Deposition of Ceramic Composites for Solid Oxide Fuel Cell
advertisement

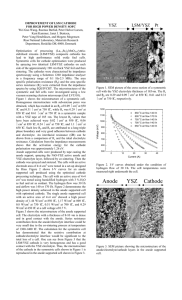
Deposition of Ceramic Composites for Solid Oxide Fuel Cell Applications using Aerosol Jet Printing Mary Sukeshini Ayyadurai University of Dayton Research Institute, Dayton, OH The Air Force Research Laboratory, Wright-Patterson Air Force Base, OH Optomec Inc., St. Paul, MN May 15, 2012 1 Outline Fuel Cell – How it works and why we need Solid Oxide Fuel Cells (SOFCs) Material properties Multi-layer configuration, electrochemistry Conventional fabrication methods Single cell fabrication and performance Aerosol Jet Printing and Processing of SOFCs Electrochemical performance of cells & microstructure • Single component- Electrolyte, YSZ • Composite Cathode layers- LSM and YSZ • Scanning Electron Microscopy(SEM) Anode Interlayer/Functional Gradation Summary, Challenges and Future Work 2 Anode cathode Hydrogen Fuel Cell H2 + ½ O2 = H2O ΔGo = -nFEo Eo= 1.23 V Cartoon scheme –Schatz Energy Research Center website Diverse Energy Sources/Fuels and Applications Stationary Conventional Fuels Biomass Natural Gas Propane Diesel Other hydrocarbons Methane Methanol Renewable sources (wind, solar, biomass) Nuclear Hydrogen Natural Gas Clean and efficient Energy conversion Fuel Cells Alkaline Direct methanol Molten carbonate Polymer electrolyte membrane Phosphoric acid Solid Oxide •Primary Power and CHP (residential, commercial and industrial) • Backup power Transportation Auxiliary Power Motive power •Trucks •Trains •Aircraft •Ships •Specialty vehicles (e.g.- fork lift) •Buses •Automobiles Coal (with carbon sequestration) Portable Power •Consumer Electronics • Battery chargers • Soldier power Source: US DOE 2010 The Promise of Renewable Hydrogen from Wind and Solar Energy -Hydrogen from renewable carbon free sources -Needs advanced photoelectrolysis and photobiological technologies PV Panels Electrolyzer Fuel Cell Compressor/ gas flow control To grid Inverter Controller Underground tank Artist’s rendition of a futuristic home – source: Interface, vol. 13 (4), 2004 5 SOFC: Material Properties Electrical properties Materials Nickel-cermet (Ni-Yttria stabilized zirconia) •el. Conductivity (el + ion) •Catalytic activity Thermomechanical properties •Porosity •Thermal expansion coefficient •Adhesion •Gas-tightness Yttria doped Zirconia (YSZ) •ionic conductivity (ionic >> el) Strontium doped lanthanum-manganate (La,Sr)MnO3 (LSM) •el. Conductivity (el + ion) •Catalytic activity •Mechanical stability •Adhesion •Thermal expansion coefficient •Porosity Color scheme in center : Courtesy -Robert Kee, Colorado School of Mines 6 Microstructure - “Triple Phase Boundary” Ni-YSZ Anode • Microstructure - particle size, Ni content, Ni dispersion in the cermet - preheating and heating temperatures - sintering temperature reaction at gas/electrode/electrolyte interface, a three phase boundary (TPB) Adsorption, surface reaction and charge transfer -dissociative adsorption of H2 (on Ni?) -surface diffusion of H or (H+) -ionization of surface H to Ni -charge transfer reactions of H+ and O2and OH- around the TPB -desorption of H2O 7 Multi-layered SOFC Configuration Cathode Cathode Interlayer LSM LSM/YSZ YSZ Electrolyte Anode Interlayer 