Technical Tips for STR Analyses
advertisement

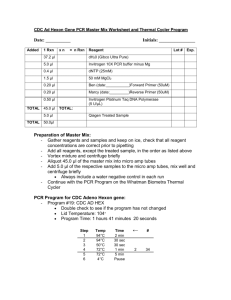
Technical Tips for STR Analyses Rita Weispfenning Publication Date: 2016 Here we present answers to a number of frequently asked questions about the effects of variation of instrumentation and consumables on the quality of PowerPlex® STR analyses. PowerPlex® Systems are highly optimized kits for human STR-based genotyping. The components of these systems and the recommended protocols were developed using specific consumables and instruments. For the best results, PowerPlex® analyses must be performed using the same conditions as those during kit development and recommended in the technical manual. Despite our best efforts to develop the most robust kits possible, small deviations from the original protocol or the use of different instrumentation or consumables can affect kit performance and interfere with the high-quality results that you have come to expect from the PowerPlex® Systems. Q: Can I use PCR plates or tubes other than those that are recommended in the PowerPlex® Technical Manuals? A: For DNA amplification using the PowerPlex® Systems, we recommend the use of either MicroAmp® Optical 96-Well Reaction Plates or 0.2ml MicroAmp® Reaction Tubes (Applied Biosystems, Cat.# 4316813 and 4316567) because those were used during the development and optimization of the PowerPlex® systems. For a successful amplification, it is important that the liquid in the wells reaches the required temperature. This is only possible when the combination of thermocycler and PCR plates is perfect. There are several suitable thermocyclers and plates available, however, not all combinations of thermocycler and plate provide optimal heat transfer. This may not be due to deficiencies in either the thermocycler or the plate, but rather to an incompatibility between the specific thermocycler and plate. For these reasons it is very important to use good quality thermocyclers (see next question) and plates known to be fully compatible with the particular thermocycler block. Even slight differences in well shape or size between the block and plate may cause reduced heat transfer efficiencies. In a study with the goal to investigate the effect of different PCR plates on peak heights and balance of PowerPlex® System profiles, four different 96 well PCR plate types (MicroAmp® Optical 96-Well Reaction Plates (Applied Biosystems, Cat.# 4316813); FrameStar® 96 Well Semi Skirted PCR Plate (4titude®, Cat.# 4ti-0770/C), Axygen® 96 Well Polypropylene PCR © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 1 Weispfenning, R. Microplate (Axygen Scientific, Cat.# PCR-96M2-HS-C) and ABgene™ Thermo-Fast™ 96-Well Semi-Skirted PCR Plate (Fisher Scientific, Cat.# AB0990)) were used in duplicate for amplification with PowerPlex® ESX 17 Fast System. In combination with the GeneAmp® PCR System 9700 thermal cycler with a gold-plated silver block, MicroAmp® PCR plates provided the highest peaks, followed by ABgene™ and Axygen® plates. FrameStar® plates provided slightly lower peak heights (Figure 1). Figure 1. PowerPlex ESX 17 Fast Peak Height and Balance Comparison of 2800M samples amplified in MicroAmp, ABgene, Axygen and FrameStar PCR plates (N=128). All PCR were performed with one single GeneAmp® PCR System 9700 thermal cycler with a gold-plated silver block by amplifying 0.5ng 2800M DNA in a 25µl reaction volume. One single Applied Biosystems 3500xl Genetic Analyzer was used for all CE-runs (1.2kV, 24 second injections). Q: Can I use any thermal cycler model for the amplification of PowerPlex® Systems? A:Thermal cyclers’ specifications are diverse, and performance can vary greatly from one model to the next. Heating blocks made of different metals or metal alloys or driven by peltiers with different specifications are likely to deliver different ramp rates and heat transfer capacities. Thus, amplification protocols for STR analysis can vary depending on the thermal cycler as well as the STR System used. The PowerPlex® Systems are optimized for use with the GeneAmp® PCR System 9700 thermal cycler with silver- or gold-plated silver sample block (Applied Biosystems) or the Veriti® 96-Well Thermal Cycler (Applied Biosystems). © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 2 Weispfenning, R. It is essential to run the PCR protocol with the recommended ramp speed by setting the correct ramp mode of each cycler. More information about the recommended thermal cyclers and mandatory ramp speeds is provided in the PowerPlex® System technical manuals and in Table 1. Table 1. Overview on Recommended Thermal Cyclers and Ramp Rates for PowerPlex Systems If you want to use a thermal cycler other than the recommended ones, please be sure that you adjust the ramp speed of each PCR protocol to match the rates listed in Table 1. We do not recommend the use of thermal cyclers with aluminum heating blocks as these blocks have different heat-transfer properties and may not reach the required temperature uniformly across the block in the programmed time. This is particularly problematic when using PowerPlex® ESX Fast and ESI Fast systems which have PCR protocols with a short denaturation step of only 5 seconds at 96°C. