Joule`s Law and the Electrical Equivalent of Heat
advertisement

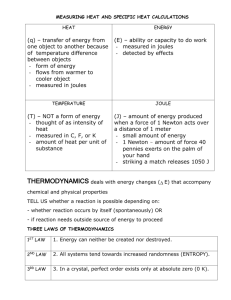
LPC Physics Joule’s Law and the Electrical Equivalent of Heat Joule’s Law and the Electrical Equivalent of Heat Purpose: To verify the conversion factor, 1 cal. = 4.18 Joules. Equipment: Double-walled calorimeter Heating coil with stirrer Power Supply Enviro-Safe Thermometer Standard Temperature Probe and LabPro Kit Rods and Clamps 2 DMMs Balance Stopwatch 400 ml beaker, ice, water Patch cords, Alligator clips Theory: The work W done (or energy expended) per unit charge in moving a charge q from one point to another is the potential difference, or voltage V V= or W q W = qV Eq. 1 The time rate of flow of charge is described in terms of current I and I= q t Eq. 2 Hence, Eq. 1 may be written W = qV = IVt Eq. 3 which represents the work done or the energy expended in a circuit in time t. This is often expressed as work or energy per time, or power P: P= W = IV t Eq. 4 The expended energy can be written in terms of the resistance R of the circuit or a particular circuit element by Ohm’s law, V=IR. Using this relationship, Eq. 3 has the various forms: W = IVt = I 2 Rt = 1 of 5 V 2t R Eq. 5 LPC Physics Joule’s Law and the Electrical Equivalent of Heat The electrical energy expended is manifested as heat energy, and is commonly called joule heat or I2R losses, I2R being the power or energy expended per time. Equation 5 shows how the joule heat varies with resistance: 1. For a constant current, I, the joule heat is directly proportional to the resistance, I2R. 2. For a constant voltage, V, the joule heat is inversely proportional to the resistance, V2 R. The energy expended in an electrical circuit as given by Eq. 5 is in the units of joules. The relationship (conversion factor) between joules and heat units in calories was established by James Joule from mechanical considerations – the mechanical equivalent of heat. You may recall that in his mechanical experiment, Joule had a descending weight turn a paddle wheel in a liquid. He then correlated the mechanical (gravitational) potential energy lost by the descending weight to the heat generated in the liquid. The result was 1 cal = 4.18 J. A similar electrical experiment may be done to determine the “electrical equivalent of heat”. By the conservation of energy, the heat equivalents of mechanical and electrical energy are the same (i.e. 1 cal = 4.18 J). Experimentally, the amount of electrical joule heat generated in a circuit element of resistance R is measured by calorimetry methods. If a current is passed through a resistance (immersion heater) in a calorimeter with water in an arrangement as illustrated in Figure 1, by the conservation of energy the electrical energy expended in the resistance is equal to the heat energy (joule heat) Q gained by the system: electrical energy = heat gained W =Q IVt = ( m w c w + mcal c cal )(T f − Ti ) or Eq. 6 where the m’s and c’s are the masses and specific heats of the water and calorimeter cup, as indicated by the subscripts. Tf and Ti are the final and initial temperatures of the system, respectively.1 DIGI 35A V C - + G + A - V Figure 1 111 The theory section of this lab was shamelessly borrowed from: Jerry D. Wilson. Physics Laboratory Experiments, 2nd Edition. Lexington MA: D.C. Heath and Company, 1986. Experiment 35. 2 of 5 LPC Physics Joule’s Law and the Electrical Equivalent of Heat Experiment: 1. Measure the mass of the empty calorimeter. 2. Fill the inner calorimeter cup with 200 ml of water, cooled to about 10o below room temperature. Be sure to record this temperature as To. 3. Measure mass of the inner calorimeter cup and water. 4. Place the lid on the calorimeter. Make sure the stirring rod does not touch the coil and short out the circuit. Suspend the thermometer (or temperature probe) such that it does not touch the coil or interfere with stirring. 5. Hook up Joule's heating coil with one DMM as an ammeter and a second as a voltmeter (see Figure 1). 6. If using a temperature probe, follow Steps 7 - 11. If using a thermometer, skip to Step 12. 7. Connect the AC adapter to the LabPro by inserting the round plug on the 6-volt power supply into the side of the interface. Shortly after plugging the power supply into the outlet, the interface will run through a self-test. You will hear a series of beeps and blinking lights (red, yellow, then green) indicating a successful startup. 8. Attach the LabPro to the computer using the USB cable that is Velcro-ed to the side of the computer box (do not unplug the USB cable from the computer!). The LabPro computer connection is located on the right side of the interface. Slide the door on the computer connection to the right and plug the square end of the USB cable into the LabPro USB connection. 9. Connect a temperature probe to an analog jack (CH1-CH4) on the LabPro. The analog jacks are located on the same side of the LabPro as the AC Adapter Port. If you are using an older temperature probe, you will need to use the DIN-BTA adapter. 10. Open the Experiments folder on the desktop and open the file joule.xmbl (or .cmbl) This will start the program Logger Pro3.3 and bring up the appropriate data file. If you do not have an auto-ID sensor (which is the likely case), a dialog box will pop up asking you to confirm the sensors being used. If you have the suggested sensor attached to the LabPro in the suggested port, click “OK”. If the “OK” button is not active, ask your instructor for help. 11. Currently, the experiment is set to collect for 180 seconds. You may wish to extend the collection period by choosing Experiment > Data Collection and changing the length of the collection time. If you still need more time, you can press Ctl+T to extend the collection period by 270 seconds. However, it is not necessary to get all the data in one run. 3 of 5 LPC Physics Joule’s Law and the Electrical Equivalent of Heat 12. Turn on the power only when the coil is under water so it doesn't burn out. 13. With no ice present, turn on the power supply. Record the current, voltage and temperature every 60 seconds until the water is heated to 10o above room temperature. Keep an accurate record of time. It is essential to stir vigorously and continuously throughout the experiment. Take turns. Analysis: 1. 2. Graph the results of m w c w (T − To )w + mcal ccal (T − To )cal vs. IV (t − t o ) . Draw the best straight line from your graph and calculate the slope and the uncertainty in the value. Be sure to use error bars on your graph. 3. Determine the conversion factor from calories to Joules, i.e.: (J/cal) x the number of cal = the number of Joules. 4. Discuss precision and error. How could the experimental procedure be altered to give more accurate results? Results: Write at least one paragraph describing the following: • what you expected to learn about the lab (i.e. what was the reason for conducting the experiment?) • your results, and what you learned from them • Think of at least one other experiment might you perform to verify these results • Think of at least one new question or problem that could be answered with the physics you have learned in this laboratory, or be extrapolated from the ideas in this laboratory. 4 of 5 LPC Physics Joule’s Law and the Electrical Equivalent of Heat Clean-Up: Before you can leave the classroom, you must clean up your equipment, and have your instructor sign below. How you divide clean-up duties between lab members is up to you. Clean-up involves: • Completely dismantling the experimental setup • Removing tape from anything you put tape on • Drying-off any wet equipment • Putting away equipment in proper boxes (if applicable) • Returning equipment to proper cabinets, or to the cart at the front of the room • Throwing away pieces of string, paper, and other detritus (i.e. your water bottles) • Shutting down the computer • Anything else that needs to be done to return the room to its pristine, pre lab form. I certify that the equipment used by ________________________ has been cleaned up. (student’s name) ______________________________ , _______________. (instructor’s name) (date) 5 of 5