Spectroscopic ellipsometry studies of II
advertisement

The University of Toledo
The University of Toledo Digital Repository
Theses and Dissertations
2010
Spectroscopic ellipsometry studies of II-VI
semiconductor materials and solar cells
Jie Chen
The University of Toledo
Follow this and additional works at: http://utdr.utoledo.edu/theses-dissertations
Recommended Citation
Chen, Jie, "Spectroscopic ellipsometry studies of II-VI semiconductor materials and solar cells" (2010). Theses and Dissertations. Paper
807.
This Dissertation is brought to you for free and open access by The University of Toledo Digital Repository. It has been accepted for inclusion in Theses
and Dissertations by an authorized administrator of The University of Toledo Digital Repository. For more information, please see the repository's
About page.
A Dissertation
entitled
Spectroscopic Ellipsometry Studies of
II-VI Semiconductor Materials and Solar Cells
by
Jie Chen
Submitted to the Graduate Faculty as partial fulfillment of the
requirements for the Doctor of Philosophy Degree in Physics
_____________________________________
Dr. Robert W. Collins, Committee Chair
_____________________________________
Dr. Patricia Komuniecki, Dean
College of Graduate Studies
The University of Toledo
December 2010
Copyright 2010, Jie Chen
This document is copyrighted material. Under copyright law, no parts of this document
may be reproduced without the expressed permission of the author.
An Abstract of
Spectroscopic Ellipsometry Studies of II-VI Semiconductor Materials and Solar Cells
by
Jie Chen
Submitted to the Graduate Faculty as partial fulfillment of the
requirements for the Doctor of Philosophy Degree in Physics
The University of Toledo
December 2010
The multilayer optical structure of thin film polycrystalline II-VI solar cells such as
CdTe is of interest because it provides insights into the quantum efficiency as well as the
optical losses that limit the short-circuit current.
The optical structure may also
correlate with preparation conditions, and such correlations may assist in process
optimization.
A powerful probe of optical structure is real time spectroscopic
ellipsometry (SE) that can be performed during the deposition of each layer of the solar
cell.
In the CdCl2 post-deposition treatment process used for thin film polycrystalline
II-VI solar cells, the optical properties of each layer of the cell change during the process
due to annealing as well as to the elevated temperature.
In this case, ex-situ SE before
and after treatment becomes a reasonable option to determine the optical structure of
CdCl2-treated CdTe thin film solar cells.
CdTe solar cells pose considerable challenges for analysis by ex-situ SE.
iii
First, the
relatively large thickness of the as-deposited CdTe layer leads to considerable surface
roughness, and the CdCl2 post-deposition treatment generates significant additional
oxidation and surface inhomogeneity. Thus, ex-situ SE measurements in reflection from
the free CdTe surface before and after treatment can be very difficult. Second, SE from
the glass side of the cell is adversely affected by the top glass surface which generates a
reflection that is incoherent with respect to the reflected beams from the thin film
interfaces and consequently depolarization if collected along with these other beams.
In
this research, the first problem is solved through the use of a succession of Br2+methanol
treatments that smoothens the CdTe free surface, and the second problem is solved
through the use of a 60° prism optically-contacted to the top glass surface that eliminates
the top surface reflection. In addition, the succession of a Br2+methanol treatment not
only smoothens the CdTe surface but also enables CdTe etching in a layer-by-layer
fashion.
In this way, it has been possible to track the optical properties of the CdTe
component layer as a function of depth from the surface toward the CdS/CdTe interface
in order to gain a better understanding of the film structure.
In this study, ex-situ spectroscopic ellipsometry was applied first to investigate the
optical properties of the TEC-15 glass substrate, and then to extract the optical properties
of thin film CdTe and CdS both as-deposited and CdCl2-treated.
After obtaining all the
optical properties of the solar cell component layer materials, a comprehensive ex-situ SE
analysis has been applied to extract the optical structure of a single thin film of
CdCl2-treated CdTe, and finally to obtain the optical structure of the CdCl2
iv
post-deposition treated CdTe solar cell.
Based on the fundamental studies in this thesis, various aspects of the solar cell
structure after the complicated CdCl2 treatment have been determined.
In future work
the role of the key parameters of CdCl2 post-deposition treatment process will be
explored including: the temperature and treatment time. As a result, a correlation will
be established between solar cell performance and film structure.
Finally, an
understanding of how solar cell structure can be optimized to achieve the highest solar
cell performance may be possible through improved control of the CdCl2 post-treatment
process.
v
Table of Contents
Abstract
iii
Table of Contents
vi
List of Tables
ix
List of Figures
xii
1
Introduction to Spectroscopic Ellipsometry
1
1.1 History…………………………………...………………...………………..…....1
1.2 Purpose…… …………………………………………………………...…..…….2
1.3 Data measured by ellipsometry…………………………………………………..3
1.4 Mathematical derivation…..……………………………………………………..5
1.5 Spectroscopic ellipsometer used in the study…………………………………..10
1.6 Data analysis……………………………………………………………………12
2
Introduction to CdTe-based Solar Cells…………………………………………..18
2.1 CdTe-based solar cell structures ……………………………………………….18
2.2 Deposition method and process steps…………………………………………..21
2.3 Application of spectroscopic ellipsometry as an analysis technique …………..22
3
Optical Properties of TEC-15 Glass…….………………………………………...26
3.1 Introduction……………………………………………………………………..26
vi
3.2 Experimental details…...………………………………………………………..28
3.3 Data analysis and results…………………...…………………………………...29
4
Verification of the Chemical Etching Process for CdTe Depth Profiling………52
4.1 Introduction……………………………………………………………………..52
4.2 Structural evolution of CdTe during etching: experimental details…………….54
4.3 Structural evolution of CdTe during etching: results and analysis ......…….......57
4.4 Detection of a-Te on etched CdTe: experiment details…………………………59
4.5 Detection of a-Te on etched CdTe: results and analysis………………………..60
5
Optical Properties of Thin Film CdTe and CdS before and after CdCl2
Post-deposition Treatment………………………………………………………...71
5.1 Introduction……………………………………………………………………..71
5.2 Optical properties of as-deposited CdTe and CdS films deposited on c-Si
substrates……..…………………………………………………………………72
5.3 Optical properties of CdCl2 post-deposition treated CdTe and CdS……………78
5.4 Etch-back profiling of CdTe thin film structure after post-deposition treatments...
…………………………………………………………………………………. 84
6
Optical Structure of As-deposited and CdCl2-treated CdTe Superstrate Solar
Cells…………………………………………………………………………………94
6.1 Introduction……………………………………………………………..………94
6.2 Experimental details…………………………………………………………….96
6.3 Results and discussion: film side and prism side measurements…..…………...97
vii
6.4 Results and discussion: through the glass measurements……………………..113
6.5 Summary………………………………………………………………………119
7
RTSE Analysis of CdTe Solar Cell Structures in the Substrate Configuration…..
………………………………………………………………………………………120
7.1 Introduction……………………………………………………………………120
7.2 Analysis of CdTe deposition on rough molybdenum…………………………121
7.3 Ex situ spectroscopic ellipsometry analysis of a CdTe solar cell in the substrate
configuration…………….……………………………………………………138
8
Spectroscopic Ellipsometry Studies of II-VI Alloy Films………………...…….152
8.1 Introduction……………………………………………………………………152
8.2 Top cell material candidates: Cd1-xMnxTe and Cd1-xMgxTe…………………...154
8.3 Bottom cell material: Cd1-xHgxTe……………………………………………..172
9
Summary and Future Directions………………………………………………...178
9.1 Summary………………………………………………………………………178
9.2 Future directions………………………………………………………………183
References
196
Appendix
A
Dielectric functions
207
viii
List of Tables
4.1 Best fit parameters and confidence limits that define Eqs. (4.1) and (4.2) for the
dielectric function of a-Te. …..……………………………………………………..63
5.1 Fitting results for single crystal and thin film polycrystalline CdTe using an analytical
model
consisting
of
four
critical
points
and
one
T-L
background
oscillator. …...………………………………………………………………………74
5.2 Fitting results for single crystal and thin film polycrystalline CdS using an analytical
model
consisting
of
three
critical
points
and
one
T-L
background
oscillator…………………………………………………………………………….75
5.3 Best fit dielectric function parameters comparing single crystal, CdCl2-treated, and
as-deposited CdTe samples. ………………………………………………..……….79
5.4 Best fit dielectric function parameters for as-deposited CdS on a fused silica prism,
CdCl2-treated
CdS
on
the
prism,
and.
as-deposited
CdS
on
c-Si. ……………………………………………………………….………….……..83
ix
6.1 Dielectric function library used in spectroscopic ellipsometry data analyses for CdTe
solar cells. ……………………………………………………………………..……98
6.2 Best fitting parameters added step by step to improve the mean square error (MSE) in
modeling
through-the-glass
SE
measurements
of
a
CdTe
solar
cell. ………………………………………………………………………………..116
6.3 Multilayer stack thicknesses, non-uniformity, and compositions, the latter expressed
in terms of volume fractions, along with parameter confidence limits for the best fit
to SE data obtained through the glass. …………………………………………….118
7.1 CdTe bulk and surface roughness layer thicknesses for the top four CdTe bulk
layers. ……………………………………………………………………………..126
7.2 Five models used to evaluate the Mo overlayer thickness using reference dielectric
functions from the literature. ……………………………………………………...134
7.3 Best fit critical point and Tauc-Lorentz oscillator parameters describing the inverted
dielectric function of polycrystalline ZnTe:Cu. The exponents µn are fixed at the
single crystal values of Table 7.4. ………………………………………………...143
x
7.4 Best fit critical point and Tauc-Lorentz oscillator parameters for single crystal ZnTe.
……………………………………………………………………………………..144
7.5 Best fitting parameters added step by step to improve the standard mean square error
(MSE) in the ellipsometric analysis of a CdTe solar cell in the substrate
configuration. ……………………………………………………………………..150
8.1 Deposition parameters used to prepare the CdxMg1-xTe and CdxHg1-xTe thin
films. ………………………………………………………………………………155
8.2 Critical point parameters of transition energy and width obtained in the fits to the
dielectric functions of Fig. 8.9. ……………………………………………………167
8.3 Critical point energies and E0 broadening parameters for two as-deposited
Cd1-xMgxTe alloys from spectroscopic ellipsometry.
Also shown are corresponding
results for as-deposited and CdCl2-treated CdTe. …………………………………172
8.4 Energy position and width of the critical point generating the strongest peak in ε2 for
as-deposited thin film Cd1-xHgxTe. ………………………………………………..174
xi
List of Figures
r
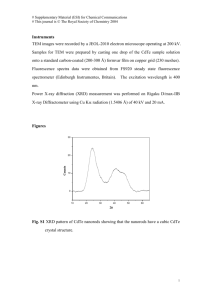
1-1 Schematic representation of the electric field vector trajectory E (rr0 , t ) for an elliptically
r
polarized light wave at a fixed position r0 versus time. Q is the tilt angle between the
ellipse major axis a and the p-axis, measured in counterclockwise-positive sense
when facing the light source.
χ is the ellipticity angle given by tan-1(b/a). …...........
……………………………..…………………………………………………………7
1-2 Reflection of a polarized light wave at an interface between two media. ……….…..9
1-3 Spectroscopic ellipsometer used in this research mounted in the ex-situ mode of
operation. …………………………………………………………………………...11
1-4 Simplified flow chart of the data analysis procedure. …………...…………………13
1-5 Optical model and physical structure of a c-Si wafer used as a substrate. .……...........
………………………………...………………………..…...………………...…….14
2-1 The substrate structure for CdTe solar cells. ……………………………………….19
xii
2-2 The superstrate structure for CdTe solar cells. ……………………………………..19
3-1 The multilayer structure of the TEC-15 glass substrate. …………………………...28
3-2 Simple model deduced from the analysis of the transmittance and ellipsometric (ψ, ∆)
spectra of Figs. 3-3 – 3-5 for the soda lime glass substrate. The surface roughness
is obtained in a best fit of the (ψ, ∆) spectra. …………………..…………………..31
3-3 Best fit simulated and experimental normal incidence transmittance spectra T vs.
photon energy for an uncoated soda lime glass substrate used in the fabrication of
TEC glasses. ………………………………………………………………………..31
3-4 Best fit simulated and experimental ellipsometric angle ψ = tan−1 (|rp/rs|) vs. photon
energy for an uncoated soda lime glass substrate used in the fabrication of TEC
glasses.
The angle of incidence is 60˚. …………………………………………..32
3-5 Best fit simulated and experimental ellipsometric angle ∆ = δp − δs vs. photon energy
for an uncoated soda lime glass substrate used in the fabrication of TEC glasses.
The angle of incidence is 60˚. ……………………………………………………...32
3-6 Index of refraction (left) and extinction coefficient (right) vs. wavelength for the
xiii
uncoated soda lime glass substrate.
The index of refraction results are derived from
the ellipsometric ψ spectrum whereas the extinction coefficient results are derived
from the transmittance spectrum.
The data values are tabulated in Appendix A. ......
………………………………………………………………………………………33
3-7 Model with best fitting parameters obtained in the analysis of the transmittance and
ellipsometric (ψ, ∆) spectra of Figs. 3.8 and 3.9 for the soda lime glass substrate
coated with a single layer of undoped SnO2. ……………………………………….34
3-8 Normal incidence transmittance T vs. photon energy for a soda lime glass substrate
coated with a single layer of undoped SnO2, the first layer in the fabrication of TEC
glasses. Experimental data (broken line) and a best fit simulation (solid line) are
shown. ………………………………………………………………………………35
3-9 Ellipsometric angles ψ and ∆ vs. photon energy for a soda lime glass substrate coated
with a single layer of undoped SnO2. Experimental data (broken lines) and best fit
simulations (solid lines) for an angle of incidence of 60˚ are shown. ………………..
………………………………………………………………………………………35
3-10 (a,b) Real and imaginary parts of the dielectric function ε1 and ε2 vs. photon energy
for undoped SnO2 that forms the first layer of TEC glasses; (c) analytical expression
xiv
for the complex dielectric function of (a,b) along with the best-fit free parameters
and their confidence limits. ………………………………………………………...36
3-11 Model adopted for the analysis of the transmittance and ellipsometric (ψ, ∆) spectra
of Figs. 3.12 and 3.13 obtained on the soda lime glass substrate coated with a single
layer of SiO2. ……………………………………………………………………….37
3-12 Normal incidence transmittance T vs. photon energy for a soda lime glass substrate
coated with a single layer of SiO2, which is used as the second layer in the
fabrication of TEC glasses; experimental data (broken line) and a best fit simulation
(solid line) are shown. ……………………………………………………………...38
3-13 Ellipsometric angles ψ and ∆ vs. photon energy for a soda lime glass substrate
coated with a single layer of SiO2, which is used as the second layer in the
fabrication of TEC glasses; experimental data (broken lines) and a best fit simulation
(solid lines) are shown. ……………………………………………………………..38
3-14 (a) Real (solid line) and imaginary (broken line) parts of the dielectric function ε vs.
photon energy for SiO2 that forms the second layer of the TEC glasses.