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 3 Weispfenning, R. Direct comparison with GeneAmp® PCR System 9700 thermal cycler and Applied Biosystems 2720 thermal cycler (with Aluminum block) when using PowerPlex® ESI 17 Fast System, reveal reduced peak heights in D2S1338 and TH01 in samples that were amplified on the 2720 thermal cycler (Figure 2). Figure 2. Peak height comparison of samples amplified using the PowerPlex® ESI 17 Fast System and the Applied Biosystems® 2720 thermal cyclers with an aluminum heating block or GeneAmp® PCR System 9700. Amplifications were performed as described in the PowerPlex® ESI 17 Fast System Technical Manual (TMD041). Panel A. The blue dye channel. Panel B. The yellow dye channel. Panel C. The green dye channel. Panel D. The red dye channel. If you have access only to a thermal cycler with an aluminum block and want to use PowerPlex® ESX Fast or ESI Fast System, we recommend that you use the alternative PCR protocol described in Table 2. The denaturation temperature is increased from 96° to 98°C to compensate for the relatively poor thermal characteristics of the aluminum block (1) . © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 4 Weispfenning, R. Table 2. PowerPlex ESX and ESI Fast Thermal Cycling Conditions Examples of PowerPlex® ESI 17 Fast profiles amplified using an Applied Biosystems® 2720 thermal cycler with aluminum block with 96°C and 98°C denaturation temperatures are shown in Figure 3. Figure 3. Peak height comparison of samples amplified using an Applied Biosystems® 2720 thermal cycler with aluminum heat block with the PowerPlex® ESI 17 Fast System and 96C and 98C denaturation temperatures. Panel A. The blue dye channel. Panel B. The yellow dye channel. Panel C. The green dye channel. Panel D. The red dye channel. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 5 Weispfenning, R. Q: Do OmniSwabs™ swabs yield better results than normal cotton swabs when pretreating samples with SwabSolution™ Reagent? A: As part of a comparison study we prepared fresh buccal swabs (11 Whatman OmniSwabs™ and 10 cotton swabs) and incubated each swab in a 1.5ml micro centrifuge tube with 1ml SwabSolution™ Reagent for 30 minutes at 70°C. We then transferred 2µl of SwabSolution™ extract from each sample directly to 23µl of PowerPlex® ESX 17 Fast PCR amplification mix and performed PCR with 26 cycles as directed in the PowerPlex® ESX 17 Fast System Technical Manual (TMD040) for initial optimization experiments. All samples provided well balanced and full profiles (Figure 4). Data obtained with OmniSwabs™ swabs and cotton swabs are comparable (2) . Figure 4. Composition of peak heights and number of peaks of buccal cells on OmniSwabs swabs (N=11) and cotton swabs (N=10) Q: Can I use any paper storage card for direct amplification with PowerPlex® Systems? A: Yes, but some need to be treated with PunchSolution™ Reagent prior to amplification. Different types of paper storage cards are available. There are FTA®-based paper storage cards such as Whatman EasiCollect™ or Fitzco Sampact® devices which are impregnated with chemicals that lyse cells, denature proteins and make DNA available for amplification. Punches of these cards can be added directly to PCR. In contrast, punches of nonFTA paper storage cards like Bode Buccal DNA Collector™ device and S&S 903 don’t contain lysis agents and denaturants and must be preprocessed with PunchSolution™ Reagent before PCR. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 6 Weispfenning, R. If you forget to treat punches of nonFTA sample types with PunchSolution™ Reagent prior to amplification, alleles at some loci (particularly TH01 and TPOX) will have reduced peak heights. Figure 5 shows a PowerPlex® 21 System profile generated from an S&S 903 blood punch without pretreatment with PunchSolution™ Reagent. Figure 5. PowerPlex® 21 System profiles from S&S 903 blood punches with and without pretreatment with PunchSolution™ Reagent. Panel A. One S&S 903 blood punch was pretreated with PunchSolution™ Reagent prior to amplification with 27 cycles of PCR. Panel B. One S&S 903 blood punch was directly added to PowerPlex® 21 Reaction Mix without prior preprocessing with PunchSolution™ Reagent and amplified for 27 cycles. Profile reveals lower average peak heights of all loci and particularly reduced peak heights at TH01 and TPOX. Please note that some PowerPlex® Systems require addition of 5X AmpSolution™ Reagent when amplifying DNA directly from paper storage card punches that were pretreated with PunchSolution™ Reagent. It is not possible to generate a full STR profile under the conditions stated in Table 3 without the addition of 5X AmpSolution™ Reagent. