Physics 20 – Unit 1 – Measurement and Data Analysis Workbook
advertisement
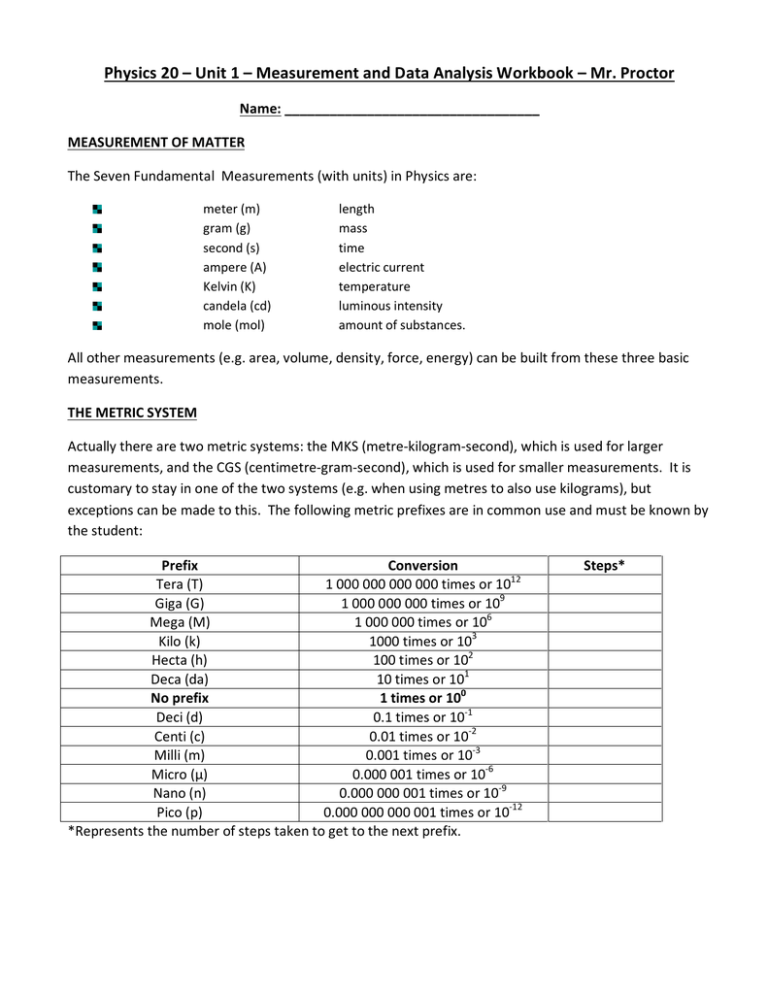
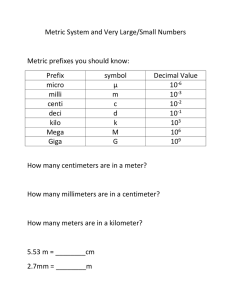
Physics 20 – Unit 1 – Measurement and Data Analysis Workbook – Mr. Proctor Name: __________________________________ MEASUREMENT OF MATTER The Seven Fundamental Measurements (with units) in Physics are: meter (m) gram (g) second (s) ampere (A) Kelvin (K) candela (cd) mole (mol) length mass time electric current temperature luminous intensity amount of substances. All other measurements (e.g. area, volume, density, force, energy) can be built from these three basic measurements. THE METRIC SYSTEM Actually there are two metric systems: the MKS (metre-kilogram-second), which is used for larger measurements, and the CGS (centimetre-gram-second), which is used for smaller measurements. It is customary to stay in one of the two systems (e.g. when using metres to also use kilograms), but exceptions can be made to this. The following metric prefixes are in common use and must be known by the student: Prefix Conversion Tera (T) 1 000 000 000 000 times or 1012 Giga (G) 1 000 000 000 times or 109 Mega (M) 1 000 000 times or 106 Kilo (k) 1000 times or 103 Hecta (h) 100 times or 102 Deca (da) 10 times or 101 No prefix 1 times or 100 Deci (d) 0.1 times or 10-1 Centi (c) 0.01 times or 10-2 Milli (m) 0.001 times or 10-3 Micro (µ) 0.000 001 times or 10-6 Nano (n) 0.000 000 001 times or 10-9 Pico (p) 0.000 000 000 001 times or 10-12 *Represents the number of steps taken to get to the next prefix. Steps* Mega, Micro, and beyond These prefixes are used the same way as the others except you must count three steps between kilo and mega and between milli and micro instead of the usual one. METRIC CONVERSIONS To do metric conversions of linear units (distance, mass, etc.): 1. Write out the metric prefixes in a vertical column from largest to smallest (refer to the chart above). 2. Start at the prefix you are at and count the number of steps to the prefix you wish to convert to. Be sure to count a step for passing the unit without a prefix. 3. Move the decimal the same number of places as the number of steps you counted. If you moved up the column, move the decimal to the left. If you moved down the column, move the decimal point to the right. Example: Convert 5.0 km to m 2. Convert 5.0 pg to Mg To do metric conversions of squared units (e.g. area): For example, convert 387.5 m2 to cm2 1. Do steps 1 and 2 under conversions of linear units. (For this example, there are two steps between metres and centimetres). 2. Double the number of steps counted and move the decimal point that amount. (In our example, twice the number of steps is four, so we move the decimal point four places to the right to get the final answer of 3 875 000 cm2). Example: Convert 387.5 m2 to km2 To do metric conversions of cubed units (e.g. volume): For example, convert 2340 cm3 to m3 1. Following steps 1 and 2 under conversion of linear units (in our example, there are 2 steps between cm and m). 