The
imaginary part of the dielectric function vanishes; (b) mathematical expression for
the dielectric function in (a) along with the best fitting parameters and their
xv
confidence limits. ……………………………………………………..……………39
3-15 Real and imaginary parts of the dielectric function ε vs. photon energy for the SiO2
that forms the second layer of the TEC glasses (solid lines) for comparison with the
reference data of a thermally-grown SiO2 on crystalline silicon. ………………..……
………………………………………………………………………………………..39
3-16 Best fit sample structure for a soda lime glass substrate coated with a two layer stack
of undoped SnO2 and SiO2, which are the first two layers used in the fabrication of
TEC glasses. ………………………………………………………………………..40
3-17 Ellipsometric angles (ψ, ∆) at an angle of incidence of 60˚ and transmittance T at
normal incidence plotted versus photon energy for a soda lime glass substrate coated
with a two layer stack of undoped SnO2 and SiO2, which are the first two layers used
in the fabrication of TEC glasses. …………………………………………………..41
3-18 Best fit multilayer stack for a complete TEC-15 glass sample.
The layered
structure includes thin undoped SnO2, thin SiO2, and thick doped SnO2:F with
surface roughness on top.
The previously-determined dielectric functions were
used for the soda lime glass and the two thin layers. ………………………………43
xvi
3-19 Normal incidence transmittance T vs. photon energy for a complete TEC-15 glass
sample consisting of a soda lime glass substrate coated with layers of undoped SnO2,
SiO2, and top-most doped SnO2:F.
Experimental data (broken line) and a best fit
simulation (solid line) are shown. ………………………………………………….43
3-20 Ellipsometric angles ψ and ∆ at a 60˚ angle of incidence plotted vs. photon energy
for a complete TEC-15 glass sample consisting of a soda lime glass substrate coated
with layers of undoped SnO2, SiO2, and top-most doped SnO2:F. The broken lines
indicate experimental spectra and the solid lines indicate the best fit
simulation. ………………………………………………………………………….44
3-21 Real and imaginary parts of the dielectric function ε1 and ε2 vs. photon energy for
doped SnO2:F that forms the top-most layer of TEC-15 glass.
These results are
obtained as a best fit analytical expression at low energies where the film is
semitransparent and by an inversion of (ψ, ∆) data at high energies where the film is
opaque. ……………………………………………………………………………..44
3-22 (a) The analytical equation for the dielectric function of the top-most SnO2:F layer
of TEC-15 that holds below 4.4 eV; also shown is (b) a table of the best fit
parameters in the equation and their confidence limits. ……………………………45
3-23 Multilayer structure with best-fit parameters for a complete TEC-7 glass sample.
xvii
The layered structure includes thin undoped SnO2, thin SiO2, and a thick layer of
doped SnO2:F with surface roughness on top.
The previously determined dielectric
functions for TEC-15 glass were used here for this TEC-7 glass sample. ……………
………………………………………………………………………………………47
3-24 Multilayer structure with best-fit parameters for a complete TEC-8 glass sample.
The layered structure includes thin undoped SnO2, thin SiO2, and a thick layer of
doped SnO2:F with surface roughness on top.
The previously determined dielectric
functions for TEC-15 glass were used here for this TEC-8 glass sample. ……………
………………………………………………………………………………………47
3-25 Transmittance T vs. photon energy for a complete TEC-7 glass sample; experimental
data (broken line) and simulated results based on the ellipsometric model (solid line)
are shown (left). The difference between the two data sets is shown at the
right. ………………………………………………………………….…………….49
3-26 Normal incidence transmittance T vs. photon energy for a complete TEC-8 glass
sample; experimental data (broken line) and simulated results based on the
ellipsometric model (solid line) are shown (left).
The difference between the two
data sets is shown at the right. ……………………………………………………...50
xviii
3-27 For TEC-7 glass, the normal incidence scattering results predicted by combining
ellipsometry and normal incidence specular transmittance are shown in comparison
with experimental normal incidence integrated scattering data from a diffuse
transmission experiment.
Different TEC-7 samples were used for the two different
data sets. ……………………………………………………………………………51
3-28 For TEC-8 glass, the normal incidence scattering results predicted by combining
ellipsometry and normal incidence specular transmittance are shown in comparison
with experimental normal incidence integrated scattering data from a diffuse
transmission experiment.
Different TEC-8 samples were used for the two different
data sets. ……………………………………………………………………………51
4-1 A schematic of optical models used to evaluate a CdTe film by optical depth profiling
during both deposition and etching processes. ……………………….…………….56
4-2 The evolution of void volume fraction within the top 100 Å of the bulk layer as a
function of CdTe bulk layer thickness obtained during the deposition and etching
processes. …………………………………………………………………………...58
4-3 Schematic of the sample structural changes that occur in the last three etching steps
for a CdTe film on c-Si.
The starting thickness of this CdTe film is 3500 Å. ………
xix
…………….…………………………………………………………………………60
4-4 Ellipsometric spectra for a smoothened CdTe film on a c-Si wafer measured at angle
of incidence of 63°.
The broken lines represent data measured before the first
additional Br2+methanol etching step, and the solid lines represent data measured
after the 6th additional Br2+methanol etching step.
the two is 18 seconds.
The total etching time between
The starting CdTe thickness before any etching was 3 µm. ...
.………………………………………………………………………………………61
4-5 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate after the 36th
and 37th etch steps for comparison.
The starting CdTe film thickness was 3500 Å. ..
.………………………………………………………………………………………64
4.6 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate with a starting
thickness of 3500 Å measured after the 37th (left) and 36th (right) etching steps (data
points). Also shown are their best fits (broken lines). ………………………..……..
.………………………………………………………………………………………64
4-7 Model and best-fit parameters used for the analysis of the ellipsometric spectra of Fig.
4.6 (left panel) collected after the 37th etching step applied to a CdTe film on a
crystalline Si substrate.
Because the CdTe film is completely removed, this
xx
analysis provides the structure of the c-Si substrate.
MSE indicates the mean
square error in the fit. ………………………………………………………………65
4-8 Model and best fit parameters used for the analysis of the ellipsometric spectra of Fig.
4.6 (right panel) collected after the 37th etching step applied to a CdTe film on a
crystalline Si substrate.
c-Si substrate.
This analysis yields the structure of the a-Te layer on the
The void volume fraction in the a-Te layer has been obtained by
expressing the a-Te layer in this study of polycrystalline CdTe as a mixture of the
a-Te obtained in a previous study of single crystal CdTe along with a void
component. …………………………………………………………………………65
4-9 Real and Imaginary parts of the dielectric function ε1 and ε2 vs. photon energy for
a-Te generated through Br2+methanol etching of a polycrystalline CdTe film. ………
.………………………………………………………………………………………65
4-10 A comparison of the a-Te optical properties deduced in this study (see Fig. 4.9) with
the literature reference optical properties of a-Te from 1.5~6 eV, the latter obtained
by etching single crystal CdTe. …………………………………………………….66
4-11 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate with a starting
thickness of 3500 Å measured after the 35th etch step (left panel).
xxi
Also shown is
the best fit and associated model deduced in the analysis of the ellipsometric spectra
in order to extract the a-Te/CdTe/c-Si structural parameters (right panel). …………...
.………………………………………………………………………………………68
4-12 Experimental and best fit spectra (left panel) along with the best fit parameters and
model (right panel) for comparison with the results of Fig. 4.11, but without
introducing an a-Te component into the model. Such a model leads to a higher
MSE. ………………………………………………………………………………..68
4-13 Ellipsometric spectra and the best fit (left panel) for a smoothened CdTe film with a
starting thickness of 3 µm obtained before the first additional etch after smoothening.
Also shown is the model and best fit parameters used in the analysis of the
ellipsometric spectra over the energy range of 2 to 6 eV in order to deduce the a-Te
volume fraction in the surface roughness layer (right panel). ………………………...
.………………………………………………………………………………………69
4-14 Experimental and best fit spectra (left panel) along with the best fit model and
parameters (right panel) for comparison with the results of Fig. 4.13, but without
introducing an a-Te component into the model.
This ellipsometric analysis is
associated with a 3 µm thick smoothened CdTe film before the first additional etch
after smoothening. ………………………………………………………………….69
xxii
4-15 Ellipsometric spectra and the best fit (left panel) for a smoothened CdTe film with a
starting thickness of 3 µm obtained after the 6th additional etch after smoothening.
Also shown is the model and best fit parameters used in the analysis of the
ellipsometric spectra over the energy range of 2 to 6 eV in order to deduce the
surface roughness thickness and the a-Te volume fraction in the CdTe structure (right
panel). ………………………………………………………………………………70
4-16 Experimental and best fit spectra (left panel) along with the best fit model and
parameters (right panel) for comparison with the results of Fig. 4.15 but without
introducing an a-Te component into the model.
This ellipsometric analysis is
associated with a 3 µm thick smoothened CdTe film after the 6th additional etch. …...
…….…………………………………………………………………………………70
5-1 The room temperature dielectric functions of single crystal CdTe (broken lines) and a
CdTe film deposited at 188°C (solid lines). The downward arrows point to the
energy values of the four critical point transitions E0, E1, E1+∆1, and E2. ……………
……………………….………………………………………………………………73
5-2 Band structure of CdTe. …...………………………………………………………..74
xxiii
5-3 The room temperature ordinary dielectric functions of single crystal (wurtzite) CdS
(broken lines) in comparison with the polycrystalline thin film CdS deposited on c-Si
at 225 °C (solid line). The three downward arrows point to the energy values of the
critical point transitions. ……………………………………………………………75
5-4 (left) Best fit analytical models of the room temperature dielectric functions for two
CdTe films of thickness approximately 1000 Å, obtained from the same deposition
but with different post-deposition processing:
as-deposited (no treatments; broken
line) and CdCl2-treated for 5 min at 387°C (solid line); (right) a comparison between
the CdCl2-treated CdTe film (solid line) and single crystal CdTe (broken line). ……..
.………………………………………………………………………………………78
5-5 A schematic of the sputtering chamber for CdTe/CdS deposition on a fused silica
prism. ……………………………………………………………………………….81
5-6 (left) Best fit analytical models for the room temperature dielectric functions of a CdS
film as-deposited on a fused silica prism measured from the prism side and on a c-Si
wafer measured from the ambient side;
(right) best fit analytical model for the
room temperature dielectric functions of CdS measured from the prism side before
and after a 30 min CdCl2 treatment at 387°C. ……………………………………...82
xxiv
5-7 Resonance energies En (upper panel) and linewidths Γn (lower panel) for the critical
point transitions in single crystal CdTe (broken lines) and in db ~ 1000 Å thick CdTe
films sputter-deposited at different temperatures (points), all measured at 15°C. ……
………………………………………………………………………………………85
5-8 Critical point energies (upper panel) and widths (lower panel) as functions of CdTe
bulk layer thickness during etching by Br2+methanol for co-deposited CdTe films
processed in three different ways: (i) as-deposited, (ii) annealed in Ar for 30 min,
and (iii) CdCl2 treated for 5 min. The deviations at low thickness are due to the onset
of semi-transparency at the E1 critical point energy. ……………………………….86
5-9 Relative void volume fractions as functions of CdTe bulk layer thickness during
etching by Br2+methanol for co-deposited CdTe films processed in three different
ways: (i) as-deposited, (ii) thermally annealed in Ar for 30 min, and (iii)
CdCl2-treated for 5 min.
For the as-deposited and annealed films, the void fraction
is scaled relative to the observed highest density film.
For the CdCl2-treated film,
the void volume fraction is scaled relative to single crystal CdTe. …………………...
.………………………………………………………………………………………88
5-10 Energy of the E1 transition (upper panel) and its width ΓE1 (lower panel) as
functions of CdTe bulk layer thickness in successive Br2+methanol etching steps for
xxv
~3000 Å thick CdTe films. The two films were processed under identical conditions
including fabrication on c-Si wafer substrates and annealing in Ar at 387°C for 30
minutes. The data for experiment #1 are the same as those depicted in Fig. 5.8. …….
.………………………………………………………………………………………90
5-11 Energy of the E1 transition (upper panel) and its width ΓE1 (lower panel) as functions
of CdTe bulk layer thickness in successive Br2-methanol etching steps for ~3000 Å
thick CdTe films.
The two films were processed under similar conditions including
fabrication on c-Si wafer substrates and CdCl2 treatment for 5 minutes.
The data
for experiment #1 are the same as those depicted in Fig. 5.8. .......................................
.………………………………………………………………………………………90
5-12 Void volume fraction as a function of CdTe bulk layer thickness in successive
Br2-methanol etching steps for ~3000 Å thick CdTe films in a second experiment for
comparison with the results in Fig. 5.9.
Two different post-deposition processing
procedures were used: (i) an anneal in Ar for 30 min, and (ii) a CdCl2-treatment for 5
min.
For the Ar annealed films, the void fraction is scaled relative to the depth at
which the highest density is observed.
For the CdCl2-treated film, the void volume
fraction is scaled relative to single crystal CdTe. The void structure for the film
annealed in Ar is attributed to structure in the as-deposited film (as in Fig. 5.8).
In
contrast, the void structure for the CdCl2 treated film is associated with extensive
xxvi
near-surface roughness. …………………………………………………………….92
6-1 Evolution of the surface roughness thickness and a depth profile of the void volume
fraction plotted versus bulk layer thickness obtained in successive Br2+methanol
etching steps that reduce the bulk layer thickness of an as-deposited CdTe
component of a solar cell. …………………………………………………………..99
6-2 (a, left) Evolution of the surface roughness thickness and a depth profile of the void
volume fraction plotted versus bulk layer thickness obtained in successive
Br2+methanol etching steps that reduce the bulk layer thickness of the CdCl2-treated
CdTe component of a solar cell; (b, right) a schematic structure suggested from (a). ..