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 7 Weispfenning, R. Table 3. Amplification Conditions Requiring 5X AmpSolution Reagent Q: Can I use any formamide for the sample preparation? A: No, the quality of formamide is critical. Using high-quality formamide with low conductivity (below 100 µS/cm) is important. Multiple freeze-thaw cycles or long-term storage at 4°C may cause breakdown of formamide into formic acid and ammonia which are negatively charged and compete with DNA during injection. This results in lower peak heights and reduced sensitivity. We recommend the use of Hi-Di™ Formamide (Applied Biosystems) and freezing it at –20° C in single-use aliquots to ensure sample quality. Q: Can I use a liquid handler with a minimum pipetting volume of 2µl to prepare my samples? A: Yes, but you must dilute the PCR product prior to adding loading cocktail to prevent reduced peak heights of the Internal Lane Standard. Doubling the volume of PCR product (adding 2µl instead of 1µl) increases the salt concentration in the tube, and the excess salt competes with the DNA fragments during electrokinetic injection. This competition decreases the sensitivity of detection for both sample allele and ILS peaks. We recommend the following volumes when preparing samples amplified with PowerPlex® Systems: 1–2µl CC5 ILS + 9.5µl Hi-Di™ formamide + 1 µl PCR product To evaluate the effect of increased PCR product volume, we prepared six different loading cocktails and varied the volume of PCR product added (see Table 4). To generate the PCR products, six samples in FTA® cards were punched in duplicate and © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 8 Weispfenning, R. amplified using the PowerPlex® 18D System as directed in the PowerPlex® 18D System Technical Manual (TMD031). We also amplified 5ng of DNA as a positive control and performed a no-template negative control. The amplified products were injected at 3kV for 5 seconds. Table 4. Composition of Different Loading Cocktails The results indicate that reducing the salt, either by reducing the volume of PCR product or diluting the PCR product with an equal volume of water, increases the ILS peak heights significantly (Figure 6). The lower salt concentration in the loading cocktail allowed more DNA fragments to be injected onto the capillary. The peak heights of allele samples did not change significantly between the different loading cocktails regardless of whether 1µl or 2µl of PCR product were added. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 9 Weispfenning, R. Figure 6. Comparison of different loading cocktails for PowerPlex 18D System Also, we have observed split peaks in profiles when more than 2µl of PCR products was added to the loading cocktail. Using a higher volume of PCR product increases the concentration of excess primers, which can hybridize with single-stranded DNA and create partially double-stranded DNA strands. Double-stranded DNA migrates faster than single-stranded DNA, creating split peaks or peaks with shoulders (3) . REFERENCES 1. McLaren, R.S. et al. (2014) Developmental validation of the PowerPlex® ESI 16/17 Fast and PowerPlex® ESX 16/17 Fast Systems. Forensic Sci. Int. Genet. 13, 195–205. 2. Kutranov, S. et al. (2015) Rapid Processing of Casework and Reference DNA Samples Using Standard Protocols. Promega Corporation website. 3. McLaren, R.S. et al. (2008) Post-injection hybridization of complementary DNA strands on capillary electrophoresis platforms: A novel solution for dsDNA artifacts. Forensic Sci. Int. Genet. 2, 257–73. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 10 Weispfenning, R. HOW TO CITE THIS ARTICLE Scientific Style and Format, 7th edition, 2006 Weispfenning, R. Technical Tips for STR Analyses. [Internet] 2016. [cited: year, month, date]. Available from: http://www.promega.com/ resources/profiles-in-dna/2016/technical-tips-for-str-analyses/ American Medical Association, Manual of Style, 10th edition, 2007 Weispfenning, R. Technical Tips for STR Analyses. Promega Corporation Web site. http://www.promega.com/resources/profiles-in-dna/ 2016/technical-tips-for-str-analyses/ Updated 2016. Accessed Month Day, Year. PowerPlex is a registered trademark of Promega Corporation. AmpSolution, PunchSolution and SwabSolution are trademarks of Promega Corporation. Applied Biosystems, GeneAmp, MicroAmp and Veriti are registered trademarks of Applied Biosystems. Bode Buccal DNA Collector is a trademark of the Bode Technology Group, Inc. FrameStar is a registered trademark of 4titude, Axygen is a registered trademark of Axygen Scientific, ABgene is a trademark of Fisher Scientific, Ltd. FTA is a registered trademark of Flinders Technologies, Pty, Ltd., and is licensed to Whatman. Hi-Di is a trademark of Applera Corporation. OmniSwab is a trademark of Whatman. © Promega Corporation, Updated 2016. http://www.promega.com/pubhub 11 Weispfenning, R.