus licheniformis. The apparent lipase activity for Geobacillus sp. was 30.4 Unit/ml and 25.86 Unit/ml for Bacillus licheniformis. Lipase or proteases that produced from these two Bacillus strains are tested on artificial fat and protein dirt clothes in presence and absence of commercial powder detergent to investigate their cleaning effect. The enzyme activity of each has been determined and the results of Amro et al. (2009) proved the possibility to use the crude enzymes alone or in combination with the powder detergent in washing purposes. The enzyme was active in pH range 7 – 9 and temperature 20 – 50 °C with optimum pH of 8 and temperature 35°C. Moreover, the enzyme activity of PA02 protease was not strongly inhibited by specific inhibitor showing the novel nature of enzyme compared to serine, cysteine, aspartyl and metalloproteases. Kinetic studies indicated that substrate specificity of PA02 protease was towards various natural and synthetic proteolytic substrates but inactive against collagen and keratin. These findings suggest protease secreted by Pseudomonas aeruginosa MCMB-327 may have application in dehairing for environment-friendly leather processing (Vasudeo et al., 2011). Vidhya et al. (2011) selected the strains positive on Skim Milk Agar (1 %) as protease producing strains and biochemically characterized. The strains were found capable of growth at temperature >40 ºC and in wide pH range of 7.0 - 12.0. The enzyme assay of strains revealed maximum activity at 50 ºC and pH 10. The enzyme production was carried out at 37 ºC for 48 hrs in fermentor containing 1 L medium having pH 8.0. The molecular weight of enzyme determined through SDS-PAGE, was 6000 kDa. The optimum pH and temperature for maximal protease activity was 9.0 and 40 ºC, respectively. The optimum protease production was achieved with 0.5 % lactose and 0.5 % yeast extract added medium. Among the inorganic nitrogen sources used, the protease production was supported by the addition of potassium nitrate. In experimentation with metal ions, the maximum protease production was observed (863.44 ± 1.63 U/ml) in the media supplemented with magnesium chloride. The maximum amount of protease production was obtained in Triton X 100 (309.275 ± 1.63 ml) added medium when compared to the other tested surfactants (Suppiah Sankaralingam et al., 2012). Georage et al. (2012) selected Bacillus sp. which demonstrated the highest protease activity and used for protease production by Shake - flask fermentation technique at 180 rpm. The maximum protease yield for 72 hrs (2.697 + 0.19 IU mc-1) was achieved under optimized culture conditions of pH 9.0, temperature of 45 °C and 5 % inoculums density with soy meal (1 %) and sugar cane bagasse (1 %) as nitrogen and carbon sources of the fermentation medium. the protease at 72 hrs incubation was significantly (p >0.05) higher that obtained from expensive substrates. The protease achieved > 85.7 = 0.08 % hydrolytic activities on the tested nitrogen wastes with soybean waste being the mostly hydrolyzed (96.3 = 0.13 %). Their results indicated the use of soy meal and sugar cane bagasse as rich substrates for maximum protease yield and the enzyme hydrolytic activity on nitrogen wastes suggests its application in environmental waste degradation. 4.3. Effect of different incubation and temperature on the Alkaline protease Temperature has a profound influence on protease production by microorganisms. The mechanism of temperature control on enzyme production is not well understood (Chaoupka, 1985). A link also exists between the enzyme synthesis and energy metabolism in Bacillus, which is controlled by temperature and oxygen uptake (Frankena et al., 1986). The microorganism utilized several carbon sources for the production of protease. Starch was the best substrate, followed by trisodium citrate, citric acid and sucrose. Among the various organic and inorganic nitrogen sources, ammonium nitrate was found to be the best. Studies on the protease characterization revealed that the optimum Available online at www.lsrj.in 11 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW temperature of this enzyme was 60 ºC. The enzyme was stable for 2 hrs at 30 ºC, while at 40 ºC and 80 ºC, 14 % and 84 % of the original activities were lost, respectively. The optimum pH of the enzyme was found to be 8.0. After incubation of crude enzyme solution for 24 hrs at pH 5.5, 8.0 and 9.0, a decrease of about 51 %, 18 % and 66 % of its original activity was observed respectively (Wellingta et al., 2004). A higher enzyme secretion by Bacillus licheniformis in the alkaline protease of Bacillus megaterium was studied by Borriss (1987). Similarly, pH 6.5 to 7.5 has been reported to be optimum for neutral proteases of Bacillus megaterium (Fartima et al., 1989). Jen-Kuo et al. (1999) optimized conditions for protease production was found when the culture was shaken at 30°C for 3 days in 100 ml of medium (phosphate buffer adjusted to pH 6.0) containing 7 % shrimp and crab shell powder (SCSP), 0.1 % K2HPO4, 0.05 % MgSO4, 1.0 % arabinose, 1.5 % NaNO3, and 1.5 % CaCl2. Under such conditions, the protease of Bacillus subtilis attained the highest activity. It was as high as 20.2 U/ml. The protease was purified in a three-step procedure involving ammonium sulfate precipitation, DEAE-Sepharose CL6B ionic exchange chromatography, and Sephacryl S-200 gel permeation chromatography. The enzyme was shown to have a relative molecular weight of 44 kDa by SDS polyacrylamide gel electrophoresis. The protease was most active at pH 8.0 and 50 °C with casein as substrate. The protease was activated by Mn, Fe, Zn, Mg and Co but inhibited completely by Hg. The protease was also inhibited by metalchelating agent such as EDTA, sulfhydryl reagents as b-mercaptoethanol, and by cysteine hydrochloride, Histidine and glycerol. The EDTA was the most effective inhibitor that