.……………………………………………………………………………………..100
6-3 (left) Depth profiles of the critical point energies of the E1, E1+∆1 and E2 transitions
in the as-deposited CdTe layer of a solar cell, plotted versus bulk layer thickness
obtained in successive Br2+methanol etching steps that reduce the bulk thickness;
(right) depth profiles of the linewidths of the E1, E1+∆1 and E2 transitions obtained in
the same experiment. ……………………………………………………………...103
6-4 (a, top left) Depth profiles of the critical point energies of the E1, E1+∆1 and E2
transitions in the CdCl2-treated CdTe layer of a solar cell, plotted versus the bulk
xxvii
layer thickness obtained in successive Br2+methanol etching steps that reduce the
bulk thickness; (b, top right) depth profiles of the linewidths of the E1, E1+∆1 and E2
transitions obtained in the same experiment; (c, bottom) a schematic structure
suggested from (b). ………………………………………………………………..104
6-5 Energies of the E1, E1+∆1, and E2 transitions as functions of CdTe bulk layer
thickness in successive etches of a CdCl2 treated CdTe solar cell that reach within
0.1 µm of the CdS/CdTe interface. ………………………………………………..106
6-6 Broadening parameters ΓE1, ΓE1+∆1, and ΓE2 as functions of CdTe bulk layer thickness
in successive etches of a CdCl2 treated CdTe solar cell that reach within 0.1 µm of
the CdS/CdTe interface. …………………………………………………………...106
6-7 Experimental pseudo-dielectric function spectra for the CdTe solar cell of Figs. 6.2
and 6.4 after the 15th etching step; also shown is the best fit using the structural
model of Fig. 6.8. …………………………………………………………………109
6-8 Structural model for the CdTe solar cell after the 15th etch step that provides the best
fit in Fig. 6.7. ……………………………………………………………………...109
6-9 Ex situ SE spectra in (ψ, ∆) (symbols) (a) from the free CdTe surface after 8
xxviii
Br2+methanol etching steps and (b) from the prism/glass side without etching. The
best fit results (solid lines) yield the structural parameters in Figs. 6.10 and 6.11,
including the thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS interface,
and CdS bulk layers, as well as the volume fractions of CdS/CdTe in the interface
layer and void in the CdS bulk layer. ……………………………………………..110
6-10 The best fit results from the free CdTe surface after 8 Br2+methanol etching steps
yielding the thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS interface, and
CdS bulk layers, as well as the volume fractions of CdS/CdTe in the interface layer
and void in the CdS bulk layer. ……………………………………………………111
6-11 The best fit results from the prism/glass side without etching yielding the
thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS interface, and CdS bulk
layers, as well as the volume fractions of CdS/CdTe in the interface layer and void in
the CdS layer. ……………………………………………………………………...111
6-12 CdS and CdTe/CdS interface layer thicknesses deduced from spectra collected
through the prism/glass (solid line) and from spectra collected from the CdTe surface
in successive etches (points, dotted line extrema). ………………………………..113
6-13 Multilayer stack used to model the thicknesses and compositions of the individual
xxix
layers of the CdTe solar cell.
The SE beam enters through the glass, and the
reflection from the top surface is blocked since it is incoherent with respect to the
reflection from the glass/film interface. …………………………………………..114
6-14 Step-by-step MSE reduction by adding one fitting parameter at a time.
Starting
with the CdTe thickness as a variable, each additional parameter was subsequently
fitted.
It was found that fitting the SnO2:F thickness provided the greatest
improvement in MSE among all 2-parameter attempts.
Similar methodology was
used for all 12 parameters. Circular points indicate the best n-parameter fit with n
given at the top and the added parameter given in Table 6.2. …………………….115
6-15 Ellipsometric spectra (points) in ψ (top) and ∆ (bottom) at an angle of incidence of
60° as measured through the glass at a single point on a 3 x 3 cm2 CdTe solar cell
sample. The solar cell was treated with CdCl2 but no back contact processing was
performed.
Also shown is a best fit (lines) using the model structure of Fig. 6.13
with the parameters listed in Table 6.3. …………………………………………...118
7-1 Time evolution of (ψ, ∆) at 5 photon energies selected from 706-point spectra
acquired during sputter deposition of CdTe on a Mo coated glass slide. The full
spectral acquisition time was 2 s and the angle of incidence was 65.68°. ………..122
xxx
7-2 Flow chart of the three-iteration <MSE> minimization procedure for CdTe film
growth on a rough Mo film substrate. …………………………………………….127
7-3 The schematic structure describing the final optical model for deposition on rough
Mo. ………………………………………………………………………………..128
7-4 The schematic structures describing the interface filling (left) and bulk layer growth
(right) models for the first interface layer. ………………………………………...129
7-5 (Left) MSE, which is a measure of the quality of the fit to RTSE data, for the
complete CdTe deposition using optical models for the CdTe film consisting of one
bulk layer (broken line) and four bulk layers (solid line).
In both cases a one-layer
model for surface roughness was employed; (right) the MSE for the model with four
bulk layers is shown on an expanded scale. ………………………………………130
7-6 Evolution of the surface roughness thickness versus deposition time determined using
a four-layer model for CdTe film growth on rough Mo.
The spikes in the surface
roughness thickness result from the consideration of each bulk layer individually
with an independent surface roughness layer.
In this case, the surface roughness
layer on the underlying layer is instantaneously transformed into an interface layer at
the vertical broken lines upon initial growth of the overlying layer, whose roughness
layer starts from zero thickness. …………………………………………………..132
xxxi
7-7 Time evolution of the CdTe overlayer volume percent during interface filling of the
underlying CdTe roughness layer for CdTe growth on Mo. ………………………132
7-8 (Left) Evolution of the individual bulk layer thicknesses versus deposition time
determined using a four-layer model for CdTe film growth on Mo; (right) evolution
of effective thickness of CdTe, including all bulk, interface, and surface layer
components. ……………………………………………………………………….133
7-9 Mo dielectric function at a nominal temperature of 200 °C acquired by inversion
assuming a Mo substrate roughness thickness of 79.6 Å (solid line).
For the
overlying CdTe, four bulk layers and a roughness layer are used to describe the best
fit model.
For the first bulk layer, the Mo/CdTe interface roughness, the CdTe bulk,
and CdTe surface roughness layer thicknesses di, db, ds, respectively, are determined
in a dynamic analysis, in which case the criterion is the minimum average MSE.
The Mo/CdTe interface roughness thickness di is taken to be the same as the Mo
substrate film roughness thickness. Also shown is the Mo dielectric function at room
temperature before heating to the deposition temperature as determined by inversion,
again assuming a Mo surface roughness layer thickness of 79.6 Å (broken line). …...
………………………………………………………………………………………134
7-10 Real (top panel) and imaginary (bottom panel) parts of the dielectric functions of the
xxxii
four layers [(a)-(d)] of a CdTe thin film deposited on rough Mo. These results are
determined from inversion, after determining the CdTe roughness and bulk layer
thicknesses through minimization of the average MSE obtained throughout the layer
analysis; (e) also shown is a comparison of the first layer dielectric function of CdTe
deduced in this study with that of CdTe deposited on a smooth c-Si substrate at a
nominal temperature of 200 °C.
In (b)-(d) comparisons are provided between the
dielectric function of a given layer and that of the layer underneath it. ………………
.……………………………………………………………………………………..137
7-11 Comparison of the surface roughness thickness at the end of the deposition for a
1496.5 Å thick CdTe film on Mo as deduced by RTSE with the relative surface
height distribution and rms roughness from AFM. ……………………………….138
7-12 A comparison of measured pseudo-dielectric functions (solid lines) for Mo thin
films deposited by sputtering (a) on glass and (b) on Kapton. Also shown are the
fits (broken lines) using a reference dielectric function for dense Mo determined
separately, and the multilayer models depicted in the insets. ……………………..140
7-13 Ellipsometric spectra (solid lines) and best fit (broken lines) using the structural
model and best fit parameters shown in the inset.
The dielectric function is
determined simultaneously using a model assuming a sum of critical point structures.
xxxiii
The resulting dielectric function is shown in Fig. 7.14. …………………………..142
7-14 Dielectric function of thin film ZnTe:Cu prepared by magnetron sputtering with 1
wt.% Cu in the ZnTe target (solid lines).
A model consisting of four critical points
in the band structure has been used in this analysis.
The data points are literature
results for single crystal ZnTe. ……………………………………………………144
7-15 Step-by-step MSE reduction by adding one fitting parameter at a time. Starting with
the CdTe thickness as a variable, each additional parameter was subsequently fitted.
It was found that fitting the CdS thickness provided the greatest improvement in
MSE among all 2-parameter attempts. Similar methodology was used for all 14
parameters. Circles connected by the solid line indicate the best n-parameter fit with
n given at the top and the added parameter given in Table 7.5. …………………..147
7-16 Ellipsometric spectra for a CdTe solar cell deposited on Mo in the substrate
configuration (points).
measurement.
The cell was exposed to a CdCl2 treatment before this
The top contact of the solar cell is not incorporated over the area
probed, leading to the structure: ambient/CdS/CdTe/ZnTe:Cu/Mo.
The solid line
depicts the optical model shown in Fig. 7.17. …………………………………….148
7-17 Optical model for a CdTe solar cell in the substrate configuration (excluding the top
xxxiv
contact) deposited on a Mo film surface.
This model and the best fit parameters
provide the solid line results in Fig. 7.16. ………………………………………...151
8-1 Current-voltage and normalized quantum efficiency spectra for a champion 16.5%
efficient CdTe/CdS thin-film solar cell. …………………………………………..152
8-2 Two-terminal tandem cell based on Cd1-xMgxTe and Cd1-xHgxTe absorbers. …………
.……………………………………………………………………………………..154
8-3 Real (a) and imaginary (b) parts of the pseudo-dielectric functions of RF sputtered
CdTe (Eg = 1.50 eV), Cd1-xMnxTe (Eg = 1.63 eV) and Cd1-xMgxTe (Eg = 1.61 eV)
films all in the as-deposited state; (c) Pseudo-dielectric function of as deposited
Cd1-xMnxTe samples after different storage times in laboratory ambient: (1)
immediately after Br2/methanol etch; (2) 3 weeks after deposition; and (3) 1.5 years
after deposition. …………………………………………………………………...158
8-4 Best fit (lines) to the second derivative of the experimental pseudo-dielectric function
(points) for the as-deposited Cd1−xMnxTe film of Fig. 8.3 (c: immediately after etch).
The three CP transitions, E1, E1 + ∆1, and E2, are indicated by arrows with best fit
energies of 3.352, 3.884, and 5.033 eV, respectively.
The composition of x=0.06
can be estimated by the empirical relationship between E1, the strongest CP in this
xxxv
case, and the composition. ………………………………………………………...160
8-5 Variation of the pseudo-dielectric function of as deposited Cd0.94Mn0.06Te with time
after Br2/methanol etching, measured in situ at room temperature during exposure to
laboratory ambient. ………………………………………………………………..161
8-6 Pseudo-dielectric functions of as-deposited and one-step and two-step CdCl2 treated
Cd0.94Mn0.06Te samples. …………………………………………………………...161
8-7 Index of refraction and extinction coefficient of amorphous TeO2. ……………….162
8-8 Pseudo-dielectric functions of as-deposited and CdCl2 treated Cd1-xMgxTe samples. ...
.……………………………………………………………………………………..163
8-9 Approximate dielectric functions, i.e., optical properties deduced with a best attempt
to eliminate surface effects, for as-deposited films and CdCl2-treated films obtained
by SE after Br2+methanol etching that improves the surface quality (points); (a)
CdTe; (b) Cd1-xMnxTe; (c) Cd1-xMgxTe; the solid lines show the results of fits to
extract critical point energies and widths.
The result for the CdCl2-treated
Cd1-xMnxTe could not be fit with a critical point parabolic band model. ……………..
.……………………………………………………………………………………..166
xxxvi
8-10 Pseudo-dielectric function obtained directly from experimental (ψ, ∆) data using a
single interface conversion formula for a Cd1-xMgxTe sample prepared from a target
of CdTe (80 wt.%) + MgTe (20 wt.%) (CGT42).
The solid line describes
experimental data and the dashed line describes the best fit result.
The deduced
bulk and surface roughness layer thicknesses are shown. ………………………...169
8-11 Best fit analytical dielectric function obtained from an analysis of the experimental
(ψ, ∆) data for the Cd1-xMgxTe sample of Fig. 8.10 prepared from a target of CdTe
(80 wt.%) + MgTe (20 wt.%) (CGT42). …………………………………………..169
8-12 Pseudo-dielectric function obtained directly from experimental (ψ, ∆) data using a
single interface conversion formula for a Cd1-xMgxTe sample prepared from a target
of CdTe (60 wt.%) + MgTe (40 wt.%) (CGT92). The solid line describes
experimental data and the dashed line describes the best fit result.
The deduced
bulk and surface roughness layer thicknesses are shown. ………………………...170
8-13 Best fit analytical dielectric function obtained from an analysis of the experimental
(ψ, ∆) data for the Cd1-xMgxTe sample of Fig. 8.12 prepared from a target of CdTe
(60 wt.%) + MgTe (40 wt.%) (CGT92). …………………………………………..171
xxxvii
8-14 Band gap of as-deposited thin film Cd1-xHgxTe as a function of the substrate
temperatures over the range from 23°C to 153°C. ………………………………..174
8-15 Dielectric functions from mathematical inversion and from the corresponding
analytical model fit for as-deposited Cd1-xHgxTe films prepared with different
substrate temperatures. ……………………………………………………………175
8-16 Comparison of the real (left) and imaginary (right) parts of the pseudo-dielectric
function of as-deposited and CdCl2 treated CdxHg1-xTe films, including results (a)
before and (b) after a single Br2/methanol etching step. ………………………….177
xxxviii
Chapter One
Introduction to Spectroscopic Ellipsometry
1.1 History
The very first ellipsometric studies were performed by Professor Paul Drude (1863~
1906), even though the term “ellipsometry” was not used at that time
[1-1]
.
Drude was
the first to derive the equations of ellipsometry, and was also the first to perform
experimental studies on both absorbing and transparent solids. The optical properties
determined in these ellipsometry studies were found to be quite accurate. In fact, when
Palik compared Drude’s results with those obtained 100 years later, the results were
amazingly close [1-2]. Because of the absence of fast computation methods made possible
by the modern computer, Drude obtained the optical properties of solids at only a few
selected wavelengths [1-1].
After Paul Drude’s tremendous impact on ellipsometry development, very little
progress was reported in the succeeding 70 years. One exception was a 1945 article
authored by Alexandre Rothen who described the half-shade method to detect the
polarization state change of light upon reflection from a specular surface, and coined the
term “ellipsometry”[1-3].
When laboratory computers became prevalent in the 1960s and
1
1970s, automated ellipsometers for diverse purposes were developed
[1-4]
. Among the
different types of automated ellipsometers developed at that time, two major types are
still widely used in the spectroscopic mode of operation: (i) the rotating element
ellipsometer
[1-5],
and (ii) the phase modulation (PM) ellipsometer
[1-6]
.
The photon
energy range of spectroscopic ellipsometry has increased significantly over the years
since D. E. Aspnes and A. A. Studna developed the first rotating analyzer spectroscopic
ellipsometer covering the full (near-infrared)-to-(near-ultraviolet) range
[1-7]
.
At the
same time, the instrument development focus was also placed on increasing the speed of
full spectroscopic measurement by incorporating a multichannel detection system in the
ellipsometer in order to acquire the entire spectral range essentially simultaneously
[1-8]
.