50Ni/50YSZ Anode support-Ni/YSZ Electrode functional layers, electrolytes and barrier layers Extension of “Triple phase boundary” into bulk of the electrode Barrier layers to avoid deleterious reactions between components Match the thermal co-efficient of expansion between components Thinner electrolytes to compensate for ohmic loss at intermediate temperature operation 8 Fabrication Methods for SOFC Components • Chemical methods - Chemical vapor deposition(1-10 µm/h) - Electrochemical vapor deposition(3-50 µm/h) - Sol-Gel (0.5 µm-1 µm for each coating) - Spray pyrolysis (5 -60µm /h) • Ceramic Powder/Wet • Physical Methods - Physical vapor -pulse laser (PLD) -sputtering (0.25-2.5 µm/h) - Laser Ablation - Molecular beam epitaxy - Atmospheric and vapor plasma spraying (100-500 µm/h) - Screen printing (10-100 µm) - Tape casting (25-200 µm) - Slip casting - Filter pressing - Slurry, spray, dip coating - Tape calendering - Transfer printing - Electrophoretic deposition 9 Characteristics of Different Methods • Chemical methods dense films high reaction temperatures limited range of compositions • Physical Methods reproducible, patterning with litho graphy/ masks, too expensive for scale-up • Ceramic Powder/Wet inexpensive,scale-up possible, prone to crack formation, inhomogenous thickness 10 Cell Fabrication: Electrolyte and Cathode Layers on Anode Support Anode support Ni- YSZ Paste or print LSM Sinter- 1200 ˚C Paste or print LSM/YSZ Paste or print electrolyte, YSZ Sinter- 1400 ˚C Anode interlayer 50/50 wt% NiO/YSZ Anode substrate- Ni -YSZ Bisque- 950 ˚C 11 Comparison of Cell with Ink-jet Printed and Pasted Cathode -Z"( cm2) 3 SEI - cell with printed cathode 2 SEI - cell with 1 pasted cathode 0 LSM (a) 650 oC 700 oC 750 oC 800 oC 850 oC 1 0 0 1 2 3 4 5 3 Cell impedance 3 650 oC 700 oC 750 oC 800 oC 850 oC ) 6 (a) 0 1 2 4 5 6 650 oC 700 oC 750 oC 800 oC 850 oC 2 3 4 (b) Cell impedance 1 0 1 2 3 4 5 Z'( cm ) 2 Z'( cm ) 3 650 oC 700 oC 5 Z'( cm ) 2 3 6 Ni/YSZ support 3 -Z"( cm2) -Z"( cm2) (a) 2 5 1 0 1 4 Z'( cm ) 1 0 3 2 3 0 2 2 2 0 6 Ni/YSZ support 2 1 YSZ Z'( cm2) 650 oC 700 oC 750 oC 800 oC 850 oC LSM LSM/YSZ YSZ -Z"( cm2) -Z"( cm2) 3 0 (b) LSM/YSZ 2 650 oC 700 oC 750 oC 800 oC 850 oC •Sukeshini, A.M., Cummins, R., Reitz, T.L., and Miller, R.M., “Ink-Jet Printing of Anode Supported SOFC: Comparison of Slurry 650 oC (b) Pasted Cathode and Printed Cathode,” Electrochem. and Solid-State Lett., 12(12), pp. B176-179 (2009). 700 oC 12 6 Comparison of Performance of Cells with Ink-jet Printed and Pasted Cathode (Fuel-H2; Oxidant- Air) 0.3 0.6 0.2 0.4 700 ˚C 0.2 650 ˚C 0.1 750 ˚C 800 ˚C 0.5 1 1.5 0.2 0.4 650 ˚C 0.4 1 0.8 printed 0.3 0.6 0.2 0.4 700 ˚C 650 ˚C 0.2 0.1 750 ˚C 800 ˚C 850 ˚C 0 0 0 0.5 1 Current Density (A/cm2) 1.5 2 Voltage (V) 1.2 Power Density (W/cm2) Voltage (V) 0.5 (b) 800 ˚C 850 ˚C 750 ˚C 0 0 0.5 Current Density (A/cm2) 1 0.1 700 ˚C 0 2 1.2 0.3 0.6 0.2 0 0.4 printed 0.8 850 ˚C 0 0 (c) 1 Power Density (W/cm2) printed 0.8 0.5 1 1.5 Current Density (A/cm2) 2 0.5 (d) Power Density (W/cm2) 0.4 Voltage (V) (a) 1 Voltage (V) 1.2 0.5 Power Density (W/cm2) 1.2 0.4 0.8 pasted 0.3 0.6 0.2 0.4 700 ˚C 0.2 650 ˚C 750 ˚C 800 ˚C 0.1 850 ˚C 0 0 0 0.5 1 1.5 2 Current Density (A/cm2) • comparable performance for both types • reproducible performance •Sukeshini, A.M., Cummins, R., Reitz, T.L., and Miller, R.M., “Ink-Jet Printing of Anode Supported SOFC: Comparison of Slurry Pasted Cathode and Printed Cathode,” Electrochem. and Solid-State Lett., 12(12), pp. B176-179 (2009). 