caused complete inhibition of protease. They concluded that this enzyme is a metal-chelator-sensitive neutral protease. The bacterium produced protease at maximum rate after 48 hrs of incubation at 37 °C with agitation speed of 170 rpm and 4 % (v/v) starter culture. The best carbon and organic nitrogen sources for this bacterium were glucose and beef extract, respectively. While, the most effective inorganic nitrogen sources were urea and lysine. Supplementation of the culture medium with Mn2+ improved the protease production substantially. Under these conditions, Bacillus cereus strain was found to produce alkaline protease at a maximum rate of approximately 2.0 µg/ml/min (Norazizah et al., 2005). Bacillus subtilis gives the maximum enzyme production by using papaya peel as the substrate with the optimized conditions of incubation time 24 hrs, temperature 300 °C, moisture content 40 % w/v, and inoculums level of 0.8 % w/v and with substrate concentration of 10 g and pH 8.0, glucose concentration 2.0 % w/v. The maximum production of protease enzyme considering all optimum conditions of various parameters was found to be 0.69 mg/ml (Meena et al., 2012). 5. ALKALINE PROTEASE EXTRACTION AND RECOVERY Enzyme extraction refers to liberation of enzymes from cells or cellular constituents. Extraction may first require mechanical, physical, chemical or combination of these methods to disrupt the cell wall or membrane. For either intra or extra cellular enzyme it may be necessary to modify the nature of liquid medium to complete the dissociation (Coxon et al., 1991). The release of intracellular enzymes from microorganisms requires violent method of cell breakage, while extra cellular enzymes from microbial cells do not require cell disruptions (Peck et al., 1990). Calcium alginate was found to be an effective and suitable matrix for higher alkaline protease productivity compared to other matrices studied. All the matrices were selected for repeated batch fermentation. The average protease production with calcium alginate was 585 U/ml which is 70 % higher production over the convention free cell fermentation. Similarly, the protease production by related batch fermentation was 380 U/ml with polyacrylamide, 498 U/ml with agar-agar and 438 U/ml with gelatine respectively (Ram et al., 2012). Sadia et al. (2013) selected fifteen positive mutants on Skim milk agar plates for shake flask Available online at www.lsrj.in 12 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW experiments. The Bacillus licheniformis mutant strain showed 81.21± 3.24 PU/mL alkaline protease activity higher than parent strain (23.57 ± 1.19 PU/mL) in optimized fermentation medium. The fermentation profile like pH (9), temperature (45 °C), inoculum size (2 ml), incubation time (24 hrs, and kinetic parameters such as U h-1, Yp/s, Yp/x, Yx/s, qs, Qs, qp also confirmed the hyper proteolytic activity of alkaline protease produced from Bacillus licheniformis mutant strain over parent strain and other mutants. Finally, the Bacillus licheniformis mutant strain was immobilized by entrapping it in calcium alginate beads and agar. Alkaline protease production and stability of biocatalyst were investigated in both free and immobilized cells. It was concluded that the immobilized cells were more efficient for enzyme production then free cells when used repeatedly. In the cell immobilization technique, the free movement of microorganisms is restricted in the process and a continuous system of fermentation can be used. This technique has been used for alkaline protease production using different carriers such as chitosan, corn cob and corn tasse. Enzyme activity before immobilization (72 hrs) was 78.3 U/ml. Corn cob with 65 % immobilization capacity and the highest enzyme activity was selected as the best carrier by various researchers. After immobilization on the corn cob enzyme, activity was obtained (119.67 U/ml) (Vida Maghsoodi et al., 2013). 6. PURIFICATION OF PROTEASE The protease enzyme was purified by ammonium sulfate precipitation and sephadex G 200 filtration. A trial for the purification of protease resulted in an enzyme with specific activity of 6381.75 (units/mg prot/ml-1) with purification folds 7.87 times. The protease activity increased as the increase in enzyme concentration; optimum substrate concentration (gelatin) was 0.5% (w/v); an optimum incubation temperature was 35 ºC. Purified protease enzyme had a maximum activity at pH 7.0 of phosphate buffer, and the optimum incubation time was 24 hrs. Data emphasized the possibility of the production and purification microbial protease enzyme for application under industrial scale (El-Safey and Abdul-Raouf, 2005). The enzyme was purified by precipitation with 55 – 60 % Ammonium sulfate, Gel filtration on Sephadex G-100 and DEAE ion exchange chromatography. The enzyme was purified 53-fold with 2 % yield. The optimum pH and temperature for catalytic activity of protease was pH 6.8 and 80 ºC respectively and 31 % activity of protease remained even after heat treatment at 100 ºC for 60 min. The relative activity of the enzyme was highly stable (90 %) at 50 ºC for 2 hrs. The half-life of the enzyme at 90 ºC, 80 ºC and 70 ºC was estimated to be 3, 4 and 6 hrs, respectively. The activation energy of -1 denaturation of purified enzyme was 21.7 k J mol . Iron, sodium, calcium, and manganese increased protease activity. On the other hand, magnesium, cobalt and zinc variably decreased the residual activity. But, cadmium and copper drastically inhibited the enzyme activity. The enzymatic activity was highly stable in the presence of 1 and 2 mM EDTA at pH 6.8 and 80 ºC. The neutral protease therefore could be defined as a highly thermostable with new properties make the present enzyme applicable for many biotechnological purposes (Hazem et al., 2012). Sathyaguru et al. (2011) showed that all the organisms were