Lab #7: Nerve Pathways and Somatosensory Physiology
advertisement

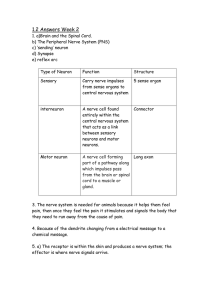
Lab #7: Nerve Pathways and Somatosensory Physiology Background The nervous system plays a central role in homeostasis. The central nervous system functions as the primary controller and integrator for most of the physiological regulatory mechanisms of the human body. The peripheral nervous system, in turn, links the central nervous system both with sensors (cells that detect environmental changes) and with effectors (cells that act on environmental changes in a way that ultimately counteracts those changes). In order for most physiological regulatory processes to work, therefore, information must be conducted through a series of neurons leading from the sensors to the central nervous system, through various regions of the central nervous system, and ultimately from the central nervous system to the effector tissue. change (Figure 7.1). This is largely because the involvement of multiple interneurons between the sensory neuron(s) and the motor neuron(s) allows integration of information from other sensory inputs, a higher degree of modulation of the signal (impeding some aspects of the signaling path while amplifying others) and the involvement of multiple structures in the body in producing a coordinated response. For example if you were to read this text aloud, sensory information would travel through three different Peripheral NS Central NS Peripheral NS Effector Sensor Nerve Pathway Structure and Spinal Reflexes Regulatory mechanisms that involve the nervous system require the transmittance of information through a series of neurons forming a nerve pathway. Most nerve pathways include neurons that perform three basic roles: a) those that conduct sensory information from the periphery into the central nervous system and perhaps through the central nervous system to a particular location, b) those that process, integrate, and interpret sensory information, and c) those that carry response information to the effector tissues, first through the central nervous system and then out through the peripheral nervous system to the appropriate muscles or glands. The specific number of neurons that information needs to travel through to go from sensor to effector can be quite variable. Differences in the lengths of nerve pathways can lead to wide variation in the complexity and precision of response to some environmental change as well as the speed of such response. Longer nerve pathways often lead to much more complex and precise responses to environmental Peripheral NS Central NS Peripheral NS Other sensory inputs Sensor Other response outputs Effector Fig 7.1. Outlines of a simple nerve pathway (top) and a complex nerve pathway (bottom). Blue arrows in the central nervous system represent an excitatory effect of the neuron on the next neuron in the pathway, whereas the red dashed arrows indicate the neuron will have an inhibitory effect on the next neuron. Note that the more complex pathway allows a greater degree of integration from multiple sensory inputs, and that based upon these sensory inputs the response will be modulate in terms of the number of motor neurons activated, the frequency of action potential generation in these motor neurons, etc. However, since more chemical synapses separate the sensor from the effector, the response time is slower in a complex pathway than in a simple one. neurons from your eye to the occipital lobe of the cerebral cortex. Information from the occipital lobe would then be relayed to a number of different areas of the brain, including the angular gyrus of the parietal lobe, regions of the temporal lobe such as Wernicke’s area (for interpretation of the symbols and formulation of words), areas of frontal lobe such as the motor cortex and Broca’s area (for controlling the muscular activity needed for vocalization), and a host of other areas that control the extrinsic eye muscles, etc. Once integration of the sensory information is made, selective stimulation and inhibition of the somatic motor neurons controlling the muscle activity of the tongue, lipids, larynx, etc. are engaged to allow the proper pronunciation of the words. Shorter motor pathways, however, tend to have much simpler responses that are localized on one particular effector organ or a small group of organs, and exhibit little integration of multiple sensory inputs in the development of a response, and thus little modulation of that response. Although complex nerve pathways enable greater control and modulation of responses to environmental change, there is a trade off with the speed of response. The slowest point of information conduction through a nerve pathway occurs at chemical synapses, where the presynaptic cell must couple the action potential to neurotransmitter release, the released neurotransmitter must diffuse across the synaptic cleft and bind to receptors on the postsynaptic cell, the binding of the messenger must be coupled to a change in the permeability of the membrane to specific ions, and the membrane must depolarize up to threshold before an action potential can be generated in the postsynaptic cell (Fig 7.2). The more chemical synapses a signal must travel through