As a result of this effort, the technique of real time spectroscopic ellipsometry (RTSE)
arose for analysis of thin film growth and materials processing.
1.2 Purpose
Spectroscopic ellipsometry is used to obtain the optical properties of materials of
interest in optical and electronic applications
[1-9]
. Once optical properties of materials
are available, thin film thicknesses can be measured using optical models for single thin
film and multilayer samples.
Advanced data analysis often enables measurement of
thickness and optical properties simultaneously
[1-10, 1-11]
.
The measurable thickness
range for ellipsometry varies from submonolayer to several microns.
For spectroscopic
ellipsometry measurements of thickness, a wide spectral range is important since the light
must penetrate through the thin film, reflect from an underlying interface, return through
2
the film, and proceed to the detector.
In studies of semiconductors, lower energy gap
materials such as CuInSe2 can be analyzed for thickness when the spectral range extends
deeper into the infrared.
For energies below the semiconductor band gap, the light
remains unabsorbed and reflects from the bottom interface of the film, enabling wave
superposition and phase shifts that allow thickness to be determined.
This demonstrates
the advantage of spectroscopic ellipsometers with an extended near-IR spectral range,
even below the 1.1 eV band gap of the most common Si diode detectors used in
ellipsometers.
A similar advantage exists for spectroscopic ellipsometers with an
extended ultraviolet spectral range when characterizing the thickness of metal thin films.
In addition to thickness, other properties of a film can be determined through
ellipsometric measurements performed in real time during the deposition process
[1-12]
.
These include roughness thickness on the surface of the film and the optical properties of
the film.
From the latter, the film density deficit (represented by a volume fraction of
voids in the layer), film crystalline quality (represented by a defect density or average
grain size), alloy composition, and temperature may be determined.
In fact, real time
measurements may also provide a depth profile of the film structure and properties, and
even area uniformity of the film.
1.3 Data measured by ellipsometry
An ellipsometric measurement provides the angles (ψ, ∆), corresponding to the
relative amplitude ratio (tanψ) and phase shift difference (∆) between the complex
3
r
r
amplitude reflection coefficients for E p and Es , the orthogonal linear electric field
components of a polarized light wave [1-13].
These electric field components are parallel
r
r
( E p ) and perpendicular ( Es ) to the plane of incidence.
(The overline arrow denotes a
complex vector in which case each vector component has a real amplitude and phase.)
r
r
The nature of E p and Es for a light wave will be further elucidated in the next section.
Thus, the quantity measured by ellipsometry is the ratio ρ% of the complex amplitude
reflection coefficients for the p-polarized field component ( R% p ) to that for the s-polarized
field component ( R% s ):
ρ% =
R% p
= tanψ ei∆ ;
%
R
(1-1)
s
where
E% p ref
R% p = inc = R% p exp ( iδ p ) ,
E%
(1-2)
E% ref
R% s = s inc = R% s exp ( iδ s ) .
E% s
(1-3)
p
Here, the notational style of these equations will be summarized.
Generally, the
subscripts p and s identify the wave characteristics for vector components parallel and
perpendicular to the plane of incidence, respectively.
For example, δp and δs represent
the phase shifts of each orthogonal electric field component upon reflection.
On the
other hand E% p ( s ) denotes the p (s) orthogonal component of the electric field amplitude.
The superscripts “ref” and “inc” in Eqs. (1-2) and (1-3) refer to the electric field
components of the reflected and incident light waves.
4
As a result, the angles ψ and ∆ are defined by:
tanψ =
R% p
,
R%
(1-4)
s
∆ = δ p − δs .
(1-5)
R% p ( s ) are also called the complex Fresnel coefficients.
As a complex variable, R% p ( s )
provides information on the amplitude change and phase shift of the p (s) field
components of the wave upon its reflection from the sample.
In fact, the complex
Fresnel coefficients provide the reflected-to-incident amplitude ratio and the
r
reflected-minus-incident phase shift for each orthogonal electric field component E p (or
r
Es ) of the polarized light wave.
1.4 Mathematical derivation
In order to understand the derivation of optical properties from the ellipsometric
angles (ψ, ∆), it is necessary to understand first the mathematics of polarized light.
When the most general state of elliptically polarized light wave transmits through or
reflects from one or more interfaces between media at a non-normal angle of incidence,
the polarization change can be defined in terms of a change in tilt angle and ellipticity
angle of the general polarization ellipse. This change depends on the angle of incidence
and the optical properties and thicknesses of the media.
The elliptically polarized state
of monochromatic light in any medium assumed to be isotropic can be described by
r
decomposing the beam into two orthogonal components which are linear and parallel ( E p )
5
r
as well as linear and perpendicular ( Es ) to the plane of incidence.
Both components are
plane waves and a superposition of such components is described by [1-14]:
r r
r
r r
E ( r , t ) = E0 exp [i (q ⋅ r − ω t ) ] ;
where
r
q
(1-6)
is the complex propagation vector,
ω
is the wave frequency,
r
and E0 determines the polarization state of the wave. In this linear p-s basis,
r
r
r
iγ
E0 = E p + Es = E p e p pˆ + Es eiγ s sˆ .
(1-7)
r
For this general polarization state of the light wave, the endpoint of the vector E0
r
traces an ellipse as a function of time t during propagation at a fixed position r0 .
complete cycle is made in a time τ =
phase velocity of v =
ω
Re(q% )
2π
ω
A
. The plane wave also travels in space with a
, and the endpoint of the field vector traverses one full
ellipse after a distance equal to the wavelength λ =
2π
.
Re(q% )
r
%ˆ.
complex magnitude of the propagation vector: q = qq
6
Here is q% defined as the
s
b
Q
r r
E (r0 , t )
χ a
p
r r
r = r0
r
Figure 1-1 Schematic representation of the electric field vector trajectory E (rr0 , t ) for an
r
elliptically polarized light wave at a fixed position r0 versus time. Q is the tilt angle
between the ellipse major axis a and the p-axis, measured in counterclockwise-positive
sense when facing the light source. χ is the ellipticity angle given by tan-1(b/a).
r
In Equation 1-6, the wavevector q defines the propagation direction.
If one
r
assumes q is parallel to the z-axis, the wave becomes:
r r
% − ωt ) ] ;
E ( r , t ) = E0 exp [i ( qz
(1-8)
where
ω
q% =
c
2
2
2
4πσ r ω % 2
ε r + i ω = c N ,
(1-9)
or
ω
q% = N% .
c
Here c is the speed of light in vacuum. At the light wave frequency ω, εr and σr denote
the real dielectric function and real optical conductivity of the medium in which the wave
7
travels, and N% is its complex index of refraction, where [1-15]
4πσ r
N% = n + ik = ε r + i
ω
.
(1-10)
Here n is the (real) index of refraction, and k is the extinction coefficient of the medium.
It should be noted that Re(q% ) =
wavelength is λ =
ω
c
n , so the phase velocity of the wave is v =
c
and the
n
2π c 2π
=
v as expected. Next q% and N% are substituted into
nω
ω
Equation 1-8 to give
r r
r
ω nz
ω kz
E (r , t ) = E0 exp −
− ωt .
exp i
c
c
(1-11)
In addition to the complex index of refraction N% , the complex dielectric function ε%
is another commonly used quantity to describe the macroscopic optical properties of
solids [1-15], where:
ε% = ε1 + iε 2 = N% 2 ,
(1-12)
ε1 = ε r = n 2 − k 2 ,
(1-13)
4πσ r
(1-14)
ε2 =
ω
= 2nk .
Ellipsometry measures the change in polarization state of the incident light caused by
reflection from one or more interfaces. When an incident linearly polarized light wave
reflects from a single interface between two media (see Fig. 1.2), the state of polarization
of the reflected beam can assume an elliptical state with the tilt and ellipticity angles
depending on the optical properties of the sample.
8
reflected wave
incident wave
p
p
.
s
Medium 0
Medium 1
.
s
θi
p
θi
s
θ% t
Plane of sample
.
Plane of incidence
p
s
transmitted wave
Figure 1-2
Reflection of a polarized light wave at an interface between two media.
For the ideal situation of a perfectly planar interface on the atomic scale with no
roughness, the optical properties of the reflecting medium can be derived from the
ellipsometric angles (ψ, ∆) as long as the optical properties of the incident medium and
the angle of incidence are known [1-13]. In the simplest case of reflection and transmission
at the perfectly planar interface between two isotropic media (see Fig. 1.2), the ratio of
the complex Fresnel reflection coefficients can be written:
N% s cos θi − na cos θ%t
R% p na cos θ%t + N% s cos θi
ρ% =
=
R% s na cos θi − N% s cos θ%t
%
%
na cos θi + N s cos θt
,
(1-15)
where na is the assumed real refractive index of Medium 0 (ambient, see Fig. 1.2), N% s is
the complex index of refraction of Medium 1 (substrate, see Fig. 1.2), θi is the angle of
incidence and θ%t is the complex angle of refraction. cos θ%t can be obtained from sin θ i ,
9
na , and N% s by using a complex form of Snell’s Law:
cos θ%t = ±
N% s 2 − na 2 sin 2 θi
.
N%
(1-16)
s
Then, eliminating cos θt from Equation 1-15 yields:
(
(
N% s 2 cos θi m na N% s 2 − na 2 sin 2 θi
R% p
ρ% =
=
R% s
N% s 2 cos θi ± na N% s 2 − na 2 sin 2 θi
ρ% =
)( n cosθ ±
)( n cosθ m
) , (1-17)
sin θ )
a
i
N% s 2 − na 2 sin 2 θi
a
i
N% s 2 − na 2
2
i
na sin 2 θi m cos θi N% s 2 − na 2 sin 2 θi
,
n sin 2 θ ± cos θ N% 2 − n 2 sin 2 θ
a
i
i
s
a
(1-18)
i
and solving for N% s 2 yields:
1 − ρ% 2
2
2
2
2
%
N s = na sin θi 1 +
tan
θ
i.
1 + ρ%
(1-19)
As a result, by using the dielectric function definition in Equation 1-12, ε%s can be
obtained from
1 − ρ% 2
ε%s = ε a sin θi 1 +
tan 2 θi .
1 + ρ%
2
(1-21)
Therefore, if one knows (i) ε a the dielectric function of the ambient; (ii) θi the angle
of incidence, and (iii) (ψ, ∆) the measured ellipsometric angles, then one can determine
the dielectric function of the reflecting medium.
1.5 Spectroscopic ellipsometer used in the study
The spectroscopic ellipsometer used for the study described in this thesis was
manufactured by J. A. Woollam Company
[1-16]
. The specific model used here was the
M-2000DI, which is a rotating-compensator multichannel ellipsometer.
10
This
ellipsometer covers the photon energy range from 0.74 to 6.50 eV. One complete set of
spectra in the ellipsometric angles (ψ, ∆) (0.74~6.5 eV) can be collected as an average
over a minimum of two optical cycles in a time of (30.7 Hz)-1 = 32 ms; thus, the single
optical period is 16 ms. Here 30.7 Hz is the mechanical rotation frequency of the
compensator. In the case of real time SE applications, specifically for monitoring the
CdTe or CdS deposition process, acquisition times from 1 to 3 seconds were chosen. In
the case of the ex-situ SE applications, the data acquisition time of 10 seconds was
chosen to ensure a higher precision in the measured (ψ, ∆) spectra. As a result of the
multichannel detection capability, this spectroscopic ellipsometer is ideal for in-situ
process monitoring and quality control, and specifically for studies of the CdTe-based
solar cells as described in this thesis.
Figure 1-3 Spectroscopic ellipsometer used in this research mounted in the ex-situ
mode of operation.
The angle of incidence is adjustable for this ellipsometer.
For ex-situ studies, the
ellipsometer is set at angles of incidence ranging from 45° to 75° at 5° intervals.
11
Measurements at different angles of incidence enable one to extract optical properties of
unknown materials with greater confidence. Analyses of all spectra apply either
numerical inversion or least-squares regression algorithms, or even combinations of these
two methods.
1.6 Data analysis
As described in Section 1.4, the ellipsometry measurement provides two angles
(ψ, ∆), which quantify the change in the state of polarization of the light wave upon
oblique reflection from the sample. Ellipsometry does not directly measure the optical
properties and thickness of a thin film; however, ψ and ∆ are functions of these
characteristics, which require data analysis for extraction [1-17]. The starting point for such
analysis is an optical model for the sample.
A general schematic of the analysis
procedure is illustrated in Fig. 1.4.
The first step in building an optical model for the sample requires identifying the
physical sequence of layers of the sample, including each layer’s thickness and optical
properties, the latter either as fixed functions, analytically defined functions with variable
parameters, or even continuously variable functions point by point.
For each such
thickness and optical property variable, it is necessary to provide an estimated value to
begin the iteration. As an example, an optical model for a simple silicon substrate
sample is shown in Fig. 1.5.
In general, building an optical model begins with the
simplest structure; however, complexities such as surface and interface roughness layers
12
can be added as required in order to improve the fit to the data and to conform with any
previously established understanding of the nature of the sample.
Measurement
(ψ, ∆) versus E
(ψ, ∆) versus θi
Construct optical
model
Assign initial
values to variables
Fit, compare
data and model
results
Results:
n, k versus E
thicknesses
Figure 1-4
Simplified flow chart of the data analysis procedure.
13
n, k, (surface roughness)
ds
n, k, (film)
db
n, k, (interface)
di
n, k, (substrate)
Figure 1-5
SiO2/void
ds
SiO2
db
SiO2/c-Si
c-Si
di
Optical model and physical structure of a c-Si wafer used as a substrate.
Calculated (ψ, ∆) spectra are first generated using the optical model with the initial
values assigned to the unknown parameters. Then these spectra are compared with the
experimental (ψ, ∆) spectra and iterative adjustments of the unknown parameters are
performed in a regression analysis intended to minimize the difference between the two
pairs of spectra. If the initial values of the unknown parameters differ substantially from
the overall best fit solution, however, then the regression algorithm may fail. The role
of this algorithm is to compute the corrections to the initial estimates that yield improved
agreement between the calculated and experimental spectra and ultimately the overall
best fit.
What can occur instead is the identification of a local minimum in the quality
of fit when plotted in the space of the unknown free parameters, and as a result the
calculated spectra may differ substantially from the experimental spectra. In contrast, if
the initial estimates are close enough to the overall best fit solution, then through iterative
corrections, the algorithm can identify the global minimum in the quality of the fit, and as
14
a result improved agreement between the calculated and experimental data is possible.
For this reason, the flow chart in Fig. 1.4 shows an iteration step not only in the
construction of the model but also in the variation of the initial values typically over a
grid in parameter space.
In addition to the simplest case of thicknesses as unknown parameters, the
least-squares regression method is commonly used to extract the complex dielectric
function of one or more materials in the model
[1-17]
.