capable of producing maximum Alkaline protease at pH 6 (8.533 to 10.133 IU/ml) and at 50 °C (8.666 to 10.666 IU/mL). The crude enzymes produced by the tested organisms were individually purified by two different methods viz., sodium alginate and ammonium sulphate-butanol methods. The purity of the protease determined in these two methods was ranged between 3.24 to 5.44 IU/ml and 3.13 to 5.55 IU/ml respectively. The partially purified enzymes were further analyzed through SDS-PAGE; accordingly the molecular weight of protein produced by the test organisms was determined in between 49.44 kDa and 50.98 kDa. Available online at www.lsrj.in 13 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW Studies of various researchers involved partial purification of the isolated Bacillus protease by protein separation technique and application of crude enzyme in detergent formulation and deharing technique. It was found that pH 9, 37°C, fructose, yeast extract jack fruit seed, zinc sulphate is optimum for protease production in the fermentation medium. The protein profile in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) revealed protein bonds around 50-75 kDa. The partially purified enzyme showed its distaining capability against blood stained cloth and deharing capability on cow skin (Mukesh et al., 2012). Aqel (2012) showed the variation between two Bacillus strains based on their ability to grow at different pH values and temperatures, pH 5 - 11 and 28 - 73 °C (HUTBS71) and pH 5 - 7 and 37 - 63 °C (HUTBS62), respectively. The purified enzyme from the two different strains also showed variation in purification folds and % yields in different steps of purification methods. Ammonium sulfate fractionation was achieved at 75 - 80 % for HUTBS71 and 55 – 60 % concentrations for HUTBS62. The purification fold and yield was 10 fold and 67 % for strain HUTBS71 and 6.5 fold and 61 % for strain HUTBS62, respectively. Sephadex G-100 purification step achieved 40-fold purification and 16.7 % yield from strain HUTBS71 and 32-fold purification and 12 % yield of protease from strain HUTBS62. DEAE ion exchange chromatography step achieved 60 fold purification and 1.7 % yield for strain HUTBS71 and 53 - fold purification and 2 % yield for strain HUTBS62. The molecular weight of purified proteases from HUTBS71 and HUTBS62 was 49 kDa and 48 kDa, respectively. The target enzyme was purified using a one-step Aqueous two-phase systems (ATPS) protocol involving 22% (w/w) polyethylene glycol (PEG)-10,000 and 18 % (w/w) citrate with a yield of 39.7 %, specific activity of 2600 U/mg and purification factor of 4.8. It was shown to have a molecular weight of 40 kDa by (SDS-PAGE). The purified thermophile enzyme was stable in alkaline pH range (9.0 - 11.0) with the optimum pH of 9.0. It was highly stable at 60 °C and retained 100 % activity even after 90 minutes, suggesting that it belong to the family of Thermophilus. Collectively, our obtained data revealed that the thermophilic protease produced by Bacillus subtilis has the potential application in industrial processes under high temperature (Mashayekhi et al., 2012). Microbes serve as a preferred source for proteases and a large proportion of the proteases are derived from Bacillus strains. To purify protease from Bacillus subtilis and also looked for its potential application in leather making process. The results of Sathiya (2013) revealed that the bacterial strain Bacillus subtilis is a potent source for protease enzyme. The purification techniques have proceeded successfully without any major difficulties and resulted in an increase in protein concentration. 7. APPLICATION OF ALKALINE PROTEASE Alkaline proteases are one of the most important groups of industrial enzymes widely used in detergent, food and leather tanning industries. Alkaline proteases can also be used on the hydrolysis of fibrous proteins such as horn, feather and hair for converting them into useful biomass other potential industrial application of alkaline protease include its utilization in peptide synthesis, resolution of racemic mixture of amino acids, hydrolysis of gelatin laws of X-ray films and also in the recovery of silver (Anwar and Saleemuddin, 1998; Kumar and Takagi, 1999). 7.1. Application of Alkaline Protease in Industries 7.1.1. Food processing industries In food industry, protease helps in processing and production of food products such as meat, milk products and beverages which requires series of enzyme treatment and alkaline protease is an important enzyme among all. Proteases are used as tonic for proper digestion for children. Protease Available online at www.lsrj.in 14 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW also plays an important role in processing tea, coffee and coco by oxidizing for producing complete product. Microbe helps in sugar fermentation for ethanol production, along with other enzymes, alkaline protease also aids in fermentation. Hence, alkaline protease plays an effective role in various streams of food processing industries which also includes meat tenderization. 7.1.2. Leather making India is one of the major countries in leather production and in Tamil Nadu, Vellore district is well known for its Leather industries. It stands at second place worldwide. Leather production involves a complex process such as soaking, dehairing, bating and tanning. Traditional method of carrying out of processing leather was done by treating with chemicals, it was less efficient and requires huge amount of chemical and also it produces enormous amount of toxic compounds to the environment so biological mean of leather processing was focused, that is treatment of raw material with enzymes. One of the major enzyme employed in this case was alkaline protease. This conventional method is environmental friendly and doesn’t cause pollution. Both fungal and bacterial proteases are used for leather processing, protease helps in hydrolysis of non collagenous part of the skin non fibrillar protein. The leather sample processed by using alkaline protease was found to have maximum softness. Thus, the use of protease in leather processing could eliminate the use of pollution causing chemicals such as sodium, lime and solvents and greatly help to prevent environmental pollution. Currently, alkaline protease with hydrated lime and sodium chloride are used for dehairing and it also aids significant low waste production. 