as it passes through the central nervous system, the slower response time will be. Therefore, the most rapid types of responses tend to be relatively simple behaviors derived from relatively short nerve pathways. The simplest, and fastest, types of nerve pathways are called reflex arcs. A reflex is a simple, stereotyped involuntary behavior that occurs in response to a specific stimulation. Reflexes are unconscious actions where evaluation, integration, and control involve the Presynaptic Cell • Influx of Ca2+ • Calmodulin Activation • Protein Kinase C activation • Synapsin Activation • Exocytosis Synaptic Cleft • Diffusion of neurotransmitter Postsynaptic Cell • Binding of neurotransmitter • Opening of ion channels (directly or through second messenger systems) • Depolarization to threshold Fig. 7.2. Diagram of a chemical synapse between two neurons and the events that must take place in order for the action potential of one neuron to induce an action potential in a second neuron. Transferring information from one neuron to another in a chemical synapse is the slow point of information transmittance through a nerve pathway spinal cord and brain stem rather than the cerebral cortex. As a result, the coordination of the responses does not directly involve the cerebral cortex, and thus the response may be made before a person becomes conscious of the stimulation or even without the person ever becoming conscious of the stimulus. The simplest and fastest of the reflex arcs are for spinal reflexes. In these particular nerve pathways, connections between sensory neurons and motor neurons are made within the spinal cord itself. Information may be relayed up to the brain for conscious perception, but the brain itself is not directly involved in the elicitation of a response. The simplest nerve pathway possible is called a monosynaptic pathway, since there is only one chemical synapse connecting the sensory portion of the pathway to the motor portion of the pathway. In such pathways, a sensory neuron synapses directly with a motor neuron, with no interneuron connecting the two. An example of such a pathway can be found in the patellar or “knee-jerk” reflex (Fig 7.3). Tapping a subject on the patellar ligament pulls Fig 7.3. The reflex arc of the patellar or “knee jerk” reflex. 1) Tapping on the patellar ligament stimulates stretch receptors (spindle fibers) imbedded in the quadriceps femoris. 2) This generates action potentials in a sensory neuron, conducting this signal to the spinal cord. 3) The sensory neuron synapses directly with an α motor neuron. The α motor neuron conducts an action potential through the same spinal nerve back to the quadriceps femoris. 5) The quadriceps contract, extending the lower leg. Illustration from www.merck.com/mmhe/ sec06/ch077/ch077c.html. See also Fig 12.27 in your textbook. the patella downward, in turn stretching the quadriceps femoris through the connecting tendons. Imbedded within the muscles are modified skeletal muscle fibers called spindle fibers (sometimes called intrafusal fibers) which have the dendritic endings of sensory neurons attached to them. Stretching the spindle fibers induces action potentials to form in the sensory neurons, which are conducted to the spinal cord. In the grey matter of the spinal cord, the sensory neuron synapses directly with the dendrites and cell bodies of specific motor neurons (α motor neurons) that lead to the contractile (extrafusal) skeletal muscles fibers in the same muscle that had been stretched. Stimulation of the muscle fibers by the motor neurons induces contraction of the quadriceps femoris, which extends the leg. Other reflex pathways may involve one or more interneurons connecting the sensory portion of the pathway to the motor portion. The inclusion of interneurons enables both inhibitory and excitatory elements to be included in reflexes. An example of an inhibitory reflex arc is that involving the Golgi tendon organs— structures imbedded in the tendons of skeletal muscles that consist of the dendritic endings of sensory neurons wrapped around the collagen bands of the tendon (Fig 7.4). Golgi tendon organs are stimulated when tension on the tendons is increased (usually due to excessive contraction of the muscle). Action potentials are propagated down the length of sensory neurons into the spinal cord, where they synapse with interneurons. The interneurons are stimulated to undergo action potentials, and in turn release inhibitory neurotransmitter to the α-motor neurons that control the contraction of the affected muscle. The inhibitory post-synaptic potentials triggered in the motor neurons slow signaling rates to the muscle, thus decreasing contractile strength and, in turn, the tension being exerted on the tendon. Since there are, in this case, two chemical synapses separating the sensory portion of the pathway with the motor portion (one between sensory neuron and interneuron, and one between interneuron and motor neuron), we refer to this reflex arc design as a disynaptic reflex arc. One should be aware that nerve pathways can branch, and this occurs even within the relatively simple nerve pathways involved in spinal reflex arcs. For example, a sensory neuron undergoing an action potential