Symmetry Operations E: Identity operation: The E operation does
advertisement
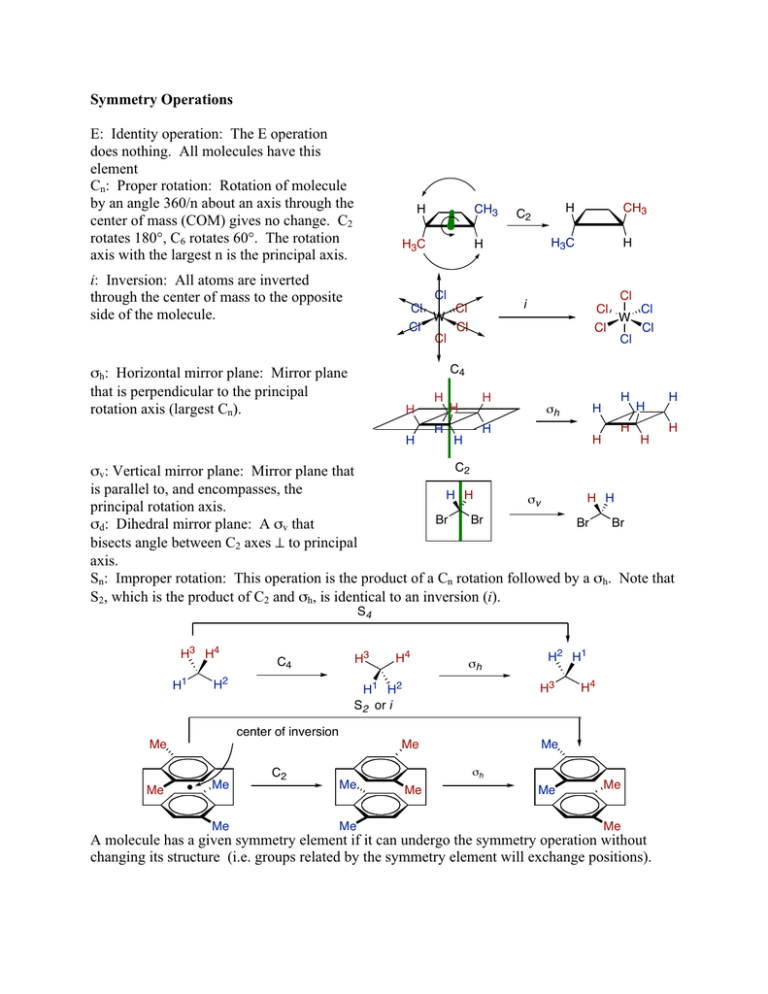
Symmetry Operations E: Identity operation: The E operation does nothing. All molecules have this element Cn: Proper rotation: Rotation of molecule by an angle 360/n about an axis through the center of mass (COM) gives no change. C2 rotates 180°, C6 rotates 60°. The rotation axis with the largest n is the principal axis. H CH3 H3C i: Inversion: All atoms are inverted through the center of mass to the opposite side of the molecule. Cl Cl W Cl CH3 H3C H Cl H C2 H i Cl Cl Cl Cl Cl W Cl Cl Cl C4 σh: Horizontal mirror plane: Mirror plane that is perpendicular to the principal rotation axis (largest Cn). H H H H H H H σh H H H H H H H H H C2 σv: Vertical mirror plane: Mirror plane that is parallel to, and encompasses, the H H H H σv principal rotation axis. Br Br Br Br σd: Dihedral mirror plane: A σv that bisects angle between C2 axes ⊥ to principal axis. Sn: Improper rotation: This operation is the product of a Cn rotation followed by a σh. Note that S2, which is the product of C2 and σh, is identical to an inversion (i). S4 H3 H4 H1 H2 H3 Me Me H4 σh C2 Me Me Me H2 H1 H3 H1 H2 S2 or i center of inversion Me Me C4 Me σh Me H4 Me Me Me A molecule has a given symmetry element if it can undergo the symmetry operation without changing its structure (i.e. groups related by the symmetry element will exchange positions). Point Group Designations Point Group C1: E. No symmetry elements are present. Cs: E, σ. Molecule has a mirror plane of symmetry Ci: E, i. Molecule has an inversion center Sn: E, Sn. In many cases there will also be a Cn/2 rotation axis, although this is not a requirement. Note that S1 is the same as a mirror plane (σ) and should be called Cs. S2 is the same as i and should be called Ci. Example F C H Cl Br C1 Br Br Br H H H Cs F Cl H H F Cl Ci Br H Br H H Br H Br S 4 CH3 Cn: E, Cn. Molecule has only a single rotation axis, with no mirror planes. Cnv: E, Cn, and n σv. Molecule vertical mirror planes corresponding to the Cn rotation. C∞v: linear unsymmetric molecule. A special case of Cnv. CH3 C2 N H H H C3v O C S C∞v Dn: E, Cn, and n C2 axes ⊥ to Cn axis: Thus a D3 molecule would have 3 C2 ⊥ to the Cn axis. Dnh: E, Cn, n C2 axes, σh. In addition to the n C2 axes there is a horizontal mirror plane. D∞h: Special case of Dnh for linear symmetric molecules. Dnd: E, Cn, n C2 axes, and n σv (also known as σd). D2 D6h O C O H H D∞h H H D3d H H H C H H H Td: tetrahedral symmetry. There are 4 C3 rotation axes. Td O O Oh: octahedral symmetry. There are six C4 axes. O C C C W C O Oh Ih: Icosahedral symmetry. There are 12 C5 rotation axes. Ih C C O O Point Group Decision Tree Yes Cnh No σh? C∞v or D∞h Cubic T, O, I Yes S2n colinear w/ Cn? Yes Cn Yes No More than one Cn (n ≥ 3) No σv? Cnv No No Linear? Find principal axes Cn is the principal axis? Yes None Cs, Ci or C1 σh? Yes Dnh Adapted from: Physical Chemistry, Joseph H. Noggle, 2nd ed., Scott Foresman & Co, Glenview, IL, 1996, pg 840. n vertical mirror planes nC2 ⊥ to Cn? No No Yes Yes σv? No Dn Dnd S2n