7.1.3. Textile Industry Silk production is the back bone of textile industry, quality of silk determines the quality of a fabric. Alkaline protease plays a major role in production of quality silk by removing gum and other impurities produced along with silk’s native form, even synthetic fabric also treated with protease for complete smooth finish. Indian sericulture field is growing enormously and hence use of protease is also been increased. Moreover, protease treatment is an environmental friendly process rather than employing chemicals for silk treatment which causes environment pollution. The proteolytic enzyme have been used to solve this problem and shown promising results not only in the production level but also quality of silk. Since, alkaline protease based degumming was ecofriendly which will be an additional advantage. Though, the conventional protease are quite efficient for degumming but having some disadvantage like thermal and chemical stability which was one drawback has to solved, also alkaline protease can hamper the quality and physical appearance of silk as silk is quite sensitive to alkali and alkaline protease. The thermostable protease basically forms Geobacillus genus has been used for the enzymatic degumming of silk which are quite resistant to various chemicals and temperature (Annavarapu et al., 2011). 7.1.4. Detergent additives Enzymes used as detergent was in practice from long back. The two German scientists namely, Rohm and Haas used human protease and sodium carbonate in washing detergents. Proteinaceous dirt binds strongly to fabric even after washing without protease. Protease helps removal of blood and other proteinaceous compounds. protease has a great role in industries as detergent for sterilization since chemical steriliants fails to remove minute trapped dirts. Hence, microbial protease commercially produced are used for cleaning large industrial boilers, surgical instruments and also for various domestic purposes Available online at www.lsrj.in 15 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW 7.2. Medical applications of Alkaline protease Alkaline proteases shows a large variety of functions in medical field, which includes from basic molecular level to whole organism therapeutic use such as haemostasis and inflammation. Alkaline proteases are used extensively in the pharmaceutical industry for preparation of medicines such as ointments for debridement of wounds. 7.2.1. Anti-Inflammatory activity Inflammation is the physiological condition occurs as a result of microbial invasion or infection which results in accumulation of immune cell along with plasma. Usual ways of treating inflammation was treatment with non-steroidal drug. However, they show several side effects. To overcome this, COXII targeting drugs were produced but thou, these specific drugs are costly. Hence, alkaline protease are used nowadays used especially Serratio pepetidase is most effective alkaline protease. Alkaline protease is available for use in management of inflammation. Additionally, a group of serine protease from Indian Earthworm has been studied for its anti-inflammatory potential. 7.2.2. Anti-Cancer activity Many alkaline protease enzymes plays a role in normal multiplication of cell count in biological process, many protease present in its inactive form ymogen requires activation by cleavage of small portion of native protein, any imbalance in this process leads to cancer. On the other hand, enzymes like caspase primarily involves in killing of abnormal cells, caspase is alkaline protease enzyme which aids in proper immune system. Advantage of using enzyme in cancer management over chemotherapeutic agents is to reduce toxicity impart by chemical based drugs. In the year 2014, a serine protease from Indian earthworm was evaluated for its antitumor activity against breast cancer cell lines and result shown tremendous scope for protease in development of anti-cancer therapeutics. In future, enzymatic treatment of cancer can be an effective remedy over other methods of treatments. 7.2.3. Clot dissolving agent Blood and thrombus clotting is an natural phenomenon which occurs as a result of hurt, were aggregation of thrombus occurs, blood clot can also be found in many cases like blood vessel disorder and it ultimately leads to severe complications. To combat these vascular hurdles, an external clot dissolving agent needed to perfuse in vascular pipeline. The available external clot dissolving agents called as thrombolytic are basically protease. May recombinant variants like Tissue plasminogen activators (t-PA), Urokinase (u-PA), Streptokinase (SK), Staphylokinase (SAK), Earthworm fibrinolyitc Enzyme (EFE) are developed for the clinical purpose. Alkaline protease plays a vital role in external protease production because of their stability and substrate selectivity. 