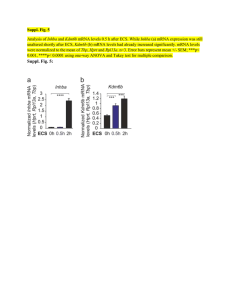
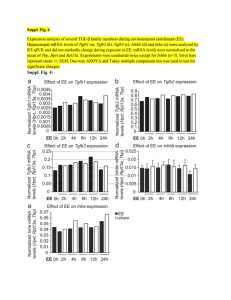
SMARTT Polymer Technology® Targets and Delivers mRNA to the Liver
Pierrot Harvie, Ph.D.
1st International mRNA Health Conference
Tübingen, October 23-24, 2013
®
®
Outline
SMARTT Polymer Technology® Enables mRNA Delivery to the Liver
o SMARTT Polymer Technology® Background
o Polymer Biodistribution and NHP Safety
o SMARTT Polymer Technology® Delivers mRNA In Vivo
o mRNA Nanoparticle Characteristics
o FLUC mRNA Nanoparticle In Vivo Activity
o mRNA Nanoparticle Stability
o Conclusion
®
2
© 2013 PhaseRx, Inc. All rights reserved.
Introduction
Introduction to PhaseRx
o Six year old biotech company,
located downtown Seattle, WA. USA
o SMARTT Polymer Technology®
delivers therapeutic macromolecules
into the cytoplasm where they can
access the drug targets
o PhaseRx has developed SMARTT
Polymer Technology® into a robust
platform for the delivery of siRNA
o SMARTT Polymer Technology®-mRNA nanoparticles deliver mRNA to the liver
in vivo, providing significant opportunities for the treatment of orphan liver
disease
®
3
© 2013 PhaseRx, Inc. All rights reserved.
SMARTT Polymer Technology®
®
4
© 2013 PhaseRx, Inc. All rights reserved.
PhaseRx SMARTT Polymer Technology®
Delivery System is a Linear, Multi-Domain Vinyl Polymer
Polymer Block 1:
Hydrophilic Block
mRNA Complexation Agent
Polymer Block 2:
Targeting Ligand
(NAG)
Endosome Release Block
Hydrophobic Monomers
Carboxylic Acid Monomers
Tertiary Amine Monomers
®
5
© 2013 PhaseRx, Inc. All rights reserved.
Active Polymer Targeting
NAG Polymers, but not Mannose Polymers, Target Hepatocytes In Vivo
NAG Targeted Polymer
6
Mannose Targeted Polymer
Cy3-siRNA/Phalloidin/DAPI
© 2013 PhaseRx, Inc. All rights reserved.
®
Polymer Biodistribution and NHP Safety
®
7
© 2013 PhaseRx, Inc. All rights reserved.
14C-Polymer-siRNA
Biodistribution/Excretion Study
Polymers Efficiently Target the Liver and are Rapidly Cleared
o Tissue distribution results from QWBA and tissue homogenates:
o Rapid and efficient targeting
o 95% of the injected polymer dose is targeted to the liver by 2 hours
o <0.5% of dose in spleen, bone marrow, lymph nodes, skeletal muscle,
small intestine mucosa, skin
o Main route of polymer clearance from liver is into bile and then feces
o 71% of dose is cleared into bile by 72 hr
®
8
© 2013 PhaseRx, Inc. All rights reserved.
Non-Human Primate Study with MET siRNA
Good KD and Tolerability with Polymer-MET siRNA Conjugate in NHPs
o Single dose study with Polymer-MET siRNA conjugate in cynomolgus monkeys
o >50% MET mRNA and protein KD was observed 7 days post dose with a dose
response
o MET siRNA polymer conjugate was well-tolerated
o No significant dose-related changes observed in serum chemistry, hematology,
coagulation, or histopathology in liver, spleen, or kidney
o No change in complement activity or increase in IL-6 cytokine levels
ALT
400
2.0
Buffer
1 mg/kg MET siRNA
1.4 mg/kg MET siRNA
2.4 mg/kg MET siRNA
300
1.5
ALT U/L
MET mRNA Relative Expression
MET mRNA at 7 Days Post Dose
1.0
200
100
0.5
0.0
Buffer
1.0 mg/kg
1.4 mg/kg
2.4 mg/kg
0
0
MET siRNA
1
2
3
4
5
6
7
8
Days Post Dose
®
9
© 2013 PhaseRx, Inc. All rights reserved.