2. Triple the number of steps counted and move the decimal point that amount (in our example, three times two is six, so we move the decimal point six times to the left to get our final answer of 0.00234 m3). Example: Convert 0.3875 cm3 to mm3 METRIC MADNESS Convert the following: a) 200 mm = ____________________ cm b) 25 dg = ____________________ g c) 105 cm = ____________________ mm d) 456 000 cl = ____________________ dal e) 6 820 hg = ____________________ mg f) 1.3 Ml = ____________________ kl g) 0.000 345 dag = ____________________ cg h) 0.34 m = ____________________ km i) 4 m2 = ____________________ cm2 j) 16 m2 = ____________________ mm2 k) 1 360 000 cm2 = ____________________ m2 l) 234 km2 = ____________________ m2 m) 67 m3 = ____________________ cm3 n) 2345 dm3 = ____________________ cm3 o) 86 km3 = ____________________ Mm3 p) 0.000 75 m3 = ____________________ dam3 q) 9 m3 = ____________________ hm3 r) 0.001 km3 = ____________________ hm3 s) 635 mm3 = _____________________________________________km3 _____________________________________________Mm3 METRIC: COMPOUND CONVERSIONS Conversions of two or more unites at once are called “compound conversions”. A common example in physics is changing m/s to km/hr or vice versa. The most reliable way of doing this is to use the units themselves to help you do your math. For each unit to be converted, find the conversion factor that will ‘cancel’ it out and replace it with the unit that you want. Example: Convert 45 km/h to m/s: There are two units to be converted, so two conversion factors will be needed: The first factor must cancel the ‘km’ and replace it with ‘m’ Do that replacing ‘km’ in the denominator and ‘m’ in the numerator Now, since you know there are 1000 m in a km, place ‘1000’ beside the ‘m’ Repeat the process, first arranging the factor so that it cancels the ‘h’ and replaces it with ‘s’ Now, since there are 3600 s in an hour, place ‘3600’ beside the ‘s’ Cancel out the units, checking that you end up with the units you wanted And, finally, do the math Compound Conversion Practice a) 16.9 km/h = __________ m/s b) 22 m/s = __________ km/h c) 18 468 cm/s = __________ km/h d) 84.9 m/s = __________ km/h e) 29.3 m/h = __________ cm/s f) 162 km/h2 = __________ m/s2 g) 0.84 m/s2 = __________ km/h2 h) 38 km/h2 = __________ m/s2 i) 0.009 254 m/s2 = __________ km/h2 j) 0.000 002 cm/s2 = __________ km/h2 ACCURACY OF MEASURED NUMBERS Science is based on accurate measurement. But every measurement contains some error. It is important to know how much error is in each measurement, especially when one scientist is using another’s data. A number of customs and rules have developed to deal with this. We begin with rules on how to measure. Rules of Accuracy: when measuring, state every digit that you are sure of, and one digit that has some uncertainty. Since everyone follows this rule, you can know the accuracy of any number simply by looking at it. The last digit is the uncertain one, and is assumed to have an uncertainty of ± 1 unless you are told otherwise. e.g. 317 means 317 ± 1 (the real value lies between 316 and 318) 48 736.4 means 48 736.4 ± 0.1 (the real value lies between 48 736.3 and 48 736.5) 0.0029 means 0.0029 ± 0.0001 (the real value lies between 0.0028 and 0.0030) If the accuracy is not ± 1, the person doing the measurement must state the accuracy. e.g. 65 ± 5 or 0.104 ± 0.002 The person making the measurement must be honest about their accuracy. Quoting a number to either more or fewer than the correct number of decimals is dishonest. The number of decimals places you go to in a measurement is going to depend on the measuring device being used and the circumstances under which you do the measurement. But when