13 Aerosol Jet Printing: Electrolyte, YSZ Pattern filling – (spiral fill) Printed YSZ Smooth corners/ adjust line width NiO/YSZ substrate Pattern filling – (raster fill) VM ToolsTM Software patterns for material deposition designed using AutoCAD®. drawing files are converted into tool paths using VMTools™ (VMT) files generated by VMT integrate stage motion with deposition Optimizing Nozzle-Substrate Distance Optical Images of printed electrolyte surface d= 9 mm d= 10 mm d= 11 mm d= 12 mm d= 5 mm d= 6 mm d= 7 mm d= 8 mm A gap of up to 8mm between the nozzle and substrate gave homogenous deposits with no particulates longer transit distances lead to coalescence of smaller droplets •Sukeshini, A.M., Gardner, P., Jenkins, T., Reitz, T.L., and Miller, R.M., “Investigation of Aerosol Jet Deposition Parameters for printing SOFC layers,” Proceedings of the ASME 8th International Conference on Fuel Cell Science, Engineering and Technology, vol. 1, pp 325-332 (2010). SEM: Surface of Printed Electrolytes printed (and sintered) electrolytes were dense grain size- 2- 5 µm •Sukeshini, A.M., Gardner, P., Jenkins, T., Reitz, T.L., and Miller, R.M., “Investigation of Aerosol Jet Deposition Parameters for printing SOFC layers,” Proceedings of the ASME 8th International Conference on Fuel Cell Science, Engineering and Technology, vol. 1, pp 325-332 (2010). SEM: Cross-sectional View of Electrolytes 20 µm YSZ electrolytes with different thicknesses printed by altering 20 µm YSZ •deposition time •speed •line spacing 20 µm YSZ 17 Composite Electrode Layer: Cathode LSM/YSZ Mass calibration curves to determine the required gas flow conditions to establish desired compositions - use single atomizer and determine individual deposition rates for a range of gas flow conditions - plot calibration curves for the two individual materials - select gas flow conditions corresponding to desired compositions - use dual atomizers simultaneously to print the composites 18 Material Mixing: Composite Cathode Vacuum pump individual components directly mixed on the fly offers potential for functional gradation •Sukeshini, A.M., Gardner, P., Jenkins, T., Reitz, T.L., and Miller, R.M., “Investigation of Aerosol Jet Deposition Parameters for printing SOFC layers,” Proceedings of the ASME 8th International Conference on Fuel Cell Science, Engineering and Technology, vol. 1, pp 325-332 (2010). Virtual Impactor (LSM) sheath gas LSM Deposition head sheath gas Virtual Impactor (YSZ) Aerosol jet YSZ exhaust gas PCM substrate platen 19 Calibration of Deposition Rate for Selection of Composition 16 YSZ Deposition rate (mg/min) 14 YSZ Atomizer1500 sccm Atomizer2000 sccm sheath gas- 3000 sccm 12 Atomizer1500 sccm 10 LSM 8 LSM Atomizer2000 sccm 6 4 600 1100 1600 Exhaust Flow (sccm) deposition rate of YSZ > LSM deposition rate likely depends on initial particle size distribution and aerosolized droplet distribution 20 SEM (Back scattered): Microstructure of Composite LSM/YSZ and LSM Layers LSM Cathode layers of different samples