SMARTT Polymer Technology® Delivers mRNA In Vivo
®
10
© 2013 PhaseRx, Inc. All rights reserved.
PhaseRx SMARTT Polymer Technology®
Polymers For mRNA Delivery
o SMARTT Polymer Technology® has demonstrated success for delivery of
siRNA to hepatocytes
o Targeting minimizes unexpected off-target activities
o Robust, manufacturable, tested in vivo in large animals
o No induction of innate immunity
o The polymer system for mRNA delivery is a linear multi-domain delivery
system that is targeted to liver hepatocytes with an N-acetylgalactosamine
ligand
o The polymers have been engineered to carry additional functionality
specifically for mRNA
o Biodegradable cationic charge moiety controls the association of the
polymer with the mRNA
o Polymer-mRNA nanoparticles are highly effective in the delivery of
mRNA to the liver
®
11
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: Self-Assembly
Self-Assembly Process Between the Polymer and the mRNA
Formulation Process:
o The mRNA and the polymer stock solutions were prepared in aqueous buffer
o Equal volumes of the polymer and mRNA solutions were mixed together
o Formulations were stored at 4oC overnight prior to injection
Formulation Characterization:
o The particle size was measured at pH 7.4
o The zeta-potential was measured at pH 7.4 and pH 4.0
o The mRNA condensation was measured by dye accessibility (SYBR® Gold )
o The mRNA integrity was accessed by agarose gel electrophoresis
®
12
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: mRNA Compaction
mRNA Condensation Using Polymer Nanoparticles
o mRNA formulated at a range of N/P ratios
o mRNA becomes inaccessible to SYBR® Gold
SYBR Gold Dye Accessibility
100
% Dye Accessibility
PRX392-3
PRX-LUC-1092
80
PRX398-1
PRX-LUC-1098
60
40
20
0
0
1.75
3.5
7
N/P
®
13
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: mRNA Compaction
PRX-LUC-1092 Compared to the Cationic Complexation Agent Alone
o PRX-LUC-1092 showed small
particle size compared to the
cationic complexation agent
o PRX-LUC-1092 has lower mRNA
compaction compared to the
cationic complexation agent
SYBR Gold Dye Accessibility
PRX-LUC-1092
100
PRX-LUC-1092
1000
Cationic complexation agent
80
Z-Average (nm)
% Dye Accessibility
Z-Average for mRNA
60
40
20
Cationic complexation agent
800
600
400
200
0
0
0
1.2
2
N/P
4
7
1.2
2
N/P
4
7
®
14
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: Size Distribution
Particle Size Distribution Before and After Polymer Addition to the mRNA
Size Distribution by Intensity
Intensity (%)
15
10
5
0
0.1
1
10
100
1000
10000
Size (d.nm)
Polymer
Record
55: PRX392 no mRNA
Polymer
mRNA w ith mRNA
Record
56:+PRX392
o A shift in the particle size distribution was observed after polymer addition
to the mRNA
®
15
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: Formulation Reproducibility
Nanoparticle Characteristics of Multiple Formulation Batches
Formulation
Z-Ave
(nm)
PRX-LUC-1092
67 ± 9
PDI
PRX-LUC-1098 59 ± 5
0.31 ±
0.04
0.45 ±
0.05
ZP pH 7.4
(mV)
ZP pH 4.0
(mV)
% Dye
Access
5±2
11 ± 2
26 ± 3
6±1
13 ± 1
12 ± 1
®
16
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: In Vivo Analysis
In Vitro/In Vivo Activity Testing
o Commercially available Luciferase mRNA
o 5’-cap, Poly A tail
o ~2000 nucleotides
o Modified mRNA
o In vitro testing
o Transfection in HeLa cells for luciferase activity/cell viability.