When an unknown complex
dielectric function can be expressed as an analytically-defined function of several
wavelength-independent parameters such as electronic resonance energies (band gaps),
resonance amplitudes (oscillator strengths), and broadening parameters (inverse
excitation lifetimes), then the fitting procedure is similar to that of fitting simply
thicknesses. All the known values of the parameters are fixed in the model, and the
wavelength independent unknown parameters are estimated, including the thicknesses
and the optical property parameters. The (ψ, ∆) spectra associated with these initial
estimates are calculated and compared with the experimental (ψ, ∆) spectra. Then, the
least-squares regression algorithm is used to adjust the unknown parameters iteratively so
as to minimize the difference between the calculated and experimental ellipsometric
spectra. A mean square error (MSE) function is used as the criterion; the iterations are
terminated when MSE attains its minimum.
If the initial estimates of the unknown
parameters are close enough to the correct values, then the global minimum can be
reached; if not, a local minimum can lead to erroneous parameter results.
15
In such analyses, the least-squares regression algorithm uses the weighted mean
square deviation given by [1-17]:
1
MSE =
2N − M
ψ cal −ψ exp
i exp i
∑
σ
i =1
ψ
N
2
2
∆ ical − ∆ iexp
+
σ exp
∆
(1-22)
where N is the number of (ψ, ∆) data pairs versus wavelength or photon energy, and M is
the number of unknown free parameters determined in the analysis.
Thus, the squares
of the differences between each pair of calculated and experimental data (ψ cal ,ψ exp ) and
( ∆ cal , ∆ exp ) at a given wavelength or photon energy indicated by the subscript i are
summed and divided by the standard deviations of the experimental data σψexp and σ ∆exp ,
respectively, for the associated wavelength.
As a result, spectral points that exhibit a
lower signal to noise ratio, typically at the highest photon energies (6.0~6.5 eV) are
weighted less heavily.
Once the fit for a given model is successful, a number of various models need to be
tested in order to improve the global fit to the data.
These models generally start with
the simplest structure, e.g., a single film on a substrate, and then progress to more
complicated ones that include surface and interface roughness.
Some complications
may be expected based on an understanding of how thin films grow.
unexpected and provide new insights.
Others may be
In particular for complicated models with many
parameters, the overall best fit parameters must be evaluated for their confidence limits
and possible pair-wise or multiple correlations.
In addition, the best fit parameters must
be physically meaningful; obviously there should be no zero or negative thickness values.
16
For an intrinsic semiconductor, the index of refraction n must decrease smoothly with
increasing λ at wavelengths longer than that associated with the band gap.
In this range,
k should remain at zero, because of the lack of absorption at photon energies below the
band gap.
Obviously, k cannot be negative; otherwise, the light would be amplified in
traversing the material.
17
Chapter Two
Introduction to CdTe-based Solar Cells
2.1 CdTe-based solar cell structures
CdTe-based solar cells can be fabricated in both substrate and superstrate
configurations [2-1, 2-2, 2-3].
In the substrate configuration, sunlight enters the active layers
of the cell before reaching the underlying substrate, and thus the substrate need not be
transparent.
A typical substrate-type deposition process would follow the sequence,
Mo/CdTe/CdS/In2O3:Sn.
Indium-tin-oxide (ITO), denoted by the chemical formula
In2O3:Sn whereby Sn is the dopant, is a transparent conducting oxide (TCO) thin film
that functions as an electrical contact as well as a window layer through which sunlight is
transmitted
[2-1]
.
In the superstrate configuration which is the configuration used by
industry, the sunlight enters the substrate first, and the substrate must be selected for low
absorption over the solar spectrum.
Typically there will be a trade-off between low
absorption and low cost in module manufacturing.
A typical deposition sequence in this
case is glass/SnO2:F/CdS/CdTe/Cu/Au. A common superstrate for the CdTe solar cell is
TEC glass manufactured by Pilkington.
TEC glass is a soda-lime glass coated with
successive layers of undoped SnO2, SiO2, and F-doped SnO2, SnO2:F, to achieve the
18
desired sheet resistance, optical properties, and chemical stability.
Another TCO used in
place of In2O3:Sn and SnO2:F in both substrate and superstrate solar cells is ZnO:Al,
aluminum-doped zinc oxide.
Schematic examples of the substrate and superstrate
configurations are shown in Figs. 2-1 and 2-2.
Ambient
front contact
ITO
CdS
CdTe
back contact
Figure 2-1
Mo
The substrate structure for CdTe solar cells.
Ambient
Cu/Au back
contact
CdTe
CdS
front
contact
SnO2:F
SiO2
TEC-15
SnO2
Soda lime glass
Figure 2-2
The superstrate structure for CdTe solar cells.
In this thesis, results for both substrate and superstrate solar cells will be presented;
however, the focus has been on solar cells using TEC-15 glass as the superstrate. This
glass has been used in module manufacturing and will be described in detail in Chapter 3.
The TEC-15 glass is ~ 3 mm thick and serves as a support for the active layers of the
solar cell.
It is transparent, rigid, and inexpensive, and has the widest applications for
ground mounted PV systems. The critical component is the TCO layer, SnO2:F, which
19
is the top-most layer of the TEC-15 glass and acts as the front contact electrode of the
solar cell.
The polycrystalline cadmium sulfide (CdS) layer is invariably an n-type
semiconductor, and serves as one side of the p-n heterojunction solar cell
[2-1]
. As a
material with a wide band gap of 2.43 eV at room temperature, CdS is transparent to
optical wavelengths as short as 510 nm.
Because its thickness is relatively small
compared to that of CdTe, typically ~1000 Å, some fraction of the photons with energy
above 2.43 eV will still pass through the CdS layer to reach the CdTe layer.
The polycrystalline cadmium telluride (CdTe) is the active absorber layer and serves
as the p-type semiconductor of the heterojunction.
It is an ideal absorber material
because its 1.5 eV band gap is very close to the theoretically calculated optimum value
for a single junction solar cell
[2-4]
under unconcentrated AM1.5 sunlight.
It is an
efficient absorber above its band gap, and its high absorption coefficient results from the
direct nature of the band gap transition. Typically, the thickness of the CdTe layer in the
solar cell ranges from 2 to 4 µm in order to absorb a larger fraction of the light between
633 nm and 832 nm.
CdS layer.
The p-n junction consists of the CdTe layer in contact with the
Because the doping level in CdTe is much lower than that in the CdS, most
of the depletion region of the device is located within the CdTe layer.
The back contact studied in this dissertation uses copper (~ 30 Å) and gold (~ 200 Å)
in forming the electrode. Due to its high conductivity, a large thickness is not needed for
the gold layer.
20
2.2 Deposition method and process steps
The deposition method pioneered at the University of Toledo utilizes the radio
frequency (RF) magnetron sputtering technique for fabrication of both the CdTe and CdS
thin films [2-5].
The CdTe or CdS sputtering target, serving as one electrode, is driven by
a RF power source.
This power source generates a plasma of ionized argon gas between
the target and the substrate platform, which serves as the second grounded electrode.
The RF potential drives the ions towards the surface of the target where they impact,
causing atoms to be dislodged from the target.
surface where they are deposited.
These atoms travel to the substrate
A magnetic field is applied to contain the plasma ions
near the surface of the target in order to increase the sputtering rate. The ions follow
helical paths around the magnetic field lines, an effect that enhances the ion density in the
plasma near the target.
This also allows the plasma to be sustained at lower pressures.
The sputtered species are predominantly neutral atoms and are not affected by the
magnetic trap.
Solar cell fabrication in the superstrate configuration begins with the deposition of a
CdS thin film with a thickness of approximately 1300 Å on a SnO2:F coated soda lime
glass substrate
[2-6]
. Typical sputtering parameters for CdS include 50 Watts RF target
power and 10 mTorr Ar gas pressure. During deposition, the substrates are held at a
nominal temperature of 300°C. After CdS deposition, a CdTe film with a standard
thickness of approximately 2.4 µm is deposited on the CdS surface using similar
deposition conditions as for CdS.
For selected depositions of this study, a nominal
21
substrate temperature of 200°C has been used for the CdTe.
After the two
semiconductor depositions, a CdCl2 post-deposition treatment is generally applied to the
substrate/CdS/CdTe stack
[2-7]
.
This treatment consists of exposure to an atmosphere of
vaporized CdCl2 with partial pressure at 3.6 mTorr, and for a standard thickness of CdTe,
is carried out for 30 minutes in a tube furnace set at 387 °C.
The CdCl2 post-deposition
treatment is applied for different durations to cells with different CdTe layer thicknesses,
while setting the same treatment temperature [2-8].
For example, for a 1 µm thick CdTe
layer the treatment time is ~ 15 min.
The solar cell is finished with an evaporated Cu-Au back contact. Cu is deposited to
30 Å thickness and Au to 200 Å thickness
[2-9]
.
The final step requires annealing the
solar cell for 45 minutes in air at 150 °C in order to diffuse the Cu into the CdTe.
In this
step, the CdTe layer near the back contact becomes more heavily p-type doped [2-9].
For
the thinner CdTe cells, the annealing time for Cu diffusion must be reduced in order to
achieve the proper dopant distribution in the CdTe layer.
2.3 Application of spectroscopic ellipsometry as an analysis technique
In the development of real time spectroscopic ellipsometry (RTSE) as a probe for
CdTe-based solar cell characterization, a step-by-step research program is being
undertaken in order to separate out the various complexities that occur in the deposition
process and in the CdCl2 post-deposition treatment process. For the first real time
analyses of CdTe and CdS deposition processes, optical properties of the deposited layers
22
must be established simultaneously with the structural parameters such as bulk layer
thickness, void volume fraction profile, and surface roughness thickness.
Such initial
analyses are typically done using ultra-smooth crystalline silicon substrates so that the
extracted optical properties are as accurate as possible
[2-10]
.
Substrates with rough
surfaces require incorporation of an interface roughness layer into the film growth model
that adds greater uncertainty to the overall analysis.
Parameterization of the deposited layer optical properties in terms of useful
characteristics such as defect density or grain size, strain, and temperature then yields an
optical property database that enables real time analysis of subsequent, more complex
deposition processes and substrate structures
[2-11]
.
In basic research studies on the
deposition process, details of the solar cell structure during its deposition beyond simple
thickness can be determined such as CdTe and CdS nucleation and coalescence
characteristics, surface roughness evolution versus thickness, void volume fraction depth
profile, deposition temperature, film stress, defect density or grain size, and CdTe and
CdS interface layer compositional depth profile at the interface between the materials.
These features can be used not only for basic research but also for process development
and troubleshooting.
In addition, the database can be applied to the ex-situ analysis of
post-deposition treatments using a bromine-methanol step-wise etching process for depth
profiling.
Finally these optical property databases established under ideal conditions of
growth on atomically smooth and well-characterized substrates such as c-Si wafers can
be applied on-line for production monitoring of the solar modules or for off-line mapping
23
of completed modules.
Data obtained on the production line could potentially be
applied to monitor and control layer thicknesses, for example, in the production process.
In addition to the deposition parameters of the CdTe film, the CdCl2 post-deposition
treatment of the CdTe solar cell significantly influences the CdTe film structure and
optical properties
[2-12]
.
The final goal of spectroscopic ellipsometry analysis is to
understand the effects of the key parameters of the CdCl2 post-deposition treatment
process, temperature and time, on the optical properties and structure of the CdS/CdTe
and relate these to the solar cell performance.
Because of the complexity of the final
film structures to be treated, the Br2+methanol stepwise etching in conjunction with
ex-situ spectroscopic ellipsometry is a unique capability for characterizing CdTe film
depth profiles, such as void fraction and grain structure.
Thus, this analysis procedure
makes it possible to perform time-reversed real time spectroscopic ellipsometry while
maintaining a smooth surface as the layers of the structure are etched away.
Such an
approach in which numerous spectra are collected as a function of thickness during
etching provides sufficient information to determine depth profiles of the film properties
with confidence.
In Chapter 3 through Chapter 5, this thesis will focus on optical property database
development.
In order to determine the structural parameters of CdTe-based solar cells,
the required database of optical properties must include not only CdTe and CdS but also
any substrate components.
Chapter 3 will describe how the four sets of optical
properties for the materials of the TEC-15 glass substrate are extracted.
24
These materials
include the soda lime glass, undoped SnO2, SiO2, and doped SnO2:F.
In Chapter 4,
validity of stepwise etching for time reversed real time spectroscopic ellipsometry will be
demonstrated for the purpose of depth profiling CdTe thin films.
Chapter 5 will
describe the determination of the optical properties of CdTe and CdS films before and
after the CdCl2 post-deposition treatment process.
These results will be applied in
Chapter 6 along with an optical model to deduce the structure for superstrate solar cells.
Results in Chapter 7 focus on the development of a database and analysis of the complete
solar cell for the substrate configuration.
In Chapter 8, the application of spectroscopic
ellipsometry will be described for the characterization of CdTe-based ternary alloys.
25
Chapter Three
Optical Properties of TEC-15 Glass
3.1 Introduction
The transparent electrically conducting (TEC) glass manufactured at low cost by
Pilkington North America is a durable, pyrolytically coated, soda lime glass.
The
coating is available in various thicknesses on 3 mm thick soda lime glass which yields an
electrical sheet resistance from 6 to 8 Ohms/square (Ω/
□ for TEC-1000.
approximately 5000 Ω/
□)
for TEC-7, up to
□ value provides the resistance in
The Ω/
Ohms when current passes from one side of a square region of the coating surface or
interface to the opposite side, irrespective of the area of the square.
Because of the
coating durability, TEC glass plates can be handled just like ordinary uncoated plates.
TEC glass can be used in many thermal and electrical applications including frost-free
refrigerator windows, defogging mirrors, touch screen displays, static-free windows,
liquid crystal displays, and superstrates and substrates for thin film photovoltaics.
Among the group of TEC glass products, TEC-15, TEC-7, and TEC-8 are the
transparent conducting oxide (TCO) coated products used most extensively in the
26
fabrication of CdTe thin film solar cells in the superstrate configuration. As a widely
used thin film transparent conductor, fluorine doped tin-oxide (SnO2:F) is the most
important layer of the three-layer TEC glass stack.
This layer forms the top contact, and
thus serves to conduct the current generated in the semiconductor layers to the external
circuit.
SnO2:F is relatively easy to deposit pyrolytically onto a heated substrate and is
quite stable chemically.
TEC-15 has the appearance of uncoated glass due to the color
suppression characteristics of the three layer stack.
Before multilayer optical analysis can be applied to spectroscopic ellipsometry data
collected on thin film CdTe-based superstrate solar cells, a library of dielectric functions
ε = ε1 + iε2 is needed that includes all the component layers.