may + - Fig 7.4. Illustration of the disynaptic reflex associated with Golgi tendon organs. Stimulation of the sensory neuron (black) induces it to stimulate the interneuron (yellow) in the spinal cord. The interneuron, in turn, inhibits the motor neuron (blue) innervating the muscle undergoing contraction Anatagonistic Muscle Stimulated Muscle + + - Fig 7.5. An example of reciprocal innervation in a muscle-stretch reflex. The sensor neuron (black) not only stimulates a motor neuron leading to the muscle that had been stretched, inducing contraction in it, but also stimulates an interneuron that inhibits a motor neuron leading to the stretched muscle’s antagonist. synapse with one or more neurons involved in the reflex response, but also synapse with interneurons in ascending tracts of the spinal cord that relay information to the brain so that the brain can process the sensory information. For example, if you place your hand on a burning hot stove, not only is a reflex arc activated to pull your hand away, but sensory information is also relayed up to the sensory cortex so that you become consciously aware that you have burned yourself. Branching of pathways is also important in coordinating the actions of multiple organs so they do not interfere with one another during the reflexive response. For example, many skeletal muscles function in antagonistic pairs, where one muscle creates a motion when it contracts that is the opposite of the motion created when its counterpart contracts. In order to generate reflexive motion in a particular direction (for example, pulling your hand away from a hot stove), not only must a specific muscle be stimulated to undergo contraction but its antagonist must be inhibited from contracting at the same time. Thus many reflexive pathways involve reciprocal innervation, where signals from a sensory neuron actually stimulate two different reflexive pathways, one that leads to the excitation of motor neurons and the contraction of a muscle, and another that inhibits action potential generation in the motor neurons leading to the antagonistic muscle (Fig 7.5). Fig. 7.6. Examples of cutaneous receptors. Image from http://www3.open.uoguelph.ca/de/ideaExchange /zoo1500/cwork/unit3/strat_skin.html Somatosensory Physiology The central nervous system must receive information regarding environmental changes occurring outside the body as well as inside in order to regulate the internal environment. A variety of sensors are distributed throughout the body which monitor environmental conditions and relay information regarding these conditions to the central nervous system. Some sensors are specialized organs that are located within a particular region of the body (e.g., eyes, vestibular apparatuses, etc.), whereas others are relatively simple structures that are distributed throughout the body. These latter sensors are referred to as somatic sensors, and are responsible for the sensations of touch, pressure, limb movement, body position, temperature, and pain. Somatic sensors typically have a simple design, consisting of either the free dendritic endings of sensory neurons or a small structure (e.g., a single cell or bundle of connective tissue) around which are wrapped the dendritic ends of sensory neurons. These sensors can be classified into two categories based on location. Cutaneous receptors are those somatic sensors that are distributed in the skin near the surface of the body, such as those responsible for the sense of touch, pressure, and temperature (Fig 7.6). Proprioceptors are sensors imbedded within muscle and associated connective tissue and which monitor tension exerted on those structures, thus enabling perception of limb movements, contractile tension and body position. The spindle fibers and Golgi tendon Heat Cold Touch Figure 7.7. A diagram of a cross section of the skin illustrating the punctate distribution of cutaneous receptors at the surface. Each sensor has an exclusive receptive field that, when stimulated with the proper stimulus, induces action potentials in a sensory neuron. organs that monitor muscle tension are examples of proprioceptors. Cutaneous receptors are designed to monitor environmental conditions at the surface of the body. Each receptor detects environmental changes in a specific location on the surface of the skin, referred to as a receptive field. For those sensors located particularly close to the surface of the skin (e.g., those responsible for sensations of light touch and those that detect the temperature of objects in contact with the skin), typically only one receptor is found in a specific region of the skin. Therefore, these cutaneous receptors tend to have a punctate distribution, where a specific region of the skin would need to be stimulated in order to detect heat, or cold, or touch (Fig. 7.7) The size and density of the receptive fields found within a region of skin can have a profound influence on sensory acuity, the ability to discriminate fine details of an object such as shape and texture. When a stimulus is applied anywhere within a receptive field, one specific sensory neuron is stimulated, which sends signals to the central nervous system. However, the central nervous system is unable to discern