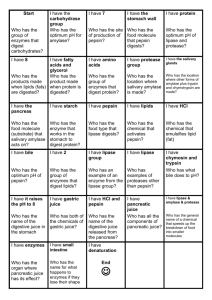
HBIO Name Directions: Read pp. 40-43 in the Dragonfly Book. Pay
advertisement
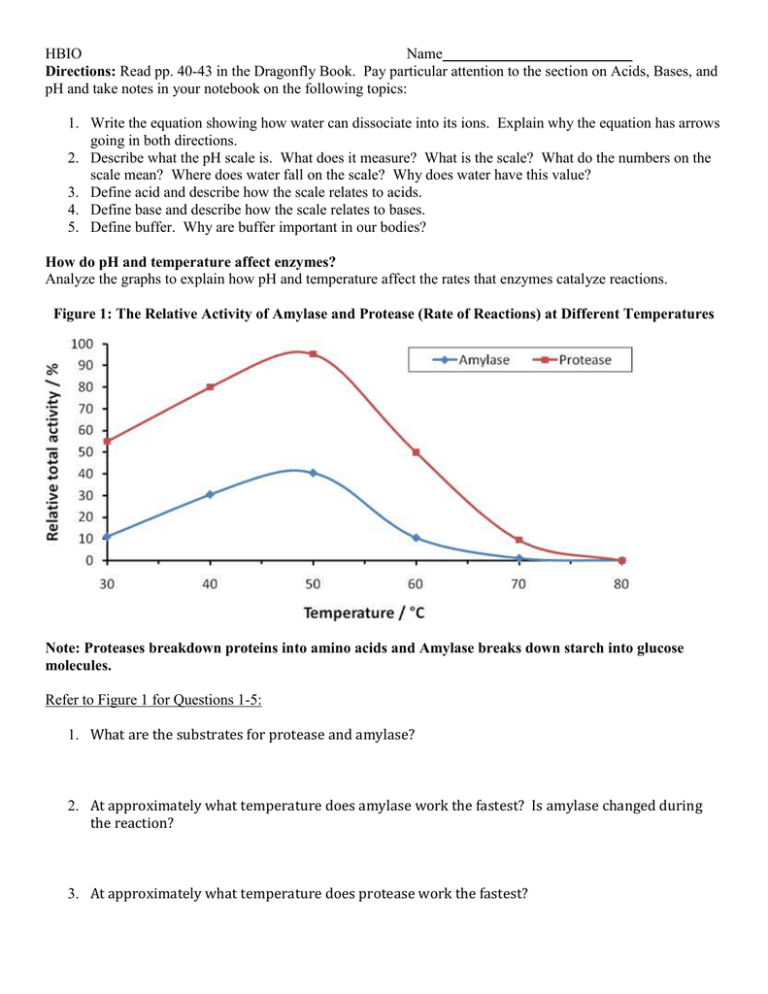
HBIO Name Directions: Read pp. 40-43 in the Dragonfly Book. Pay particular attention to the section on Acids, Bases, and pH and take notes in your notebook on the following topics: 1. Write the equation showing how water can dissociate into its ions. Explain why the equation has arrows going in both directions. 2. Describe what the pH scale is. What does it measure? What is the scale? What do the numbers on the scale mean? Where does water fall on the scale? Why does water have this value? 3. Define acid and describe how the scale relates to acids. 4. Define base and describe how the scale relates to bases. 5. Define buffer. Why are buffer important in our bodies? How do pH and temperature affect enzymes? Analyze the graphs to explain how pH and temperature affect the rates that enzymes catalyze reactions. Figure 1: The Relative Activity of Amylase and Protease (Rate of Reactions) at Different Temperatures Note: Proteases breakdown proteins into amino acids and Amylase breaks down starch into glucose molecules. Refer to Figure 1 for Questions 1-5: 1. What are the substrates for protease and amylase? 2. At approximately what temperature does amylase work the fastest? Is amylase changed during the reaction? 3. At approximately what temperature does protease work the fastest? HBIO Name 4. Which enzyme generally works at a faster rate? Use data from the graph to explain your answer. 5. What do you think the relative activity of amylase would be if the graph started at 0oC? Explain your reasoning. (Hint: Think about Brownian movement.) 6. Why is the activity of both amylase and protease 0% at 80oC? (You may need to do a little research to answer this question.) 7. Summarize how temperature affects the rate at which enzymes catalyze chemical reactions. HBIO Name Figure 2: The Effect of pH on Trypsin and Pepsin Activity (Relative Rates Reactions are Catalyzed) Note: Pepsin is a type of protease that works in the stomach and trypsin is a protease that works in the small intestines. Refer to Figure 2 to answer Questions 68. What pH is trypsin most active? What pH is pepsin most active? 9. Based on the data in Figure 2, what can you infer about the pH of the stomach and the pH of the small intestines. 10. Catalase is the name of the enzyme that was in the liver we tested a couple days ago. It is found in almost all of our cells, as it breaks down the toxic hydrogen peroxide that is produced as a waste in our cells. Your blood pH is approximately 7.2. Based upon this information, infer the optimal pH for catalase. Explain your reasoning. 11. Based upon what you have learned about pH, what happens to an enzyme if the pH gets too high or low for it? 12. Summarize how pH affects the rate at which enzymes catalyze reactions.