Solution for the Homework 4
advertisement
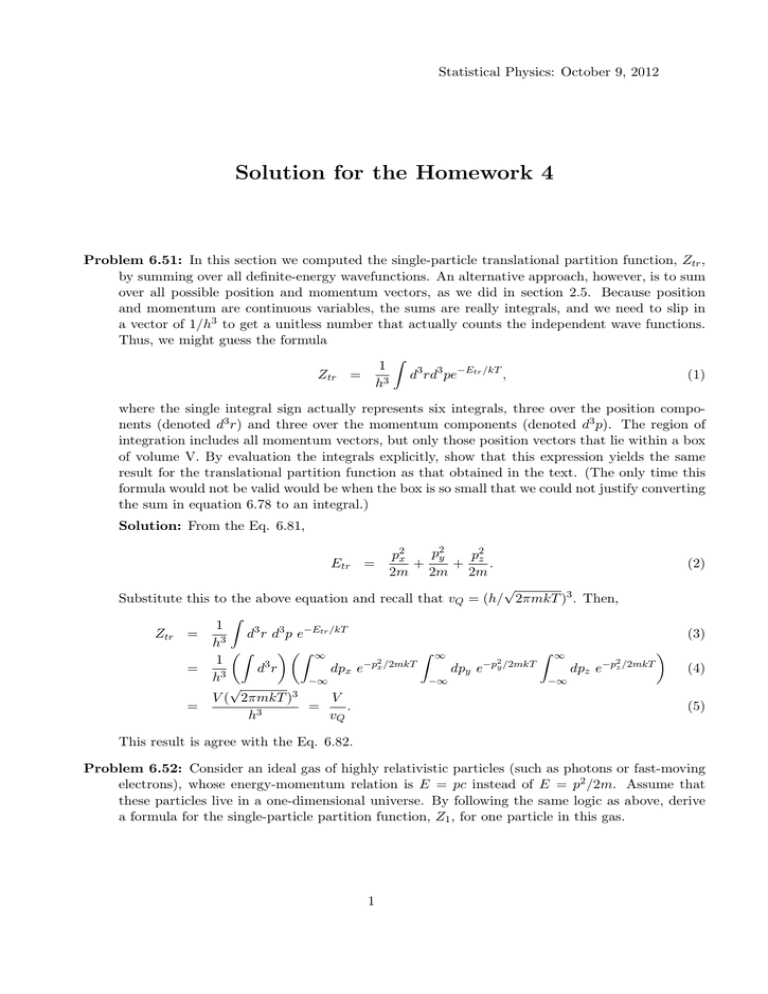
Statistical Physics: October 9, 2012 Solution for the Homework 4 Problem 6.51: In this section we computed the single-particle translational partition function, Ztr , by summing over all definite-energy wavefunctions. An alternative approach, however, is to sum over all possible position and momentum vectors, as we did in section 2.5. Because position and momentum are continuous variables, the sums are really integrals, and we need to slip in a vector of 1/h3 to get a unitless number that actually counts the independent wave functions. Thus, we might guess the formula ∫ 1 d3 rd3 pe−Etr /kT , (1) Ztr = h3 where the single integral sign actually represents six integrals, three over the position components (denoted d3 r) and three over the momentum components (denoted d3 p). The region of integration includes all momentum vectors, but only those position vectors that lie within a box of volume V. By evaluation the integrals explicitly, show that this expression yields the same result for the translational partition function as that obtained in the text. (The only time this formula would not be valid would be when the box is so small that we could not justify converting the sum in equation 6.78 to an integral.) Solution: From the Eq. 6.81, Etr = p2y p2x p2 + + z . 2m 2m 2m √ Substitute this to the above equation and recall that vQ = (h/ 2πmkT )3 . Then, ∫ 1 Ztr = d3 r d3 p e−Etr /kT h3 ) (∫ ) (∫ ∞ ∫ ∞ ∫ ∞ 1 3 −p2x /2mkT −p2y /2mkT −p2z /2mkT = d r dpx e dpy e dpz e h3 −∞ −∞ −∞ √ V ( 2πmkT )3 V = = . 3 h vQ (2) (3) (4) (5) This result is agree with the Eq. 6.82. Problem 6.52: Consider an ideal gas of highly relativistic particles (such as photons or fast-moving electrons), whose energy-momentum relation is E = pc instead of E = p2 /2m. Assume that these particles live in a one-dimensional universe. By following the same logic as above, derive a formula for the single-particle partition function, Z1 , for one particle in this gas. 1 Statistical Physics: October 9, 2012 Solution: Since the energy should be a positive, let Etr = |p|c. Consider the partition function with the one-dimensional case and put the given energy. Then, ∫ 1 Z1 = dr dp e−Etr /kT (6) h (∫ )∫ ∞ 1 = dr dp e−|p|c/kT (7) h −∞ ∫ L 2L ∞ 2kT L ≡ = dp e−pc/kT = , (8) h 0 ch lQ where lQ = ch/2kT . Problem 7.2: In a real hemoglobin molecule, the tendency of oxygen to bind to a heme site increases as the other three heme sites become occupied. To model this effect in a simple way, imagine that a hemoglobin molecule has just two sites, either or both of which can be occupied. This system has four possible states (with only oxygen present). Take the energy of the unoccupied state to be zero, the energies of the two singly occupied states to be -0.55eV, and the energy of the doubly occupied state to be -1.3eV (so the change in energy upon binding the second oxygen is -0.75eV). As in the previous problem, calculate and plot the fraction of occupied sites as a function of the effective partial pressure of oxygen. Compare to the graph from the previous problem (for independent sites). Can you think of why this behavior is preferable for the function of hemoglobin? Solution: There are four states and the grand partition function of this system is Z = 1 + 2e(0.55eV +µ)/kT + e(1.3eV +2µ)/kT . (9) Then the occupancy of this system is n = N /N = ( ) 1∑ N (s)P(s) = e(0.55eV +µ)/kT + e(1.3eV +2µ)/kT /Z, 2 s and the chemical potential of ideal gas is µ = −kT ln (V Zint /N vQ ). n 1.0 0.8 0.6 0.4 0.2 0.05 0.10 2 0.15 0.20 P Hbar L (10) Statistical Physics: October 9, 2012 The internal partition function of diatomic molecule is rotational partition function and then Zint = kT /η where η = 0.00018eV (this is not a constant for the temperature and pressure, so I just approximate). Then the occupancy vs. pressure is like below figure. When the pressure of oxygen is high like in the lung, heme sites prefer to occupied by oxygen and the pressure of oxygen is lower than before like in the cell, heme sites prefer to unoccupied. This process moves the oxygen. Problem 7.5: Consider a