you measure a series of numbers under the same conditions, they will always be to the same number of decimal places. e.g. This column of numbers was gathered under the same conditions with the same ruler: 16.4 cm 28.3 cm 45 cm 52.9 cm 67.85 cm It makes no sense that one those (45 cm) is ten times less accurate, and another (67.85 cm) is ten times more accurate than the others. This is an example of dishonest reporting of accuracy. The most accurate number is one with the most number of significant figures (the most measured numbers that have certainty). SIGNIFICANT FIGURES (http://www.physics.uoguelph.ca/tutorials/sig_fig/SIG_dig.htm) You will often need to estimate the accuracy of numbers. Most people think that a number that goes to more decimal places is always more accurate, but that is not true. The truth is that the more measured digits a number has, the more accurate it is. An honest experimenter will never write down digits that weren’t actually measured except when he/she is forced to use the digit ‘zero’ as a place holder. A measured digit is called a ‘significant’ digit, and the more significant digits a number has, the more accurate it is. In order to help you see how many significant figures a number has, five rules were developed. The rules are all centred on the problem of whether each zero was really measured, or is merely a place holder. Here are the rules: 1. Any number that is not zero is always significant. (1,2,3,4,5,6,7,8,9) 2. Zeros ‘trapped’ between other numbers are significant. For example, 3005 has four significant figures. Since the ‘5’ and the ‘3’ were measured, the zeros between them must have been measured too. ‘Trapped’ zeros are always significant. This is true whether there is a decimal point there or not (e.g. 12.078 has five significant figures). 3. Zeros at the beginning of a decimal number are not significant. For example, 0.009087. Here, the first two zeros are only there to hold the place, so they are not significant. The rest of the number, ‘9087’, is significant, so 0.009087 has four significant figures. Any number that begins with a decimal point and zeros falls under this rule. 4. Zeros at the end of a decimal are significant For instance, .0098000 has five significant figures. Here, the first two zeros are not significant but what about the last three? They are not there to hold the place, so they must be there because they were measured. Therefore, those zeros are significant, and the number .0098000 has five significant figures. 5. Zeros at the end of a whole number are not significant unless you are told differently. This is a difficult situation. Whole numbers ending with zero are genuinely ambiguous in their accuracy. For example, the number 300 could mean: 300 ± 1 (between 299 and 301) and have three significant figures, or 300 ± 10 (between 290 and 310) and have 2 significant figures, or 300 ± 100 (between 200 and 400) and have 1 significant figure. Different people interpret this rule differently, but the majority of experts feel that it is best to call none of these zeros significant unless told otherwise, so this is the rule we will follow. The number 300, therefore, has only 1 significant figure. Remember that these five rules are there so you can estimate the accuracy of a number at a glance, simply by counting the number of significant figures it has. Rule for Multiplying