where, precisely the stimulus was applied within a receptive field, since the same neuron is stimulated regardless of what specific point in the receptive field is stimulated. To detect the multiple points of stimulation needed to discern the shape and texture of objects, then, the stimulus must be applied to multiple receptive fields. Smaller receptive fields, therefore, enable more precision in determining where the surface of the skin is being stimulated and where it is not. Thus, regions of the skin that have small receptive fields tend to have greater degrees of sensory acuity than do areas with larger receptive fields (Fig 7.8). Receptive field size, in turn, is related to the density of sensory receptors in a region of the skin. For example, some regions of the skin (e.g., the back of the lower leg) have relatively low densities of touch receptors. Thus each touch receptor is responsible for a relatively large area of the surface of the skin, and hence the receptive field for each touch receptor is large. However, other regions of the skin (e.g., the lips and finger tips) have very high densities of sensory receptors, and thus the receptive field size for each receptor is comparatively small. Sensors can vary widely in the way they respond to prolonged stimulation. Many sensors will undergo sensory adaptation, adjusting their rates of action potential generation in response to chronic stimulation (Fig 7.9). Sensors that readily undergo sensory adaptation are called Large Fields, Low Density Small Fields, High Density Fig 7.8. A diagram illustrating the effect of receptor field size on acuity for the sensor of touch. The grey four-pointed arrow is an object placed in contact with the skin, and receptive fields filled in black are the fields stimulated by contact with the object. Notice that the pattern of receptive field stimulation for the small fields better reflects the actual shape of the object than does that for the large receptive fields. Membrane potential Phasic receptor Membrane potential Tonic receptor Fig 7.8. Reponses to sustained stimulation in phasic and tonic receptors. phasic receptors. Other sensors, however, show little sensory adaptation with continuous stimulation, and continue to generate action potentials at a constant rate as long as the stimulus is applied. These sensors are called tonic receptors. Somatosensory receptors, like all sensory receptors, function as transducers. They respond to changes in the environment by generating action potentials in sensory neurons. Ultimately, it is the signals delivered into specific regions of the brain or spinal cord from specific sensory neurons that enable the central nervous system to perceive environmental changes, and not the application of the stimulus itself. Sensors are designed to be most sensitive to specific stimuli (the so-called “adequate stimulus” for that sensor), but it is possible for other types of stimuli to cause a sensor to evoke an action potential in a sensory neuron if the stimulus is strong enough. As a result, it is possible to perceive a stimulus as being a different stimulus. For example, receptors that detect cold temperatures can also be stimulated by the chemical methanol. It is also possible for a stimulus to be perceived at a location other than where it is actually being applied, a phenomenon called referred pain. For example, stimulating the endings of a severed nerve in an amputee could lead to the sensation that the limb nonexistent limb is being stimulated (so-called “phantom limb pain”). Moreover, the pain receptors of many visceral organs synapse with the same interneurons in the spinal cord as do pain receptors from regions at the surface of the body. Thus when damage occurs to the visceral organ, the pain is perceived as originating from a particular region near the surface of the body (Fig. 7.9). Angina pectoris—the pain in the left chest, shoulder, and arm that is associated with heart disease, is one such example. Fig 7.9. Sites where pain originating from visceral organs are perceived cutaneously. Yellow = diaphragm/pericardium/heart; red = heart; violet = digestive tract; orange = liver/gall bladder; green = kidney/ureters; black = pelvic organs. Photograph from http://www.med.umich.edu/lrc/coursepages/M1 /anatomy/html/surface/abdomen/referred.html. (Dude! Put some pants on!) Experimental Procedures Experiment I: Spinal reflexes. Using a rubber mallet, test one of your lab group members for their stretch reflexes (see Fig 7.10). a) 1) Patellar reflex: The subject should be seated and relaxed, with their lower legs dangling and their feet off the floor. The examiner should locate the patellar ligament, and with a relatively loose grip on the hammer, the examiner applies a brisk tap to the ligament. If the subject does not respond well, have the subject lock fingers and pull his/her arms apart to amplify the response. 