o In vivo testing using IVIS Lumina System for live luciferase imaging
o Formulation Screening
o IV injections via tail vein
o 1 mg/kg Luc mRNA
®
17
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: In Vivo Activity
In Vivo Luminescence Imaging - Controls
Buffer
Free Luc mRNA at 1 mg/kg
Imaging 3 hours post i.v. dose
IVIS Lumina II can only image 3 mice at a time
®
18
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: In Vivo Activity
Luciferase mRNA Expression in Liver with PRX-LUC-1092
1 mg/kg Luc mRNA/ PRX-LUC-1092
Imaging 3 hours post i.v. dose
®
19
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: In Vivo Activity
Luciferase mRNA Expression in Liver – Luminescence Values
Luminescence (Photon/sec)
In Vivo Luminescence
1.0×10 8
1.0×10 7
1.0×10 6
1.0×10 5
1.0×10 4
3 hr
6 hr
Buffer
3 hr
6 hr
Luc mRNA
only
3 hr
6 hr
PRX-LUC-1092
3 hr
6 hr
PRX-LUC-1098
®
20
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: Innate Immunity
No Cytokine Induction using PhaseRx mRNA Nanoparticles
Serum IFN-α at 6 hours Post IV Dose
300
pg/mL
200
100
LOD
0
Buffer
PRX-LUC-1092
PRX-LUC-1098
PhaseRx mRNA nanoparticles did not show any TNF-α or IL-6 in serum at 6 h
®
21
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles: Stability
Nanoparticle Characteristics Before and After a Freeze Thaw Cycle
Formulation
Z-Ave
(nm)
ZP pH7.4
ZP
pH 4
%
dye Access
PDI
PRX-LUC-1092 stored 24h at 4oC
65
0.303
5
13
20
PRX-LUC-1092 stored 24h at -80oC
70
0.309
5
11
20
Size Distribution by Intensity
Intensity (Percent)
10
8
6
4
2
0
0.1
1
10
100
1000
10000
Size (d.nm)
Record 28: PRX-LUC-1092 24h at 4C
Record 29: PRX-LUC-1092 After 24 h at -80C
®
22
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles
mRNA Nanoparticle is Active After a Freeze-Thaw Cycle
1.0×
Luminescence (Photon/sec)
10 9
In Vivo Luminescence Imaging 3 hr
Single Dose, IV Injection
1.0×10 8
1.0×10 7
1.0×10 6
1.0×10 5
1.0×10 4
Buffer
-80oC
4oC
PRX-LUC-1092
®
23
© 2013 PhaseRx, Inc. All rights reserved.
Polymer-mRNA Nanoparticles
Summary
o mRNA delivery to liver obtained with two different polymer nanoparticle
formulations
o Luciferase expression in liver 2-3 logs above background
o No increase in the IFN-α, TNF-α and IL-6 plasma level at 6h
o Particle size is less than 100 nm
o Formulation stability after freeze thaw cycle at -80oC
o Formulation stability for 1 week at 4oC
o Metabolism study shows >95% targeting to the liver and the polymer is
rapidly cleared via the biliary route
o NHP safety data indicate a favorable polymer safety profile
o Next Steps:
o Optimize formulation parameters
o Test new constructs
o Evaluate other mRNA targets
®
24
© 2013 PhaseRx, Inc. All rights reserved.
Acknowledgments
PhaseRx Contributing Team
Biology:
o Mary Prieve, Ph.D.
o Allen Li, M.D., Ph.D.
o Tod Brown, Ph.D.
o Oleksandr Baturevych, MSc.
o Amber Paschal, MSc.
Chemistry:
o Sean Monahan, Ph.D.
o Mike DeClue, Ph.D.
o Russell Johnson, Ph.D.
o Debashish Roy, Ph.D.
o Maher Qabar , Ph.D.
Executive Team:
o Paul Johnson, Ph.D. CSO
o Robert Overell, Ph.D. President and CEO
®
25
© 2013 PhaseRx, Inc. All rights reserved.
Contact Information
Pierrot Harvie, Ph.D.
Phone: 206.805.6303
Email: Pierrot@phaserx.com
410 W. Harrison Street, Suite 300
Seattle, WA 98119
www.phaserx.com
®
26
© 2013 PhaseRx, Inc. All rights reserved.
®
®