7.3. Research Applications of Alkaline proteases 7.3.1. Nucleic acid isolation Cell composed of complex structure with rigid cell membrane. In order to isolate nucleic acid from the cell its membrane has to be lyzed and all other molecules, contaminants has to be removed. Alkaline proteases are like proteolytic enzyme aid in obtaining protein free nucleic acid proportion. The most widely used proteolytic enzyme in nucleic acid purification is Proteinase K. The Proteinase K also quickly inactivates the nucleases which might degrade the nucleic acids present in the sample. It also helps in preventing degradation of DNA or RNA and hence, high yield can be achieved. Available online at www.lsrj.in 16 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW 7.3.2. Cell Isolation and Tissue Dissociation Cytology studies rely primarily on isolation of desire cell which are surrounded by other molecules and extracellular matrix. The commonly used method of cell isolation is done by treating with enzymes there are several enzymes available in market for the detachment of cultured cells, cell dissociation and cell component or membrane -associated protein isolation. Besides the polysaccharidases, nucleases and lipases, the proteases are the most important enzymes used widely to dissociate cells from tissues, depending on desire type of cell, enzyme with high specificity are employed. Collagenase, elastase, amidase, chymotrypsin and trypsin are some of the proteolytic enzymes used in cell isolation process. 7.3.3. Cell Culturing Cell adhered to the culture plate during cell culture can be separated by treating the culture with trypsin i.e., trypsinization. However, trypsin treatment can lead to cleavage of membrane proteins and receptors, which can cause significant changes in the expression level of different proteins so the effect should be considered and minimized. 7.4. Alkaline proteases in Effluent treatment One of major cause of water and soil pollution of this modern era is because of improper waste water management and ineffective method of treating solid waste processing and industrial effluent waste. The better way of treating this waste can be done by microbes with xenobiotic property, alkaline protease plays a major role in waste management. Kumar and Takagi (1999) reported an enzymatic process using a Bacillus subtilis alkaline protease in the processing of waste feathers from poultry slaughter houses. 7.5. Alkaline proteases in Silver recovery Silver recovery from photographic films and x-ray films involves burning the films directly oxidation of metallic silver followed by electrolysis stripping the silver-gelatin layer using microbial enzymes especially protease which breaks the gelatin layer embedded with silver in films approximately 1.5 % to 2.0 % (by weight) silver in its gelatin layers. By using this method, pollution free stripping can be done. 8. REFERENCES 1) Absida V.A. (1985). Some extracellular enzymes associated with tomato fruit spoilage molds. Mycopathologia, 9: 101 - 108. 2) Adams MWW and Kelly RM. (1998). Finding and using thermophilic enzymes. Trends Biotechnol., 16:329-32. 3) Akbalık G. (2003). “Screening for industrially important extracellular enzymes from Alkalophilic Bacillus genus” Izmir Institute of Technology, Biotechnol Department, Izmir., 4) Amro A. Amara, Soheir R. Salem and Mohamed S. A. (2009). Shabeb. The Possibility to Use Bacterial Protease and Lipase as Biodetergent. Journal of Biotechnology and Biochemistry, 4: 104 - 114. 5) Annamalai N, Rajeswari M.V and Thavasi R. (2013). Optimization, purification and characterization of novel thermostable, haloalkaline, solvent stable protease from Bacillus halodurans CAS6 using marine shellfish wastes: a potential additive for detergent and antioxidant synthesis. Bioprocess and Biosystem Engineering, 7: 873 – 883. 6) Annavarapu R, Ravi V, Neerja Reddy M, and Sambasiva Rao K.R.S. (2011). Thermostable Bacterial Available online at www.lsrj.in 17 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW Protease - A New Way for Quality Silk Production. International Journal of Bioscience and Biotechnology, 4 (8): 223 - 234. 7) Anwar M. N and Saleemudin A. L. (1998). Some properties of protease of the fungal strain Aspergillus flavus, International Journal of Biology, 8(2):162 - 164. 8) Aqel H. (2012). Phenotypic and protease purification of two different thermophilic Bacillus strains HUTBS71 and HUTBS62. Annals of Biological Research, 3: 1747 - 1756. 9)Arun Kumar S, Vinay S and Jyoti S. (2011). Production of Protease and Growth Characteristics of Aspergillus sydowii. Nature and Science, 9 (5): 1338 - 1349. 10) Ashok Raja C and Prabhahar C. (2012). Screening and optimization of Alkaline Protease productivity from Bacillus sp. from Tannery Effluent. Journal of Pharmaceutical and Biological Archives, 3: 244 - 248. 11) Asokan S and Jayanthi C. (2010). Alkaline protease production by Bacillus licheniformis and Bacillus coagulans. Journal of Cell and Tissue Research, 10: 2119 - 2123. 12) Bajaj B. K and Sharma P. (2011). An alkali-thermotolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotechnology, 28: 725 - 732. 13) Battaglino R.A, Huergo M, Pilosof A.M.R. and Bartholomai G.B. (1991). Culture requirement for the production of protease by Aspergillus oryzae in solid state pigmentation. Applied Microbiology and Biotechnology, 35: 292 - 296. 14) Beg Q.K, Gupta R. (2003). Purification and characterization of an oxidation stable, thiol-dependant serine alkaline protease from Bacillus mojavensis. Enzyme Microbial Technology, 32(2): 294 - 304. 15) Bhaskar N, Sudeepa E.S, Rashmi H.N and Selvi A.T. (2007). Partial purification and characterization of protease of Bacillus proteolyticus CFR3001 isolated from fish processing waste and its antibacterial activities. Bioresource Technology, 98: 2758 – 2764. 16) Borris R. (1987). Biology of enzyme. In: Rehm H and Reed G. (eds.), Biotechnol, weinheim, verlag chemie., 35 - 62. 17) Cazetta M.L, Celligoi, Buzato M.A and Scarmino I.S. (2007). Fermentation of molasses by Zymomonas mobilis: effects of temperature and sugar concentration on ethanol production. Bioresource Technology, 98: 2824 – 2828. 18) Chaoupka J. (1985). Temperature as a factor regulating the synthesis of microbial enzymes. Microbiology Science, 2: 59 - 90. 19) Chauhan B and Gupta R. (2004). Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochemistry, 39: 15 – 22. 