In this chapter, an ex-situ
spectroscopic ellipsometry investigation is described that provides the four sets of optical
properties for the material components of TEC-15 glass.
From bottom to top, these
materials include the soda lime glass substrate, undoped SnO2, SiO2, and SnO2:F.
In
determining the optical properties of these materials, optical models were used to analyze
the measured ellipsometry and transmittance spectra.
Because all the TEC glasses
exhibit the same multilayer structure, the optical properties deduced for each layer of the
TEC-15 glass are assumed to be applicable for the corresponding layers of the TEC-7 and
TEC-8 glasses in order to determine their thicknesses.
For the TEC-7 and TEC-8
glasses, comparisons of the experimental and calculated transmission spectra, the latter
based on an ellipsometric analysis, will be presented in this Chapter as well. These
comparisons provide information on light scattering, so-called haze, and macroscopic
27
roughness.
A schematic of the multilayer structure of TEC-15 glass is shown in Fig.
3-1.
Surface roughness
SnO2:F
SiO2
SnO2
Soda lime glass
Figure 3-1
3.2
The multilayer structure of the TEC-15 glass substrate.
Experimental details
The primary effort was focused on TEC-15 glass which exhibits the thinnest SnO2:F
layer, and thus the smoothest surface among the three glasses explored. As a result, the
measured data are least affected by scattering due to macroscopic roughness.
In
addition to ex-situ ellipsometric spectra, normal incidence transmittance spectra were
collected for the TEC-15 components.
The purpose of the latter spectra was to seek
higher accuracy extinction coefficient values for the components of the TEC-15 glass.
The ellipsometry measurement is sensitive to small changes in the polarization state of
the light, which in turn is measurably affected by even a monolayer change in the
thickness of a thin film.
Because the ellipsometer measures the change in p-s ratio of
the electric fields of the light wave upon reflection from the sample surface, it is not very
sensitive to the extinction coefficient k, when the k-value is small (≤ 0.1).
As a result, a
transmission measurement, which can provide more accurate values of k when its
28
magnitude is low, is used to supplement the ellipsometry measurement.
The two data
sets are analyzed together using the same optical model.
TEC glass samples were provided by Pilkington North America; five samples were
prepared for this study.
These samples include (i) uncoated soda lime glass, (ii)
SnO2/(soda lime glass), (iii) SiO2/(soda lime glass), (iv) SiO2/SnO2/(soda lime glass), and
(v) fully coated TEC-15 glass: SnO2:F/SiO2/SnO2/(soda lime glass).
separate samples of TEC-7 and TEC-8 glass were provided.
In addition,
Variable angle
spectroscopic ellipsometry and normal incidence transmittance measurements were
applied to study all samples.
Each sample was cut into two pieces.
For the first piece, the backside of the sample
was roughened to remove the beam returning from the back side glass/air interface.
This beam will distort the ellipsometry spectra in ways that are difficult to model due to
the incoherence of this beam relative to the top side reflection.
The ellipsometry data
were acquired at angles of incidence of 45°, 60° and 75°, and each acquisition time at a
given angle was 10 seconds.
The second sample piece was kept “as-is” and measured
using normal incidence transmittance.
The spectral range of all ellipsometry and
transmission measurements was 0.75~6.5 eV.
3.3
(i)
Data analysis and results
Uncoated soda lime glass
The deduced substrate structure, which includes the bulk glass and a thin surface
29
roughness or modulation layer, is shown in Fig. 3-2. The best fit value for the thickness
of this layer (13 Å) is also given in Fig. 3-2.
The transmittance spectrum measured on
the soda lime glass was first analyzed in order to determine its extinction coefficient k.
By fixing the index of refraction spectra of the soda lime glass using results from a
reference database as a first estimate
[3-1]
, numerical inversion could be applied to the
transmittance spectra in order to deduce the extinction coefficient k.
Then, the index of
refraction spectrum could be extracted by fitting ellipsometry spectra (ψ, ∆) as measured
on the back-surface roughened glass in a procedure that also provides the surface
modulation layer thickness.
In this fitting procedure, the spectrum in k was fixed as that
obtained from transmittance analysis.
This process was iterated by repeating the
inversion of the transmittance spectra using the index of refraction and sample structure
deduced from the ellipsometry spectra.
As a check of the final results, an analysis of
both the transmittance and ellipsometric spectra was also applied in order to deduce n and
k simultaneously using a 100% weighting level of transmittance relative to ψ and ∆.
Once the optical properties have been determined in this analysis procedure, they have
been fitted by smooth analytical functions.
The results are tabulated in Appendix A.
Experimental spectra for the transmittance, ψ and ∆ (broken lines), and their best fit
simulations (solid lines) using the final structure and optical properties are shown in Figs.
3-3, 3-4, and 3-5.
30
surface roughness
soda lime glass
13.1 ± 0.1 Å
3 mm
MSE = 1.85
Figure 3-2 Simple model deduced from the analysis of the transmittance and
ellipsometric (ψ, ∆) spectra of Figs. 3-3−3-5 for the soda lime glass substrate. The
surface roughness is obtained in a best fit of the (ψ, ∆) spectra.
1.0
Soda Lime Glass substrate
simulation
exp.
Transmittance
0.8
θi =0
o
0.6
0.4
0.2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-3 Best fit simulated and experimental normal incidence transmittance spectra
T vs. photon energy for an uncoated soda lime glass substrate used in the fabrication of
TEC glasses.
Considering Fig. 3-5, the ellipsometric spectra ∆ should be 0° throughout the spectral
range when the surface layer thickness is negligible and absorption is weak as is the case
for soda lime glass. The 13 Å surface roughness or modulation layer is justified by the
observation of a non-zero ∆ spectrum. This layer may also include contributions due to
surface contamination and/or differences in the chemical nature of the glass near the
surface.
As a result, the relatively poor quality of the fit to ∆ is likely to be due to
inadequacies in the simple Bruggeman effective medium theory model for the optical
31
properties of the thin modulation layer.
The analytical expressions that best fit the index
of refraction and extinction coefficient spectra for the uncoated soda lime glass are shown
in Fig. 3-6.
simulation
exp.
6
θi = 60
o
ψ (degree)
5
4
3
2
soda lime glass substrate
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-4 Best fit simulated and experimental ellipsometric angle ψ = tan−1 (|rp/rs|) vs.
photon energy for an uncoated soda lime glass substrate used in the fabrication of TEC
glasses. The angle of incidence is 60˚.
soda lime glass substrate
∆ (degree)
20
10
simulation
exp.
o
θi = 60
0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-5 Best fit simulated and experimental ellipsometric angle ∆ = δp − δs vs.
photon energy for an uncoated soda lime glass substrate used in the fabrication of TEC
glasses. The angle of incidence is 60˚.
32
1.7
Soda lime glass substrate
Soda lime glass substrate
Extinction Coefficient
Index of Refraction
1E-4
1.6
1E-5
1E-6
1.5
1E-7
0
200
400
600
800
1000
1200
1400
1600
1800
0
200
400
600
800
1000
1200
1400
1600
1800
Wavelength (nm)
Wavelength (nm)
Figure 3-6 Index of refraction (left) and extinction coefficient (right) vs. wavelength for
the uncoated soda lime glass substrate. The index of refraction results are derived from
the ellipsometric ψ spectrum whereas the extinction coefficient results are derived from
the transmittance spectrum. The data values are tabulated in Appendix A.
(ii)
SnO2/(soda lime glass)
Ellipsometry and transmittance spectra were measured on the soda lime glass
substrate coated with a single film of undoped SnO2. The analysis of this coated soda
lime glass substrate was performed similarly to that of the uncoated glass and used the
optical properties of the soda lime glass shown in Fig. 3-6.
The deduced sample
structure is shown in Fig. 3-7, including the bulk glass and a two-layer (roughness/bulk)
model for the film. The best fit structural parameters obtained in the analysis are also
included in Fig. 3-7.
The experimental and best fit simulated transmittance and
ellipsometric (ψ, ∆) spectra are given in Figs. 3-8 and 3-9, and the optical properties of
the undoped SnO2 used in the best fit simulations are shown in Fig. 3-10.
These optical
property results are also tabulated in Appendix A.
The surface roughness layer on the film depicted in Fig. 3-7 is modeled as a mixture
33
of the underlying undoped SnO2 and void with a variable composition.
The
parameterized expression used for the optical properties of the SnO2 in the fits of Figs.
3-8 and 3-9 employs the parameters given along with their confidence limits in Fig. 3-10.
The absorptive properties of the film can be assessed from the imaginary part of the
dielectric function ε2 shown in Fig. 3-10 (b).
Due to the thinness of the SnO2 layer, the
absorption associated with values of ε2 below 0.01 is not definitive, however, and can be
approximated as 0.
In fact, an ε2 value of 0.01 at 2.0 eV, corresponds to a k-value of
0.0026, an absorption coefficient of 5.3 x 102 cm-1, and a single pass absorbance of
0.16% in a 310 Å film.
As a result, no significant influence on any of the optical
characteristics of the TEC glass stack results from this approximation.
surface roughness
160 ± 1 Å
0.52 ± 0.01 / 0.48 ± 0.01 SnO2 / void
SnO2
226 ± 2 Å
Soda Lime Glass
3 mm
MSE = 3.81
Figure 3-7 Model with best fitting parameters obtained in the analysis of the
transmittance and ellipsometric (ψ, ∆) spectra of Figs. 3-8 and 3-9 for the soda lime glass
substrate coated with a single layer of undoped SnO2.
34
1.0
SnO2/SLG
simulation
exp.
θi = 0
Transmittance
0.8
o
0.6
0.4
0.2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-8 Normal incidence transmittance T vs. photon energy for a soda lime glass
substrate coated with a single layer of undoped SnO2, the first layer in the fabrication of
TEC glasses. Experimental data (broken line) and a best fit simulation (solid line) are
shown.
300
SnO2/SLG
o
θi= 60
16
SnO2/SLG
o
θi = 60
simulation
exp.
12
∆ (degree)
ψ (degree)
200
8
100
0
simulation
exp.
4
0
1
2
3
4
5
6
-100
7
Photon Energy (eV)
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-9 Ellipsometric angles ψ and ∆ vs. photon energy for a soda lime glass
substrate coated with a single layer of undoped SnO2. Experimental data (broken lines)
and best fit simulations (solid lines) for an angle of incidence of 60˚ are shown.
35
10
4.4
(b) SnO2
(a) SnO2
1
4.0
ε2
ε1
0.1
3.6
0.01
1E-3
3.2
0
1
2
3
4
5
6
Photon Energy (eV)
0
7
1
2
3
4
5
Photon Energy (eV)
6
7
(c) Parameterization of dielectric function:
2
ε1 + iε2 = ε∞ − ADrudeΓDrude/(E2+iΓDrudeE) +
∑ (ε
1,T-L,n
+ iε 2,T-L,n ) + ε1,Gaussian + iε 2,Gaussian
n=1
∞
∫
Here: ε1,T-L,n =
Egn
ξε 2,T-L,n (ξ)
2
ξ −E
2
dξ , and
ε 2,T-L,n
ε 2,T-L,n
∞
ε1,Gaussian =
σ=
−(
2
ξε (ξ)
p ∫ 2 2 2 d ξ , ε 2,Gaussian = A Gaussian (e
π 0 ξ −E
E − EGaussian 2
)
σ
− e
−(
E + EGaussian 2
)
σ
E > E gn
,
E ≤ E gn
) , and
Γ Gaussian
2 ln(2)
An (eV)
n=1
A n E 0n C n (E − E gn ) 2 1
= 2
⋅
2 2
2 2
(E − E 0n ) + C n E E
=0
E0n (eV)
Cn (eV)
Egn (eV)
ADrude (eV) ΓDrude (eV)
ε∞
20.564±1.380 4.825±0.052 2.647±0.063 3.236±0.025
5.303±4.020 0.026±0.020 0.357±0.213
n=2
68.155±8.90018.992±1.31011.845±1.200 1.557±0.027
AGaussain (eV) EGaussian (eV) ΓGaussian (eV)
0.228±0.016 4.384±0.006 0.522±0.024
Figure 3-10 (a,b) Real and imaginary parts of the dielectric function ε1 and ε2 vs. photon
energy for undoped SnO2 that forms the first layer of TEC glasses; (c) analytical
expression for the complex dielectric function of (a,b) along with the best-fit free
parameters and their confidence limits.
36
(iii)
SiO2/(soda lime glass)
Transmittance and ellipsometry spectra on a soda lime glass substrate coated with a
single layer of SiO2 were measured and fit simultaneously at the 100% weighting level of
transmittance relative to ψ and ∆.
fabrication of TEC glasses.
The SiO2 is used as the second layer in the
The adopted sample structure, including the bulk glass and
a two-layer (roughness/bulk) model for the film is shown in Fig. 3-11.
structural parameters and their confidence limits are also shown.
The best fit
The surface roughness
is assumed to be a 0.5/0.5 volume fraction mixture of the underlying SiO2 and void.
The experimental transmittance and ellipsometric spectra along with their best-fit
simulations are shown in Figs. 3-12 and 3-13. The optical properties of the SiO2 used in
these simulations are shown in Fig. 3-14(a), and the data are tabulated in Appendix A.
A parameterized expression for the optical properties of the SiO2 is used as shown in Fig.
3-14(b), based on a two term Sellmeier expression and a separate pole term.
Figure
3-15 shows the reference dielectric function for thermally-grown SiO2 on crystalline Si
[3-2]
in comparison with the deduced dielectric function of the SiO2 film on the soda lime
glass.
Surface roughness
35 ± 2 Å
SiO2
315 ± 4 Å
Soda Lime Glass
3 mm
MSE = 1.24
Figure 3-11
Model adopted for the analysis of the transmittance and ellipsometric (ψ, ∆)
37
spectra of Figs. 3-12 and 3-13 obtained on the soda lime glass substrate coated with a
single layer of SiO2.
1.0
simulation
exp.
SiO2/SLG
o
θi= 0
Transmittance
0.8
0.6
0.4
0.2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-12 Normal incidence transmittance T vs. photon energy for a soda lime glass
substrate coated with a single layer of SiO2, which is used as the second layer in the
fabrication of TEC glasses; experimental data (broken line) and a best fit simulation
(solid line) are shown.
7.5
25
SiO2/SLG
o
θi= 60
SiO2/SLG
o
θi= 60
20
∆ (degree)
ψ (degree)
7.0
6.5
15
10
6.0
5
simulation
exp.
5.5
0
1
2
3
4
5
6
simulation
exp.
7
Photon Energy (eV)
0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-13 Ellipsometric angles ψ and ∆ vs. photon energy for a soda lime glass
substrate coated with a single layer of SiO2, which is used as the second layer in the
fabrication of TEC glasses; experimental data (broken lines) and a best fit simulation
(solid lines) are shown.