LSM/YSZ YSZ cathode interlayer, LSM/YSZ about 12 µm thick and porous LSM layer about 3 µm thick and more dense than desired highly reproducible 21 VI Curves for Button Cells (Fuel-H2; Oxidant- Air) 0.3 (a) 1 0.2 0.6 0.4 0.1 850 ⁰C 0.2 700 ⁰C 650 ⁰C 0 0 0.2 750 ⁰C 800 ⁰C 0 0.4 0.6 0.8 1.2 (b) (b) 0.8 0.2 0.6 0.4 0.2 700 ⁰C 650 ⁰C 0 0 0.2 750 ⁰C 800 ⁰C 0.1 850 ⁰C 0 0.4 0.6 0.8 Current Density (A/cm2) LSM LSM/YSZ (a) YSZ The cells have open circuit voltages (OCV) ranging from 1.16-1.20V, depending on the temperature. 0.3 1 Voltage (V) (a) 1 Power Density (W/cm2) Voltage (V) 0.8 Power Density (W/cm2) 1.2 LSM The maximum power density at 850(b) LSM/YSZ2. ˚C for both cells is 250 mW/cm YSZ 1 22 Cathode Optimization Identically printed and processed YSZ, LSM/YSZ but different LSM printing/processing LSM LSM/YSZ YSZ (a) (b) (c) (d) Identical for all four cells Ni/YSZ Ink composition Thickness Sintering Temperature (a) LSM Ink- 34 wt %, 40 passes, - 1200 ⁰C (b) LSM Ink- 34 wt %, 60 passes, - 1200 ⁰C (c) LSM Ink- 17 wt %, 40 passes, - 1200 ⁰C (d) LSM Ink- 34 wt %, 40 passes, - 1150 ⁰C Aerosol Jet Printing and Microstructure of SOFC Electrolyte and Cathode Layers", ECS Transactions, Vol. 35(1), "Solid Oxide Fuel Cells 12 (SOFC-XII)", pp 2151-2160, (2011). 23 0.6 0.6 0.4 0.4 0.4 0.4 0.2 0.2 0.2 0.2 700 ⁰C 700 ⁰C 00 00 00 11 0.1 0.1 800 800 800⁰C⁰C 800⁰C ⁰C 700 ⁰C 700 750 ⁰C⁰C⁰C 750 ⁰C 750 750 ⁰C 650 ⁰C 650 ⁰C 650 650⁰C⁰C 00 11 0.3 40 40 0.3 40passes passes 40 passes passes 850 ⁰C sintering temp.1200 temp.850sintering ⁰C sintering temp.-1200 1200⁰C ⁰C sintering temp.1200 ⁰C ⁰C solids wt %34 solids %34 solidswt wt %34 solids wt %- 34 0.2 0.2 850 850⁰C⁰C 0.6 0.6 0.4 0.4 0.5 0.5 11 0.5 0.5 1.5 1.5 1.5 1.5 22 0.8 0.8 0.6 0.6 0.6 0.6 0.2 0.2 0.4 0.4 0.4 0.4 00 0.5 0.5 11 0.8 0.8 0.8 0.8 0.6 0.6 850 850⁰C⁰C 800 800⁰C⁰C 0.4 0.4 850 850⁰C ⁰C 0.3 0.3 800 800⁰C ⁰C 0.6 0.6 750 750⁰C⁰C 0.4 0.4 0.4 0.4 0.2 0.2 0.2 0.2 750 750⁰C ⁰C 0.2 0.2 650 700⁰C⁰C 650⁰C ⁰C700 0.5 0.5 0.5 0.5 11 0.8 0.8 0.3 0.3 0.6 0.6 0.2 0.2 0.4 0.4 11 dd 1.5 1.5 22 2 Current (A/cm ) ) (A/cm Current Density CurrentDensity Density (A/cm Current Density (A/cm2)) 1.5 1.5 1.5 1.5 22 00 22 1.5 1.5 22 00 22 40 40 40passes passes 40 passes passes sintering temp.1150 sintering temp.sintering temp.1150⁰C ⁰C sintering1150 temp.1150 ⁰C ⁰C solids wt solids solidswt wt%%-34 34 solids wt %%- 34 34 0.5 0.5 0.5 0.5 0.4 0.4 0.4 0.4 dd 0.8 0.8 0.3 0.3 0.6 0.6 0.2 0.2 0.4 0.4 0.2 0.2 650 650⁰C⁰C 11 11 850 850⁰C⁰C 0.1 0.1 0.2 0.2 00 00 11 700 700⁰C ⁰C 00 00 1.2 1.2 0.4 0.4 0.1 0.1 650 650⁰C⁰C 00 cc cc 1.2 0.5 1.2 0.5 (V) Voltage(V) Voltage 11 40 40 40passes passes 40 passes passes sintering temp.1200 sintering temp.sintering temp.1200⁰C ⁰C sintering1200 temp.1200 ⁰C ⁰C solids wt solids solidswt wt%%-17 17 solids wt %%- 17 17 0.5 0.5 (W/cm2)2) Density(W/cm PowerDensity Power 1.2 1.2 11 0.2 0.2 22 2 Current (A/cm ) ) (A/cm Current Density CurrentDensity Density (A/cm Current Density (A/cm2)) (W/cm22)) Density (W/cm Power Density Power (V) Voltage (V) Voltage 1.2 1.2 (V) Voltage(V) Voltage (V) Voltage (V) Voltage 22 2 Current (A/cm ) ) (A/cm Current Density CurrentDensity Density (A/cm Current Density (A/cm2)) 0.5 0.5 0.3 0.3 0.1 0.1 00 00 00 0.4 0.4 0.1 0.1 0.2 0.2 00 00 22 0.4 0.4 0.3 60 60 0.3 60passes passes 60 passes passes sintering temp.1200 sintering temp.sintering temp.