20) Chutmanop J, Chuichulcherm S, Chisti Y and Sirinophakun P. (2008). Protease production by Aspergillus oryzae in solid state fermentation using agro industrial substrates. Journal of Chemical Technology and Biotechnology, 83: 1012 – 1018. 21) Coxon R.D. Harwood C.R and Archibaid A.R. (1991). Protein export during growth of Bacillus subtilis. The effect of extracellular deficiency. Letters in Applied Microbiology, 12: 91 - 94. 22) Debananda S. Ningthoujam and Pintubala Kshetri. (2010). A Thermostable Alkaline Protease from a Moderately Halo-alkali thermotolerant Bacillus Subtilis Strain SH1. Journal of Basic and Applied Sciences, 4: 5126 - 5134. 23) Ellaiah P, Adinarayana K, Rajyalaxmi P and Srinivasulu B. (2003). Optimization of process parameters for alkaline protease production under solid state fermentation by alkalophilic Bacillus sp. Asian Journal of Microbiology, Biotechnology and Environmental Science, 5: 49 - 54. 24) El-Safey E.M and Abdul-Raouf U.M. (2004). Production, Purification and Characterization of Protease enzyme from Bacillus subtilis. International Conferences for Development and the Available online at www.lsrj.in 18 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW Environment in the Arab World, Assiut University. 25) Enshasy E.H, Abuol-Enein A, Helmy S and Azaly E. (2010). Optimization of the industrial Production of alkaline protease by Bacillus licheniformis in different production scales. Australian Journal of Basic and Applied Sciences, 2: 583 – 593. 26) Esakkiraj P, Sankaralingam S, Usha R, Palavesam A and Immanuel G. (2011). Solid- state protease production using anchovy waste meal by moderate halophile Serratia proteamaculans AP-CMST isolated from fish intestine. Annals of Microbiology, 61: 749 – 755. 27) Fartima M.G, Pagliarini N.S and Manachini P.L. (1989). Heat resistant protease from Pseudomonas fluorescens. Indian Journal of Food Science, 3: 63 - 70. 28) Fikret U, Ilknur P, Goksel K and Ebru Ince Y. (2011). Optimal conditions for production of extracellular protease from newly isolated Bacillus cereus strain CA15. Journal of Biological Science, 1 - 9. 29) Frankena J, Koningstein G.M, Van Verseeved H.W and Stouthamer A.H. (1986). Different limitation in chemostat culture on growth and production of exocellular protease by Bacillus licheniformis. Applied Microbiology and Biotechnology, 24:106 - 112. 30) Ganesh Kumara C and Hiroshi T. (1999). Microbial alkaline proteases: From a Bioindustrial view point. Journal of Advance Biotechnology, 561 – 594. 31) Geetha M, Saranraj P, Magalakshmi S and Reetha D. (2012). Screening of Pectinase producing bacteria and fungi for its pectinolytic activity using fruit wastes. International Journal of Biochemistry and Biotech Sciences, 1(1): 30 - 42. 32) Geethanjali S and Anitha S. (2011). Optimization of protease production by bacillus subtilis isolated from Mid Gut of Fresh Water Fish Labeo rohita. Journal of Fish and Marine Science, 3 (1): 88 - 95. 33) George Okafor U.O and Mike-Anosike E.E. (2012). Screening and optimal protease production by Bacillus sp. SW-2 using low cost substrate medium. Journal of Microbiology Research, 7: 327 - 336. 34) Gitishree Das and Prasad M.P. (2010). Isolation, purification & mass production of protease enzyme from Bacillus subtilis. International Journal of Microbiology, 026 - 031. 35) Godfrey T and West S. (1996). Introduction to Industrial enzymology. Industrial enzymology 2nd edition, 1-8. 36) Gomma M.A, Abouzied M.M and E1-Habashy M. (1990). Enzymes and its applications. Egyptian Journal of Food Science, 16: 9. 37) Gupta R, Beg Q.K and Khan S. (2002). An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Applied Microbiology and Biotechnology, 60: 381 - 395. 38) Hazem A, Farouk Al-Quadan Tahani K and Yousef. (2012). A novel neutral protease from thermophilic Bacillus strain HUTBS62. Journal of Bioscience and Biotechnology, 1: 117 - 123. 39) Ibrahim Noor A and Yusoff N. (2013). Thermostable Alkaline Serine Protease from Thermophilic Bacillus Species. International Research Journal of Biological Science, 2(2): 29 - 33. 40) Jen-Kuo Y, Ing-Lung S, Yew-Min T and San-Lang W. (2000). Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme and Microbial Technology, 26: 406 – 413. 41) Kavi Karunya S, Reetha D, Saranraj P and John Milton D. (2011). Optimization and purification of chitinase produced by Bacillus subtilis and its antifungal activity against plant pathogens. International Journal of Pharmaceutical and Biological Archives, 2(6): 1633 - 1638. 42) Krishnan R, Ashok kumar M and Saravanan G. (2012). Isolation of alkaline protease from Bacillus subtilis AKRS3. Journal of Biotechnology, 13415 - 13427. 43) Kuberan T, Sangaralingam S and Thirumalai Arasu V. (2010). Isolation and optimization of Protease Available online at www.lsrj.in 19 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW producing Bacteria from halophilic soil. Journal of Biological Science and Research, 1:163 - 174. 44) Kumar C.G and Takagi H. (1999). Microbial alkaline protease from a Bioindustrial view point. Biotechnology Advances, 17: 561 - 594. 45) Lakshmi G and Prasad N.N. (2013). Isolation and Screening of alkaline protease producing bacteria and induction of overproducing Bacillus licheniformis mutants through UV irradiation. International Journal of Applied Sciences, 2 (3): 120 - 137. 46) Li X, Xu T, Ma X, Guo K, Kai L, Zhao Y, Jia X, Mad Y. (2008). Optimization of culture conditions for production of cis-epoxysuccinic acid hydrolase using response surface methodology. Bioresource Technology, 99: 5391 – 5396. 47) Marcela J. Gonzalez, Fernanda Gorgoroso, Stella M. Reginensi and Jorge A. (2013). Identification of closely related Bacillus subtilis and Bacillus amyloliquefaciens isolated from dairy farms and milk powder. Journal of Microbiology, Biotechnology and Food Science, 2 (5): 2326 - 2331. 