2) Gastrocnemius (Achilles) reflex: The subject should be seated and relaxed, with their lower legs dangling and their feet off the floor. The examiner should hold the bottom of the subject’s foot with one hand, and with a relatively loose grip on the rubber mallet, apply a brisk tap to the Achilles tendon. If the subject does not respond well, have the subject lock fingers and pull his/her arms apart to amplify the response. b) c) Fig 7.10. Depictions of the techniques used for triggering stretch reflexes: a) patellar reflex, b) gastrocnemius (Achilles) reflex, c) biceps brachii reflex. Note that for most of these reflexes the examiner is holding the portion of the limb that will be moved as a result of the muscle contracting. Illustrations are from http://medicine.tamu.edu/ neuro/reflex.htm 3) Biceps brachii reflex: The subject should be seated and relaxed. The examiner should palpate the anterior distal end of the humerus to locate the biceps tendon, the place his/her thumb over the tendon. Have the subject cross his/her legs and squeeze them together (this should amplify the response). With a relatively loose grip on the hammer, the examiner applies a brisk tap to his/her thumbnail. Flick the hammer repetitively to test several times. Experiment II: Mapping cutaneous receptors. Draw a 2 cm × 2cm square on the back or the hand or the ventral forearm of the subject with a pen (Fig 7.11). Have the subject close their eyes. Place a metal probe into a beaker of ice water to chill it, then remove it from the water and dry the probe with a paper towel. Lightly touch the probe to different points in the square, and have the subject tell you when they feel a cold sensation. Mark the location of cold sensation with a dark dot (●) with a pen. Repeat the procedure with a probe that had been heated in hot water Fig 7.11. The mapping of cutaneous receptors in a small region of skin. Illustration is from http://www.mcrel.org/ whelmers/whelm25.asp then blotted dry and mark points where the subject feels heat with an open circle (○). Repeat once again with a single bristle from a paint brush for, and have the subject tell you when they feel the bristle in contact with their skin, and mark the location with a small “x” (×). Record the distribution of the sensors in the subject’s skin on your datasheet. Experiment III: Two-point touch discrimination. This procedure is used to examine acuity for the sensor of touch in different area of the skin. Two points are applied to the skin simultaneously (Fig 7.12). If they fall in two separate receptive fields, then two points of contact will be felt. However, if both touch the skin in the same receptive field, then only one point of contact will be felt. By carefully adjusting the distance between the two points so that they are as far apart as possible when only one point is felt, the approximate diameter of the receptive field can be estimated. Use the following procedure to measure receptive field size for the following regions of the skin: the tip of the index finger, the palm of the hand, the medial lower arm, and the nape of the neck. Obtain a pair of calipers, and move the tines of the calipers 2-2.5 cm apart. Have a member of your group serve as a subject, and have them close their eyes. Select a location on their body, and lightly touch both tines of the calipers to their skin simultaneously. Ask the subject if they feel one point or two. If they feel two points, move the tines slightly closer together and repeat. Repeat this procedure until the subject can only feel one point. Measure the distance between the two tines to the nearest millimeter. This distance is roughly equal to the diameter of the receptive fields for touch in that area of the skin. Fig 7.12. Two-point touch discrimination. When the skin is touched at two points that fall in different receptive fields, then two different sensory neurons will be stimulated , and two separate points of contact will be perceived. However, if the skin is touched at two points in the same receptive field, then only one sensory neuron will be stimulated, and only one point of contact will be perceived Experiment IV: Thermosensory adaptation. In this experiment, different groups of cutaneous thermoreceptors (which tend to be phasic) will undergo sensory adaptation. The result of differential adaptation of thermoreceptors, in turn, will illustrate how the same environmental conditions can be perceived in different ways based on the sensitivity of different sensors. Place one hand into each of two water baths (one hot, one cold) simultaneously for 1 minute. Afterwards, place both hands simultaneously into a bath containing lukewarm water. Describe the sensation you feel in each hand. Experiment V: Referred pain. Select one person from your group. Obtain a large axe. Position the subject’s arm on the counter surface and restrain the subject. With a single, sweeping downward blow of the axe, attempt to sever the lower Identify the person in your group with the poorest cardiovascular health, and have them eat a couple of Monster Thickburgers©, drink a pot of strong coffee, chain smoke a carton of cigarettes, and take a couple doses each of Vioxx and Fen-Phen. Then have them run wind sprints around the Tap firmly on the ulnar nerve where it crosses the median epicondyle of the elbow with your finger or with the rubber mallet (Fig 7.13). Describe where the tingling sensation is perceived. Figure 7.13. Illustration depicting stimulation of the ulnar nerve at the median epicondyle of the elbow. Image from http://www.nsbri.org/ HumanPhysSpace/focus7/ep_metabolism.html