and Dividing: round off the answer to the least number of significant figures from the question. Rule for Adding and Subtracting: the final answer will contain the same number of significant digits to the least precise place value. Keep One Extra Digit in Intermediate Answer: When doing multi-step calculations, keep at least one more significant digit in intermediate results than needed in your final answer. For instance, if a final answer requires two significant digits, then carry at least three significant digits in calculations. If you round-off all your intermediate answers to only two digits, you are discarding the information contained in the third digit, and as a result the second digit in your final answer might be incorrect. (This phenomenon is known as "round-off error.") The Two Greatest Sins Regarding Significant Digits: 1. Writing more digits in an answer (intermediate or final) than justified by the number of digits in the data. 2. Rounding-off, say, to two digits in an intermediate answer, and then writing three digits in the final answer. SIGNIFICANT FIGURES PRACTICE 1. How many significant figures are there in each of the following? i) 0.0030 a) 3.43 _____ j) 2 b) 8007 _____ c) 17.05 _____ k) 323.70 d) 173.764 _____ l) 1 000 000 e) 0.0006 _____ m) 0.00106 f) 0.10006 _____ n) 90 010 g) 700 _____ o) 0.0100004 h) 8400 _____ p) 0.00908060 _____ _____ _____ _____ _____ _____ _____ _____ 2. Which of the following numbers is the most accurate and which is the least accurate? a) 0.0007 c) 5.0 e) 0.4005 f) 0.35000 b) 3100 d) 1.02 3. These were all measured with a metre stick. Correct the errors made recording the data: 34.9 cm 16.25 cm 18.4 cm 9.3 cm 39 cm 11.5 cm 22.1 cm 4. Perform the following, rounding off the answers to the least number of significant figures: a) 2.55 x 3.2 = f) 0.002903 / 0.0029 = b) 2.0000 x 4.00 = g) 16.409 x 2.00 = c) 30.0 x 6.45 = h) 17340 x 0.799236 = d) 0.0030 x 6.45 = i) 6.090 x 0.07 = e) 846.923 x 3.920 = j) 0.000 004 x 0.34 = k) 56873.5 / 986230 = q) 17.66 / 0.90 = l) 0.9 x 0.99 = r) 560 000 / 9862 = m) 0.2 / 75.8 = s) 0.00753 / 18.45 = n) 0.009834 x 567.23 = t) 0.09600 x 235.48 = o) 0.9900 / 0.00700 = u) 7500 / 125 = p) 600.0 x 4.9309 = v) 144 / 36 = 5. Perform the following, rounding off the answers to the least number of decimal places: a) 6.24 + 1.358 = j) 678.9 + 34.08 = b) 245.6 – 0.2999 = k) 0.45 – 0.34 = c) 74 + 0.000 265 = l) 26 + 0.098 = d) 8000 – 6 = m) 234.5673 – 0.0045 = e) 16.93 + 7.9 = n) 789 230 + 4500 = f) 12.060 + 0.00923 = o) 0.876 + 0.000 99 = g) 800 – 0.14 = p) 7.567 – 4560 = h) 0.000 709 + 0.012 = q) 0.00350 – 0.0124 = i) 273 000 + 3.62 = r) 112 + 5620 = 6. Solve the following using all rules of significance: a) 89.23 x 0.000 650 = i) 2.3850 x 85.0 = b) 16.7342 + 98 452.35 = j) 1.0 + 1.00 = c) 0.0053 – 2.9 = k) 180 / 90 = d) 5638.238 / 14 000 = l) 16.4 – 200 = e) 0.0050230 x 4.0 = m) 200 x 0.025 = f) 843 + 1600.5 = n) 0.00600 + 0.00400 = g) 5600 / 3.5894 = o) 3.0 / 5673.9 = h) 63.248 – 0.08456 = SCIENTIFIC NOTATION 1. Writing Numbers in Scientific Notation 1. Write down the first significant digit, followed by a decimal point. 2. Write down all the other significant digits, followed by ‘x 10’. 3. Count the number of times the decimal moved from the original form of the number to the way you have it written now. That will be the exponent. This exponent will be negative if the original was less than one. Examples: 245 000 0.067 2. Multiplying and Dividing Numbers in Scientific Notation 1. Multiply or divide the numerical parts of the two numbers. 