48) Mashayekhi F, Mazar, Shahbaz Mohammadi H, Ebrahimi-Rad M, Gregorian A, and Omidinia E. (2012). Isolation, Purification and Characterization of a Thermophilic Alkaline Protease from Bacillus subtilis. Journal of Science and Islamic Republic of Iran, 23 (1): 7 - 13. 49) Meena V, Sree Satya N, Surya Prakash D.V and Sumanjali A. (2012). Production of protease through SSF by Bacillus subtilis NCIM 2724. Journal of Chemistry Biology and Physical Science, 4: 1929 - 1935. 50) Mohamed A, Hassan, Bakry M, Haroun, Amro Amara A and Ehab Serour A. (2013). Production and characterization of Keratinolytic Protease from New Wool-Degrading Bacillus species isolated from Egyptian Ecosystem. Bio Med Research International, 7 (5): 890 – 898. 51) Montgomeryd D.C and Runger G.C. (2002). Applied Statistics and Probability for Engineers, 3rd ed. John Wiley and Sons, New York. 52) Mrunmaya K.P, Mahesh Kumar S and Kumananda T. (2013). Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. International Journal of Microbiology, 5 (2): 159 - 165. 53) Mukesh Kumar D.J, Rameetha R, Lincy L, Priyadarshini H, Sandhivachittybabu S and Kalaichelvam P.T. (2012). Destaining and dehairing capability of partially purified Bacillus subtilis protease from optimized fermentation medium. Journal of Society of Applied Science, 9 (3): 613 - 620. 54) Naidu M.A and Saranraj P. (2013). Bacterial Amylase: A Review. International Journal of Pharmaceutical and Biological Archives, 4(2): 274 - 287. 55) Nascimento W.C and Martins M. L. (2004). Production and properties of an extracellular protease from thermophilic Bacillus sp. Brazilian Journal of Microbiology, 35 (1 - 2): 91 - 96. 56) Nihan S and Elif Demirkan. (2011). Production of Protease by Bacillus sp. N-40 isolated from soil and its enzymatic properties. Journal of Biological Environment, 5 (14): 95 - 103. 57) Nisa R, Patoomporn Chim-Anagae and Anuvat Jangchud. (2010). Optimization of silk degumming protease production from Bacillus subtilis C4 using placket -burman design and response surface methodology. Science Asia, 36:118 - 124. 58) Norazizah S, Sayangku Norariati Aris, Raja Noor Zaliha, Abd Rahman, Mahiran Basri and Abu Bakar Salleh. (2005). Optimization of environmental and nutritional conditions for the production of Alkaline Protease by a newly isolated bacterium Bacillus cereus Strain146. Journal of Applied Science Research, 1(1): 1 - 8. 59) Ogino H, Watanabe F, Yamada M, Nakagawa S, Hirose T, Noguchi A, Yasuda M and Ishikawa H. (1999). Purification and characterization of organic-stable protease from organic solvent-tolerant Pseudomonas aeruginosa. Journal of Bioscience and Bioengineering, 87: 61 - 68. 60) Olajuyigbe F.M and Ajele J.O. (2005). Production dynamics of extracellular protease from Bacillus Available online at www.lsrj.in 20 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW species. African Journal of Biotechnology, 4(8): 776 - 779. 61) Ozgur K and Nilufer C. (2011). Partial purification of Protease by A Novel Bacterium, Bacillus cereus and enzymatic properties. Journal of Biology and Chemistry, 39: 39 – 43. 62) Pandey A. (2003). Solid-state fermentation. Biochemical Engineering Journal, 13: 81 – 84. 63) Parawira W and Zvauya R. (2012). Production and characterisation of protease enzyme produced by a novel moderate thermophilic bacterium (EP1001) isolated from an alkaline hot spring, Zimbabwe. Journal of Microbiology Research, 6: 5542 - 5551. 64) Peck K, Janssen H. W, Morgan N and Daniel R. M. (1990). Enhancement of in vitro stability and recovery of a proteinase produced by an extreme thermophilic. In: Fermentation Technologies, Industrial Applications, Elsevier Science Publications, New York: 97 - 102. 65) Prabhavathy, G, Rajasekara M, Pandian S and Senthilkumar B. (2013). Identification of industrially important Alkaline protease producing Bacillus subtilis by 16s rRNA sequence analysis and its applications. Journal of Research in Pharma and Biomedical Science, 2229 - 3701. 66) Raga Aruna Binda D, Silpa S, Rajesh B and Bhaskar Reddy I. (2013). Isolation and identification of a novel strain Bacillus stratophericus DF producing alkaline protease and optimization of enzyme production. International Journal of Science and Engineering Research, 8 (7): 444 - 458. 67) Rajesh Patel, Mital Dodia and Satya P. Singh. (2005). Extracellular alkaline protease from a newly isolated haloalkaliphilic Bacillus sp.: Production and optimization. Process Biochemistry, 40: 3569 – 3575. 68) Ram B, Jagadeesh C and Bose K. (2012). Production of alkaline protease from Bacillus subtilis by difeerent entrapment techniques. J Biochem Tech., 4(1):498-501. 69) Randa A, Abusham, Raja Noor Zaliha R. A, Rahman, Abu Bakar Salleh and Mahiran Basri. (2009). Optimization of physical factors affecting the production of thermo-stable organic solvent-tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain. Journal of Microbial Cell Factories, 9 (7): 1475 -2859. 70) Reddy L.V.A, Young-Jung W, Jong-Sun Y, Hwa-Won R. (2008). Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett–Burman and response surface methodological approaches. Bioresource Technology, 99: 2242 – 2249. 71) Roja Rani M, Lalitha kumari B, Hanuma sri M and Siva Prasad S. (2012). Isolation and screening of alkaline protease producing bacteria and induction of overproducing Bacillus licheniformis mutants through UV irradiation. Journal of Pharmacy, 001-014. 72) Romero F, Garcia L. A and Diaz M. (1998). Protease production from whey at high concentration by Serratia marcescens. Resource in Environmental Biotechnology, 2: 93 - 115. 73) Ruann Janser Soares D.C and Helia Harumi S. (2013). Production and biochemical characterization of protease from Aspergillus oryzae: An evaluation of the physical–chemical parameters using agroindustrial wastes as supports. Biocatalysis and Agricultural and Biotechnology, 6 (9): 813 – 821. 