38
2.5
2.0
ε1, ε2
1.5
1.0
0.5
(a) SiO2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
(b) Parameterization of the real part of the dielectric function:
ε1 = εoffset + A0/(E02−E2) + Apole/(E2pole−E2); ε2 = 0;
A0 (eV2)
E0 (eV)
Apole (eV2)
Epole (eV)
εoffset
248.876 ± 1.458
14.544 ± 0.003
0.033±0.001
0
1.000 ± 0.009
Figure 3-14 (a) Real (solid line) and imaginary (broken line) parts of the dielectric
function ε vs. photon energy for SiO2 that forms the second layer of the TEC glasses.
The imaginary part of the dielectric function vanishes; (b) mathematical expression for
the dielectric function in (a) along with the best fitting parameters and their confidence
limits.
2.5
ε1
2.0
ε1, ε2
1.5
1.0
ε1 TEC SiO2
ε2 TEC SiO2
ε1 thermal SiO2
0.5
ε2 thermal SiO2
ε2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-15 Real and imaginary parts of the dielectric function ε vs. photon energy for
the SiO2 that forms the second layer of the TEC glasses (solid lines) for comparison with
the reference data of a thermally-grown SiO2 on crystalline silicon[3-2].
39
(iv)
SiO2/SnO2/(soda lime glass)
For the next set of results to be described, the SiO2 layer was deposited instead on the
SnO2/(soda lime glass) structure.
The ellipsometry and transmittance data were fit
simultaneously using 100% weighting of transmittance relative to ψ and ∆ for this sample,
as well.
The optical properties of the soda lime glass, the undoped SnO2, and the SiO2
used in the best-fit simulation are those shown in Figs. 3-6, 3-10, and 3-14, respectively.
Figure 3-16 shows the optical model applied in this case along with the best fit
parameters and their confidence limits.
Figure 3-17 shows the best fit to the
ellipsometric spectra ψ and ∆, obtained using the model of Fig. 3-16.
Surface roughness
144 ± 2 Å
(0.63 ± 0.01)/(0.37 ± 0.01) SiO2/void
SiO2
253± 3 Å
SnO2
466 ± 1 Å
Soda Lime Glass
3 mm
MSE = 4.03
Figure 3-16 Best fit sample structure for a soda lime glass substrate coated with a two
layer stack of undoped SnO2 and SiO2, which are the first two layers used in the
fabrication of TEC glasses.
40
300
SiO2/SnO2/SLG
simulation
exp.
40
θi= 60
200
o
∆ (degree)
ψ (degree)
32
24
16
θi= 60
8
0
o
simulation
exp.
1
2
3
4
5
6
SiO2/SnO2/SLG
-100
0
0
100
7
0
1
Photon Energy (eV)
3
4
5
6
7
Photon Energy (eV)
1.0
simulation
exp.
SnO2/SiO2/SLG
o
θi= 0
0.8
Transmittance
2
0.6
0.4
0.2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-17 Ellipsometric angles (ψ, ∆) at an angle of incidence of 60˚ and
transmittance T at normal incidence plotted versus photon energy for a soda lime glass
substrate coated with a two layer stack of undoped SnO2 and SiO2, which are the first two
layers used in the fabrication of TEC glasses.
The effective thicknesses of these layers as indicated in Fig. 3-16, including surface
roughness and bulk components, are larger than those of the individual layers.
For the
SnO2 layer of the bi-layer the effective thickness is the bulk layer thickness, so that
deff(SnO2) = 466 Å.
For the SiO2, one must also include the contribution of the
roughness layer, so that deff(SiO2) = 344 Å.
41
For the individual layers, deff(SnO2) = 309
Å and deff(SiO2) = 333 Å.
(v)
TEC-15 glass with SnO2:F/SiO2/SnO2/ (soda lime glass) structure
The assumed sample structure, including the bulk glass and a four-layer model for
the TEC-15 multilayer stack is shown in Fig. 3-18.
The stack includes two ideal films
for the undoped SnO2 and the SiO2 and two layers -- roughness/bulk -- for the top-most
doped SnO2:F film.
Figure 3-18 also shows the best-fit structural parameters of the
model along with their confidence limits.
The previously-determined dielectric
functions were used for the soda lime glass, the thin undoped SnO2, and the SiO2.
The
dielectric function of the SnO2:F and structural parameters were deduced simultaneously
in this analysis.
In Figs. 3-19 and 3-20, the experimental transmittance and
ellipsometric spectra are shown along with the simulations obtained as the best fit using
at a 100% weighting level of transmittance relative to ψ and ∆.
The optical properties
of the top-most doped SnO2:F used in the simulation are shown in Fig. 3-21 and tabulated
in Appendix A.
The minimum ε2 value of 0.02 near 2.5 eV corresponds to a k value of
0.0027, an absorption coefficient of 6.8 x 102 cm-1, and a single pass irradiance loss of
2.5%, the latter value typical of high quality transparent conducting oxides.
The
analytical expression for the complex dielectric function valid for photon energies below
4.4 eV is given in Fig. 3-22 along with the best fit parameters and their confidence limits.
This expression includes a two term Sellmeier expansion, one Lorentz oscillator, and one
Drude contribution.
The dc electrical conductivity can be deduced from the Drude
amplitude AD according to σdc = ADε0/ħ = 2.87 x 103 (Ω⋅cm)−1, leading to a resistivity of
42
□.
3.48 x 10-4 Ω⋅cm and a sheet resistance of ~ 10 Ω/
Surface roughness
273 ± 3 Å
0.60 ± 0.005 / 0.40 ± 0.005 SnO2:F / void
SnO2:F
3533 ± 6 Å
SiO2
222 ± 3 Å
SnO2
287 ± 3 Å
Soda Lime Glass
3 mm
MSE = 64.65
Figure 3-18. Best fit multilayer stack for a complete TEC-15 glass sample. The
layered structure includes thin undoped SnO2, thin SiO2, and thick doped SnO2:F with
surface roughness on top. The previously-determined dielectric functions were used for
the soda lime glass and the two thin layers.
1.0
SnO2:F/SiO2/SnO2/SLG
simulation
exp.
Transmittance
0.8
o
θi= 0
0.6
0.4
0.2
0.0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-19 Normal incidence transmittance T vs. photon energy for a complete
TEC-15 glass sample consisting of a soda lime glass substrate coated with layers of
undoped SnO2, SiO2, and top-most doped SnO2:F. Experimental data (broken line) and
a best fit simulation (solid line) are shown.
43
40
SnO2:F/SiO2/SnO2/SLG
simulation
exp.
o
θi= 60
300
∆ (degree)
30
ψ (degree)
SnO2:F/SiO2/SnO2/SLG
simulation
exp.
o
θi= 60
20
200
100
10
0
0
-100
0
1
2
3
4
5
6
0
7
1
2
3
4
5
6
7
Photon Energy (eV)
Photon Energy (eV)
Figure 3-20 Ellipsometric angles ψ and ∆ at a 60˚ angle of incidence plotted vs. photon
energy for a complete TEC-15 glass sample consisting of a soda lime glass substrate
coated with layers of undoped SnO2, SiO2, and top-most doped SnO2:F. The broken
lines indicate experimental spectra and the solid lines indicate the best fit simulation.
SnO2:F
7
6
SnO2:F
exact inversion
of (ψ, ∆) data
fit to analytical
expression
fit to analytical
expression
1
exact inversion
of (ψ, ∆) data
5
ε2
ε1
4
3
0.1
2
1
0
0
1
2
3
4
5
6
0.01
7
Photon Energy (eV)
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 3-21 Real and imaginary parts of the dielectric function ε1 and ε2 vs. photon
energy for doped SnO2:F that forms the top-most layer of TEC-15 glass. These results
are obtained as a best fit analytical expression at low energies where the film is
semitransparent and by an inversion of (ψ, ∆) data at high energies where the film is
opaque.
44
(a) Parameterization of the dielectric function E < 4.4 eV:
ε1 + iε2 = εoffset + A1/(E12−E2)+ ALΓLE0/(E20−E2−iΓLE) − ADΓD/(E2+ iΓDE);
(b) Sellmeier:
A1 (eV2)
E1 (eV)
207.561±4.905
8.857±0.001
εoffset
1.003±0.070
Lorentz:
AL
E0 (eV)
1.332±0.240
4.686±0.077
ΓL (eV)
0.389±0.061
Drude:
AD (eV)
21.311±0.608
ΓD (eV)
0.089±0.002
Figure 3-22 (a) The analytical equation for the dielectric function of the top-most
SnO2:F layer of TEC-15 that holds below 4.4 eV; also shown is (b) a table of the best fit
parameters in the equation and their confidence limits.
(vi)
TEC-7 and TEC-8 glasses with SnO2:F/SiO2/SnO2/ (soda lime glass) structure
The sample structures deduced for TEC-7 and TEC-8 glasses are shown in Figs. 3-23
and 3-24.
The best-fit structural parameters are included in the figures, as well. The
dielectric functions previously-determined from TEC-15 glass were used for the soda
lime glass, the thin undoped SnO2, thin SiO2, and thick doped SnO2:F layers of the
TEC-7 and TEC-8 samples. The incorporation of two additional fitting parameters was
attempted in this case because of the larger surface roughness and bulk SnO2:F film
thicknesses for the TEC-7 and TEC-8 glasses relative to TEC-15. One parameter is the
volume fraction of voids in the SnO2:F layer, which is incorporated due to the expected
coarser microstructure of the SnO2:F layers for the TEC-7 and TEC-8 glasses.
other parameter describes the thickness non-uniformity of the layers.
The
Thickness
non-uniformity arises due to the variation of one or more film thicknesses across the
45
sample surface.
If the film thickness varies over the area of the light beam, then the beam on the film
surface can be divided into components by a grid selected such that the thickness
variation is negligible over a given beam component. The resulting grid size must be
larger than the lateral coherence of the beam, estimated to be ~5-10 µm, otherwise
interference between neighboring beam components will occur.
In the absence of
interference, the irradiances associated with all beam components will add and the
resulting reflected beam will be partially polarized.
parameter can then be determined in the analysis.
A thickness non-uniformity
This parameter describes the
percentage variation in film thickness within the overall probe beam area according to the
definition [(dmax−dmin)/dave]×100%.
For the TEC-7, the observed ~7% non-uniformity is
not of the macroscopic variety, i.e. not a variation in thickness from one side of the beam
to the other, but rather is associated with surface roughness having an in-plane scale that
exceeds the lateral coherence length.
As support for this interpretation, when thickness
non-uniformity is added as a free parameter into the TEC-15 fitting procedure, however,
the best-fit value is zero within confidence limits.
This result is consistent with the fact
that this film has an in-plane roughness scale that is smaller than the TEC-7 and TEC-8,
which in turn is consistent with its thinner surface roughness layer.
46
Surface roughness
409 ± 4 Å
0.55 ± 0.005 / 0.45 ± 0.005 SnO2:F / void
SnO2:F
4708 ± 19 Å
0.97 ± 0.005 / 0.03 ± 0.005 SnO2:F / void
SiO2
222 ± 5 Å
SnO2
302 ± 6 Å
Soda Lime Glass
3 mm
Thickness non-uniformity 7.4% ± 0.8%; MSE = 87.6
Figure 3-23 Multilayer structure with best-fit parameters for a complete TEC-7 glass
sample. The layered structure includes thin undoped SnO2, thin SiO2, and a thick layer
of doped SnO2:F with surface roughness on top. The previously determined dielectric
functions for TEC-15 glass were used here for this TEC-7 glass sample.
Surface roughness
609 ± 7 Å
0.53 ± 0.01 / 0.47 ± 0.01 SnO2:F / void
SnO2:F
5672 ± 52 Å
0.92 ± 0.01 / 0.08 ± 0.01 SnO2:F / void
SiO2
294 ± 14 Å
SnO2
335 ± 14 Å
Soda Lime Glass
3 mm
Thickness non-uniformity 10.7% ± 0.8%; MSE = 156.2
Figure 3-24 Multilayer structure with best-fit parameters for a complete TEC-8 glass
sample. The layered structure includes thin undoped SnO2, thin SiO2, and a thick layer
of doped SnO2:F with surface roughness on top. The previously determined dielectric
functions for TEC-15 glass were used here for this TEC-8 glass sample.
The experimental normal incidence transmission data measured for the TEC-7 and
TEC-8 samples and the data calculated on the basis of the optical models deduced by
ellipsometry are shown in Fig. 3-25 (left) and Fig. 3-26 (left). Due to the existence of
47
thicker surface roughness layers on the TEC-7 and TEC-8 samples, some amount of
scattering is expected in the normal incidence transmission measurement. In theory, the
transmission spectra calculated on the basis of the model deduced by ellipsometry should
be approximately equal to the summation of the experimental normal incidence specular
transmission data and the total normal incidence scattering data integrated over all solid
angles.
In comparison with the close agreement between the calculated and experimental
transmission spectra for the TEC-15 sample (Fig. 3-18), the corresponding data sets for
TEC-7 and TEC-8 samples differ considerably below 3.5 eV.
Considering the values of
the microscopic surface roughness thicknesses of three TEC samples, these results can be
easily explained.
TEC-15 glass has the smallest microscopic surface roughness
thickness among three samples ~275 Å, which means it has the smallest macroscopic
roughness as well, and thus, the smallest scattering loss.
In this case, the experimental
data are closest to the calculated results and in fact, it was valid to use the transmittance
as a data component to be fitted in the analysis of Fig. 3-18. TEC-8 glass has the largest
microscopic surface roughness thickness, thus the largest macroscopic roughness
thickness, and as a result the largest scattering loss. This explains why the experimental
transmission for the TEC-8 glass exhibits the greatest deviation from the calculated
results.
Figures 3-25 (right) and 3-26 (right) show the differences between the calculated and
experimental transmission spectra for the TEC-7 and TEC-8 glass samples.
48
This
difference approximates the total normal incidence scattering data integrated over all
angles.
It is easily recognized that the transmission loss in TEC-8 glass is larger
because of a stronger scattering effect than that in TEC-7 glass.
In addition, in Figs. 3-27 and 3-28 the normal incidence scattering data for these
samples as predicted by the combination of ellipsometry and normal incidence specular
transmission has been compared with experimental normal incidence integrated
scattering data.
The experimental scattering data were measured at Pilkington in a
diffuse transmission experiment using a different pair of TEC-7 and TEC-8 samples than
the ones measured in this investigation.
From the comparison in Figs. 3-27 and 3-28,
the experimental scattering results are lower than the predictions over the 400 – 600 nm
and 1200 – 1500 nm wavelength ranges in both cases.