2. Add (for multiplying) or subtract (for dividing) the exponents. 3. Round off the answer so that it has the same number of significant figures as the original number with the fewest significant figures. 4. If necessary, shift the decimal point and adjust the exponent so that the answer is still in proper scientific notation. Examples: (3 x 104) x (2 x 105) = (10.2 x 106) / (2.0 x 10-3) = (4 x 10-3) / (8.00 x 10-6) = 3. Adding and Subtracting Numbers in Scientific Notation 1. Shift the decimal and change the exponent one number so they both have the same exponent. If you shift the decimal point to the left, the exponent increases and if you shift the decimal point to the right, the exponent decreases. 2. Add or subtract the numerical parts of the two numbers. The exponent is not changed. 3. Round off the answer so that it has the same number of decimal places as the number with the fewest number of decimal places as shown when both numbers have the same exponent. 4. If necessary, shift the decimal point and adjust the exponent so that the answer is in scientific notation. Examples: 4.0 x 106 + 3.01 x 105 = 4.00 x 102 – 2.0 x 101 = 6.3 x 10-5 – 3.0 x 10-4 = 7.89 x 103 + 3.0 x 103 = SCIENTIFIC NOTATION PRACTICE 1. Write the following numbers in scientific notation: a) 678 g) 0.100 b) 3.99 h) 0.10 c) 8 888 000 i) 0.010 d) 23 000 j) 0.000 000 046 e) 23 000 ± 10 k) 0.000 047 0 f) 23 000 ± 1 l) 0.000 407 800 0 2. Calculate the following and give your answer to the correct number of significant figures: a) (3.89 x 102) x (7.77 x 103) = b) (9.45 x 108) x (3.23 x 10-3) = c) (4.744 x 10-6) x (2.300 x 10-4) = d) (8.3299 x 105) x (6.754 x 10-9) = e) (6.892 x 102) / (4.70 x 104) = f) (2.22 x 106) / (6.7 x 103) = g) (6.91845 x 10-6) / (5.4293 x 10-5) = h) (3 x 10-1) / (6.994327 x 10-6) = i) (6.87 x 101) + (3.63 x 101) = j) (2.4964 x 106) + (4.927 x 105) = k) (8.290 x 1016) + (6.852 x 1014) = l) (9.99 x 10-5) + (8.48368 x 10-6) = m) (8.359 x 103) – (2.554 x 102) = n) (3.79747 x 1022) – (1.1 x 1020) = o) (4.2754 x 100) – (8.467 x 101) = p) (3.4800 x 10-6) – (4.5 x 10-5) = EQUATIONS AND TRANSPOSITION OF FORMULAE Most of the Mathematics of Physics/Engineering/Science consists of relationships between various physical quantities. These are expressed as Mathematical equations. Examples (i) (ii) Area of a circle, radius r = Coulombs Law. The force of attraction between two charged particles is = Where Q1 & Q2 are the charges, r is their distance apart, k is a physical constant. Equations such as the above which represent frequently used results are known as formulae. When using a formula connecting physical quantities it is of course important to use a consistent set of physical units, but in these notes we are solely interested in the Mathematical rules for manipulating equations. There is only one rule for changing the appearance of an equation. Whatever you do to one side of the equation you must do the same to the other side Example: Solve for x = x − y Try a few for practice. Solve for the variable between the (): 1. = 2. = () 6. ( = () 3. = 332 + 0.6 ρ 4. = √ρ $ 5. = ($%&)& ()*")λ + () 7. 8. (ρ" ) " 0. 0- () " () " = - + . (/- ) . = − - (ℎ - ) 9. 2" 3" ∆" + 2 3 ∆ = 0 (2" )