74) Sadia J, Munazzah M, Shazia Anwer B, Rao Irfan and Saqib Mahmood. (2013). Hyper-production of Alkaline protease by mutagenic treatment of Bacillus subtilis M-9 using Agroindustrial Wastes in submerged fermentation. Journal of Microbiology and Biochemical Technology, 5(3): 074 - 080. 75) Saranraj P and Naidu M.A. (2014). Microbial Pectinases: A Review. Global Journal of Traditional Medicinal System, 3(1): 1 - 9. 76) Saranraj P and Stella D. (2013). Fungal Amylase: A Review. International Journal of Microbiological Research, 4(2): 203 - 211. Available online at www.lsrj.in 21 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW 77) Saranraj P, Stella D and Reetha D. (2012). Microbial cellulases and its applications: A review. International Journal of Biochemistry and Biotech Sciences, 1(1): 1 - 12. 78) Sathiya G. (2013). Production of protease from Bacillus subtilis and its application in leather making process. International Journal of Research in Biotechnology and Biochemistry, 8 (5): 2277 3827. 79) Sathyaguru P, Ethiraj K, Ramasamy R, Palanisamy I, Suyambu A, Arunachalam P and Grasian I. (2011). Production and partial purification of protease by selected bacterial strains using raw milk as substrate. Malaysian Journal of Microbiology, 7(4): 192 - 200. 80) Sen S and Sathyanarayana T. (1993). Optimization of alkaline protease production by thermophilic Bacillus licheniformis. Indian Journal of Microbiology, 33: 43 - 47. 81) Senthilkumar P. K, Uma C and Saranraj P. (2012). Amylase production by Bacillus sp. using cassava as substrate. International Journal of Pharmaceutical and Biological Archives, 3 (2): 274 – 280. 82) Sharma S and Aruna K. (2011). Optimization of protease production by Thermophilic Bacillus subtilis WIFD5 isolated from a milk power sample. Journal of Microbial World, 10 (3): 241 - 247. 83) Singhal P, Nigam V.K and Vidyarthi A.S. (2012). Studies on production, characterization and applications of microbial alkaline proteases. Journal of Advanced Biotechnology, 23 (4): 653 - 669. 84) Sinhan N and Sathyanarayana T. (1991). Alkaline protease production by thermophilic Bacillus licheniformis. Indian Journal of Microbiology, 31: 425 - 430. 85) Siva Sakthi S, Kanchana D, Saranraj P and Usharani G. (2012). Evaluation of Amylase activity of the Amylolytic fungi Aspergillus niger using cassava as substrate. International Journal of Applied Microbiology Sciences, 1 (1): 24 - 34. 86) Siva Sakthi S, Kanchana D, Saranraj P and Usharani G. (2012). Evaluation of Amylase activity of the Amylolytic fungi Aspergillus niger using cassava as substrate. International Journal of Applied Microbiology Sciences, 1 (1): 24 - 34. 87) Siva Sakthi S, Saranraj P and Rajasekar M. (2011). Optimization for cellulase production by Aspergillus niger using paddy straw as substrate. International Journal of Advanced Scientific and Technical Research, 1 (1): 69 – 85 88) Srinivas Naik L, Kopuri Aruna N, Sreevennela P and Venkata Ramana Devi. (2013). Isolation and biochemical characterization of protease isolated from Bacillus sp. International Journal of Pure and Applied Microbiology, 3 (3): 94 -101. 89) Srinubabu G, Lokeswari N and Jayaraju K. (2007). Screening of nutritional parameters for the production of protease from Aspergillus oryzae. E - Journal of Chemistry, 4 (2): 208 - 215. 90) Sugumaran K.R, Ponnusami V, Gowdhaman D, Gunasekar V and Srivastava S.N. (2012). Thermostable alkaline protease production from Bacillus thuringiensis MTCC 1953: Optimization and kinetic studies. International Journal of Chem Tech Research, 18 (5): 198-202. 91) Suppiah Sankaralinga, Ramasamy R and Arunachalam P. (2012). Optimization of culture conditions for the production of an extracellular Alkaline protease from Vibrio mimicus. In Conference on Environment, Energy and Biotechnology,3 (3): 444 - 458. 92) Valerie De Jonghe, An Coorevits, Jan De Block, Els Van Coillie, Koen Grijspeerdt, Lieve Herman, Paul De Vos and Marc Heyndrickx. (2009). Taxinogenic and spoilage potential of aerobic spore-formers isolated from raw milk. Journal of Food Microbiology, 3 (4): 111 – 119. 93) Vasudeo Z, Smita N and Pradnya K. (2011). A novel Extracellular protease from Pseudomonas aeruginosa MCM B-327: enzyme production and its partial characterization. New Biotechnology, 2 (8): 335 - 342. Available online at www.lsrj.in 22 COMMERCIAL PRODUCTION AND APPLICATION OF BACTERIAL ALKALINE PROTEASE: AN OVERVIEW 94) Verma O. P, Prashansa Kumari, Shruti Shukla and Abha Singh. (2011). Production of Alkaline protease by Bacillus subtilis (MTCC 7312) using submerged fermentation and optimization of process parameters. Journal of Experimental Biology, 1 (3): 124 - 129. 95) Vida Maghsoodi, Akhtar K, Parvin N, Soheila Y and Mohammad Amin Sabzevari S. I. (2013). Alkaline protease production by immobilized cells using Bacillus licheniformis. Asian Journal of Biological Research, 20 (3): 607 – 610. 96) Vidhya Pallavi R, Kuberan T and Arumugam S. (2011). Screening of Thermophilic Bacillus cereus for the optimization of mass production of alkaline protease enzyme. Journal of Biological Science Research, 2: 24 - 29. 97) Wang H.Y, Liu D.M, Liu Y, Cheng C.F, Ma Q.Y, Huang Q and Zhang Y.Z. (2006). Screening and mutagenesis of a novel bacillus pumilus strain producing alkaline protease for dehairing. Applied Microbiology, 44: 1 - 6. 98) Wang S.L, Yang C.H, Liang T.W and Yen Y.H. (2008). Optimization of conditions for protease production by Chryseobacterium taeanense TKU001. Bioresource Technology, 99: 3700 – 3707. 99) Wellingta C.A, Nascimento D and Leal Martins M. L. (2004). Production and properties of An Extracellular protease from thermophilic Bacillus sp. Brazilian Journal of Microbiology, 35: 91 - 96. 100) Yang J, Shih I, Tzeng Y and Wang S. (1999). Production and purification of protease from Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microbial Technology, 26: 406 - 413. Available online at www.lsrj.in 23