-1200 1200⁰C ⁰C sintering temp.1200 ⁰C ⁰C solids wt %34 solids %34 solidswt wt %34 solids wt %- 34 0.2 0.2 850 850 850⁰C⁰C 850⁰C ⁰C 800 800 800⁰C⁰C 800⁰C ⁰C 750 750 750⁰C⁰C 750⁰C ⁰C 650 650 ⁰C 650⁰C⁰C 700 650 ⁰C⁰C⁰C 700 700 700⁰C ⁰C 0.1 0.1 0.2 0.2 00 11 0.8 0.8 0.3 0.3 bb bb 0.5 0.5 (W/cm2)2) Density(W/cm PowerDensity Power 0.8 0.8 0.4 0.4 0.5 0.5 00 650 700 650⁰C ⁰C 700⁰C⁰C 750 ⁰C 700 ⁰C 700750 ⁰C ⁰C 750 800 ⁰C 800⁰C ⁰C 750 ⁰C 850 850⁰C ⁰C 0.1 0.1 0.1 0.1 00 00 0.5 0.5 0.5 0.5 11 11 0.2 0.2 800 800⁰C ⁰C 00 00 0.3 0.3 (W/cm2)2) Density(W/cm PowerDensity Power 0.8 0.8 aa aa 1.2 1.2 (W/cm22)) Density (W/cm Power Density Power 11 1.2 0.5 1.2 0.5 (W/cm22)) Density (W/cm Power Density Power 11 0.5 0.5 (W/cm2)2) Density(W/cm PowerDensity Power (V) Voltage(V) Voltage 1.2 1.2 (W/cm22)) Density (W/cm Power Density Power (V) Voltage (V) Voltage 1.2 1.2 (V) Voltage(V) Voltage (V) Voltage (V) Voltage VI Curves of Different Printed Cells 1.5 1.5 22 2 Current (A/cm ) ) (A/cm Current Density CurrentDensity Density (A/cm Current Density (A/cm2)) 1.5 1.5 22 00 22 24 Anode Interlayer : Functional Gradation Scheme Electrolyte, YSZ 50/50 Anode support non graded Ni/ YSZ Electrolyte, YSZ 17/83 33/67 50/50 Anode support compositionally graded interlayer with NiO/YSZ. 25 VI Curves for Cells with Graded and Non-graded anode Functional Layer Two identical sets Voltage (V) 0.8 0.2 0.6 non-graded anode interlayer 0.4 0.1 0.3 1 graded anode interlayer Voltage (V) graded anode interlayer Power Density (W/cm2) 0.3 1 0.8 0.2 0.6 non-graded anode interlayer 0.4 0.1 0.2 0.2 0 0 0 0.2 0.4 0.6 0.8 1 Current Density (A/cm2) 0 0 0 0.2 0.4 0.6 Current Density (A/cm2) 0.8 1 • reproducible performance • graded cells show better performance compared to non-graded cells 26 material submitted for publication Power Density (W/cm2) 1.2 1.2 Summary, Challenges and Conclusion SOFC anode , cathode, and electrolyte components were deposited using aerosol jet printing Layer thickness and microstructure and hence electrochemical performance of cells incorporating printed components was reproducible Functionally graded composite anode and composite cathode layers were printed and incorporated in button cells Cells with functionally graded anode interlayers performed better than cells with non-graded anode interlayer Low voltage scanning electron microscopy was used as a diagnostic tool for evaluating material mixing and elucidation of phases Material mixing on anode side requires further optimizationmodify inks, improve processing? Current overall cell performance is not satisfactory- requires further printing/processing optimization Additive deposition manufacturing methods enable printing of dense and porous SOFC Layers. The potential for electrode patterning, reproducibility of microstructures, and ease of mass manufacture make these deposition methods very advantageous compared to traditional methods of screen printing and spraying. 27 Acknowledgments Thomas Jenkins (UES Corp.) Paul Gardner (AFRL) Frederick Meisenkothen (UES Corp.) Optomec Inc., for a grant through a AFRL contract #FA8650-09-C2016 Dr. Thomas Reitz, Dr. Ryan Miller, AFRL for facilities and funding 28