system consisting of a single impurity atom/ion in a semiconductor. Suppose that the impurity atom has one “extra” electron compared to the neighboring atoms, as would a phosphorus atom occupying a lattice site in a silicon crystal. The extra electron is then easily removed, leaving behind a positively charged ion. The ionized electron is called a conduction electron, because it is free to move through the material; the impurity atom is called a donor, because it can “donate” a conduction electron. This system is analogous to the hydrogen atom considered in the previous two problems except that the ionization energy is much less, mainly due to the screening of the ionic charge by the dielectric behavior of the medium. (a) Write down a formula for the probability of a single donor atom being ionized. Do not neglect the fact that the electron, if present, can have two independent spin states. Express your formula in terms of the temperature, the ionization energy I, and the chemical potential of the “gas” of ionized electrons. Solution: There are three states, one with energy 0 is ionized, and the others with energy -I are not. In this case, the grand partition function is Z = 1 + 2e(I+µ)/kT . (11) So, the probability of a single donor atom being ionized is Pion = 1 1+ 2e(I+µ)/kT . (12) (b) Assuming that the conduction electrons behave like an ordinary ideal gas (with two spin states per particle), write their chemical potential in terms of the number of conduction electrons per unit volume, Nc /V . Solution: From the Eq. 7.10 with Zint = Ze = 2, ( ) 2V µ = −kT ln N c vQ (13) (c) Now assume that every conduction electron comes from an ionized donor atom. In this case the number of conduction electrons is equal to the number of donors that are ionized. Use this condition to derive a quadratic equation for Nc in terms of the number of donor atoms (Nd ), eliminating µ. Solve for Nc using the quadratic formula. (Hint: It’s helpful to introduce some abbreviations for dimensionless quantities. Try x = Nc /Nd , t = kT /I, and so on.) Solution: The answer of (a) is exactly same as the ratio of conduction electrons and donor ions. And a chemical potential is given by the result of (b), Nc Nd = 1 1+ 2e(I+µ)/kT 3 1 = 1+ N v eI/kT cV Q (14) Statistical Physics: October 9, 2012 This is just quadratic equation, so we can easily get the result with the condition that the number of conduction electron should be positive: [ ] √ V Nd I/kT Nc = −1 + 1 + 4vQ e . (15) V 2vQ eI/kT (d) For phosphorus in silicon, the ionization energy is 0.044eV. Suppose that there are 1017 P atoms per cubic centimeter. Using these numbers calculate and plot the fraction of ionized donors as a function of temperature. Discuss the results. Solution: Put the values and then we can plot the Nc /Nd as a function of T. NC Nd 1.0 0.8 0.6 0.4 0.2 0 100 200 300 400 500 T Problem 7.6: Show that when a system is in thermal and diffusive equilibrium with a reservoir, the average number of particles in the system is N = kT ∂Z , Z ∂µ (16) where the partial derivative is taken at fixed temperature and volume. Show also that the mean square number of particles is N2 = (kT )2 ∂ 2 Z . Z ∂µ2 Use these results to show that the standard deviation of N is √ kT (∂N /∂µ), σN = (17) (18) in analogy with Problem 6.18. Finally, apply this formula to an ideal gas, to obtain a simple expression for σN in terms of N . Discuss your result briefly. 4 Statistical Physics: October 9, 2012 Solution: In the previous section, we are done this for energy. Similarly, starts from the grand partition function: ∑ Z = e−(E(s)−µN (s))/kT . (19) s Then, [ ] e−(E(s)−µN (s))/kT kT ∂Z N (s) = = Z Z ∂µ s [ ] ∑ e−(E(s)−µN (s))/kT (kT )2 ∂ 2 Z = N 2 (s) = . Z Z ∂2µ s ∑ N N2 (20) (21) So, √ σN = (kT )2 ∂ 2 Z − Z ∂2µ ( kT ∂Z Z ∂µ √ )2 = (kT )2 ∂ 2 Z ∂Z ∂ (kT )2 + = 2 Z ∂ µ ∂µ ∂µ Z √ kT ∂N . (22) ∂µ Let’s consider the ideal gas with the number of particle is N = N . From the Eq. 6.93, we can calculate the σN for ideal gas: ∂N = ∂µ ( ∂µ ∂N )−1 = N kT ⇒ √ σN = N. (23) This means that the deviation of particles is same with the average of particles and this case is called Poisson distribution. 5