One possible origin of this
difference may arise from the small collection aperture of the ellipsometer used to
measure the normal incidence specular transmittance relative to the apertures used to pass
the
0.20
1.0
TEC-7 transmission
simulation
exp. data
θi = 0 °
0.15
θi = 0°
0.6
0.10
∆T
Transmittance, T
0.8
Difference between TEC-7 simulation
and exp. data
0.4
0.05
0.2
0.00
0.0
0
1
2
3
4
5
6
-0.05
7
0
1
2
3
4
5
6
7
Photon Energy (eV)
Photon Energy (eV)
Figure 3-25 Transmittance T vs. photon energy for a complete TEC-7 glass sample;
experimental data (broken line) and simulated results based on the ellipsometric model
(solid line) are shown (left). The difference between the two data sets is shown at the
right.
49
1.0
TEC-8 transmission
0.8
and exp. data
0.30
θi = 0°
θi = 0 °
0.25
0.6
0.20
∆T
Transmittance, T
0.35 Difference between TEC-8 simulation
simulation
exp. data
0.4
0.15
0.10
0.05
0.2
0.00
0.0
-0.05
0
1
2
3
4
5
6
0
7
1
2
3
4
5
6
7
Photon Energy (eV)
Photon Energy (eV)
Figure 3-26 Normal incidence transmittance T vs. photon energy for a complete TEC-8
glass sample; experimental data (broken line) and simulated results based on the
ellipsometric model (solid line) are shown (left). The difference between the two data
sets is shown at the right.
specularly reflected and transmitted beams in the measurement at Pilkington with an
integrating sphere.
In such a case, near-specular scattering would be considered true
scattering by ellipsometry but rather as part of the specular beam in the integrating sphere
measurement, and hence not collected.
This difference due to near-specular scattering
amounts to about 5% for TEC-7 and closer to 10% for TEC-8 at the high and low
energies.
Another possible origin of the difference may arise from different optical properties
of the SnO2:F layer in the three different types of TEC glass.
If the absorbance in
TEC-7 and TEC-8 are underestimated with the use of the TEC-15 optical properties, then
the measured transmittance of the TEC-7 and TEC-8 would be lower than that predicted
by ellipsometry.
This effect would contribute a positive term to the ∆T spectra in Figs.
3-25 and 3-26 that is not accountable by scattering.
In fact, it is expected that the
increasing ∆T values at long wavelengths visible most clearly in Figs. 3-27 and 3-28 are
generated by a larger Drude contribution to the absorption in the TEC-7 and TEC-8
50
glasses compared to TEC-15.
Improvements in the analysis of TEC-7 and TEC-8 using
the approach developed here for TEC-15 will be the subject of future research.
TEC-7
Predicted scattering by ellipsometry
and specular transmission
Total integrated scattering
0.3
θi = 0°
0.2
0.1
0.0
Measured scattering by diffuse transmission
0
200
400
600
800
1000
1200
1400
Wavelength (nm)
Figure 3-27 For TEC-7 glass, the normal incidence scattering results predicted by
combining ellipsometry and normal incidence specular transmittance are shown in
comparison with experimental normal incidence integrated scattering data from a diffuse
transmission experiment. Different TEC-7 samples were used for the two different data
sets.
TEC-8
Predicted scattering by ellipsometry
and specular transmission
0.4
θi = 0 °
0.3
0.2
0.1
0.0
Measured scattering by diffuse transmission
0
200
400
600
800
1000
1200
1400
Wavelength (nm)
Figure 3-28 For TEC-8 glass, the normal incidence scattering results predicted by
combining ellipsometry and normal incidence specular transmittance are shown in
comparison with experimental normal incidence integrated scattering data from a diffuse
transmission experiment. Different TEC-8 samples were used for the two different data
sets.
51
Chapter Four
Verification of the Chemical Etching Process for CdTe Depth Profiling
4.1 Introduction
A critical step in the fabrication of CdTe-based solar cells is the CdCl2 vapor
treatment performed near 400 °C in the presence of oxygen.
the solar cell efficiency by a factor of two or more [4-1, 4-2].
This process step improves
Comparisons of as-deposited
and CdCl2 vapor-treated CdTe thin film materials have revealed significant near-surface,
interface, and bulk property differences.
Considering the near-surface, the CdCl2
post-deposition treatment generates much thicker roughness and oxidized layers on the
treated film in comparison with the as-deposited film.
Evidently the exposure to
chlorine and oxygen in the high temperature (~ 400 °C) environment during the treatment
leads to three-dimensional grain growth and oxidation of the surfaces of the large grains.
In addition, many bulk film and interface parameters that may impact the solar cell
performance are modified in the post-deposition CdCl2 treatment.
These include the
physical characteristics such as thickness, density, and grain size and orientation for both
the CdTe and CdS films, as well as the composition profile of the interface region
between the two films.
Changes also occur in the optical and electronic properties upon
52
treatment including the dielectric functions of the CdTe, CdS, and interface region, the
concentration and nature of the defects which control the free electron concentration, and
the mobility of photoinjected carriers.
Real time spectroscopic ellipsometry can be used to obtain dynamic information on
film growth and modification through analysis of the data acquired during thin film
deposition or post-deposition processing.
During the CdCl2 post-deposition treatment,
however, the properties of several layers change simultaneously.
In fact, the layers that
may change in their properties and thickness upon CdCl2 treatment include CdTe and
CdS, as well as the surface and interface layers: TCO/CdS, CdS/CdTe, and CdTe/oxide.
In order to separate out all the changes that occur during the post-deposition treatment
process, real time spectroscopic ellipsometry is avoided in favor of ex-situ spectroscopic
ellipsometry, accompanied by chemical etching for depth profiling.
A comparison of
the depth profiling results with those obtained in real time on the as-deposited film
provides an effective comparison before/after deposition.
Depth profiling of the CdCl2-treated thin films by successive Br2+methanol etches of
the CdTe thin film layer has been applied in this thesis research.
In previous research
the Br2+methanol etch has been successful in removing overlayers including native
oxides and surface roughness from opaque single crystal materials, such as CdTe, and
HgxCd1-xTe
[4-3]
.
By performing spectroscopic ellipsometry during the etching process,
one can determine the extent to which oxide and roughness removal has been successful
by monitoring the maximum magnitude of the imaginary part of the pseudo-dielectric
53
function denoted <ε2> after each etching step.
The pseudo-dielectric function is
calculated from the ideal single-interface ambient/bulk model. Thus, as the overlayers
are removed, the pseudo-dielectric function maxima determined from the (ψ, ∆) spectra
increase and approach the true values of the complex dielectric function of the opaque
CdTe film in this case.
Thus, upon complete removal of the overlayers, an inverted
form of the complex Fresnel amplitude reflection ratio R% p / R% s yields the complex
dielectric function spectra of the opaque film directly from the (ψ, ∆) spectra.
In order to adapt successfully an etching technique previously demonstrated for
single crystals to the polycrystalline films of this study, it must be verified that the
chemical etching process does not modify the underlying film structure that one is
attempting to measure.
In the following paragraphs, an experiment is described to
evaluate the validity of the etching method to be used for the CdCl2 treated CdTe-based
solar cell structure as described in Chapters 5 and 6.
In fact, optical depth profiling
results for the CdTe film structure from real time spectroscopic ellipsometry are
compared with the corresponding results from ex-situ spectroscopic ellipsometry during
etch back.
4.2 Structural evolution of CdTe during etching: experimental details
Magnetron sputtering of the CdTe thin film used in this study was performed at a
radio frequency (rf) target power of 60 W, an Ar pressure of 18 mTorr, and an Ar flow
rate of 23 sccm.
The substrate was a native oxide-covered crystalline Si (c-Si) wafer
54
held at a nominal temperature of 200°C [4-4]. The true temperature of the starting surface
just prior to deposition was estimated to be 130 ± 5°C, as determined in situ from an
analysis of the c-Si E1 and E2 critical points.
deposition due to its smoothness.
A c-Si wafer substrate was used for this
As a result, complications were avoided due to
substrate-induced surface roughness that evolves into interface roughness during
overlying film growth.
The design of the commercial rotating-compensator multichannel ellipsometer used
in this study is similar to that first developed to study the growth of Si:H-based materials
and solar cells [4-5].
The spectral range of the ellipsometer extends from ~0.75 to 6.5 eV,
and complete spectra in the ellipsometric angles (ψ, ∆) can be collected as an average
over a minimum of two optical cycles in a time of (30.7 Hz)-1 = 32.6 ms.
is the mechanical rotation frequency of the compensator.
Here 30.7 Hz
In the real time experiment
performed on the deposition of the CdTe film to be etched, complete (ψ, ∆) spectra were
collected in 1.95 seconds as an average over 60 such optical cycle pairs.
A total of 705
spectral files were collected, corresponding to a set of time points from 2.38 s to 3216.3 s,
with a step of 4.56 s. Each spectral file includes 706 values of ψ and ∆, spanning
photon energies from 0.743 to 6.50 eV.
Thus, during the 3213.9 s deposition time, the
instrument acquired (705)*(706)*(2) = 9.9546x105 experimental data values, filling about
15 Mbytes of computer memory. During the acquisition time for one set of (ψ, ∆)
spectra, an average thickness of CdTe of 2.4 Å accumulates at the bulk layer deposition
rate measured here (1.21 Å/s).
Analyses of all spectra utilized a specialized
(mathematical inversion) / (least-squares regression) algorithm developed previously [4-6].
The angle of incidence for the RTSE measurement was 65.61°.
55
Figure 4-1 A schematic of optical models used to evaluate a CdTe film by optical depth
profiling during both deposition and etching processes.
The large data set accumulated in this way during the deposition of the CdTe film was
analyzed over the energy range of 5~6.5 eV in order to achieve the highest depth
resolution possible.
The absorption depth ( α −1, where α is the absorption coefficient)
of single crystal CdTe decreases with increasing photon energy, reaching 100 Å at 5 eV.
A schematic of the optical models used in the analysis of the experiment including the
deposition and etching processes is shown in Fig. 4-1.
The chemical etch of the CdTe
thin film was performed using a Br2+methanol solution prepared with 0.05 volume
percent Br2. Initially, the etching time was fixed at 3 seconds for each etch step, then
increased to 30 seconds and fixed.
56
4.3 Structural evolution of CdTe during etching: results and analysis
In order to verify the validity of the above approach, the same sample was studied in
real time during deposition and ex-situ during sequential etching, without a CdCl2
treatment in between the two measurements.
A simple two-layer roughness/bulk model
was applied to analyze SE data collected in real time during deposition as well as ex-situ
after each chemical etch step.
Figure 4-2 (solid line) shows the evolution of void
volume fraction in the top ~100 Å of the CdTe bulk layer as a function of the bulk layer
thickness (the latter as deduced from lower energy data) during the deposition process.
The abrupt upward step in void fraction represents a sharp microstructural transition,
characteristic of the relaxation of high compressive strain that occurs in the early stage of
film growth.
This structural transition is only observed at low substrate temperatures (<
200 °C) and the resulting void structures that propagate throughout the film are likely to
be detrimental to the final solar cell performance.
The discrete points in Fig. 4-2
collected after each etch step match the deposition results (solid line) very well −− given
the confidence limits and depth resolution of the analysis.
Thus, for each CdTe bulk
thickness, the sample has the same structural profile as measured during both the
deposition and etching processes.
This result shows that the etching process is the
reverse of the deposition process, and that etching does not modify the underlying film
structure, even when the underlying structure is inhomogeneous and varies significantly
with depth.
57
CdTe growth process
CdTe etch process
void volume fraction, fv
0.25
0.20
depth resolution
1/α (5eV) ~ 100 Å
0.15
0.10
one-layer surface
roughness model
0.05
analysis range
for fv: 5.0~6.5 eV
0.00
-0.05
0
500
1000
1500
2000
2500
3000
3500
bulk layer thickness, db (Å)
Figure 4-2 The evolution of void volume fraction within the top 100 Å of the bulk layer
as a function of CdTe bulk layer thickness obtained during the deposition and etching
processes.
One detail of interest must be considered in view of these results.
Previous reports
have shown that a thin amorphous Te (a-Te) layer remains on the top surface of CdTe
single crystals after a Br2+methanol etch step [4-7,4-8].
The data of Fig. 4-2 show that this
thin a-Te layer, if it does exist for the polycrystalline CdTe film, has only a weak effect on
the determination of the sub-surface void fraction within the sample as long as the CdTe
layer is much thicker than the a-Te layer.
Such conditions are satisfied due to the
expected thinness of the a-Te layer using the previous report as a guide.
Another detail
must be addressed considering that, for the data acquired in these two processes, the
samples are at different temperatures, 130 °C for deposition and 25 °C for etching.
For
the film structure during deposition, thermal expansion will occur at the elevated
temperature, and as a result, a difference in the thickness scales will exist relative to the
58
film structure at room temperature.
Given the thermal expansion coefficient of single
crystal CdTe of 5.9x10−6 (°C)−1 at 25 °C, the thickness change that a 2000 Å thick CdTe
film undergoes upon heating to 130°C is (2000 Å)[5.9x10−6 (°C)−1](105 °C) = 1.2 Å,
which is inconsequential in this study.
4.4 Detection of a-Te on etched CdTe: experiment details
Two experiments have been designed in order to detect a-Te on etched CdTe films
and to extract its optical properties.
In the first experiment, a 3 µm thick CdTe film was
deposited onto a c-Si wafer, and smoothened by Br2+methanol etching from a starting
surface roughness thickness of 500 Å to 50 Å, as characterized by spectroscopic
ellipsometry over the photon energy range from 0.75 to 1.5 eV.
Once the surface
roughness has been reduced to its minimum, additional Br2+methanol etch steps have
been applied to this sample with spectroscopic ellipsometry measurements performed
before the first additional etch step and after each successive etch.
The very rough
region at the surface of the starting CdTe is removed through successive 3 second etches,
leaving a smooth surface which increases the sensitivity of the analysis to the surface and
underlying CdTe.
In the additional etches, also set at 3 second durations, the smooth
CdTe film was removed in a layer-by-layer fashion with a relatively constant roughness
thickness.
The additional etches and measurements have provided the information
needed to evaluate the presence of an a-Te layer in the Br2+methanol etching process.
In the second experiment, a 3500 Å thick CdTe film was deposited on a c-Si wafer, and a
59
total of 37 Br2+methanol etch steps was applied to this sample until the CdTe thin film
was completely removed from the c-Si wafer.
Spectroscopic ellipsometry
measurements were performed after each etch step. These measurements have been
used to extract the optical properties of the a-Te film generated in the Br2+methanol
etching process.
The concept of this second experiment is shown in Fig. 4-3 through a
schematic of the sample structural changes.
These layered structures are applied in the
modeling of the spectroscopic ellipsometry data.
35th etching
36th etching
37th etching
a-Te
CdTe
SiO2
a-Te
SiO2
SiO2
C-Si
C-Si
C-Si
Figure 4-3 Schematic of the sample structural changes that occur in the last three
etching steps for a CdTe film on c-Si. The starting thickness of this CdTe film is 3500 Å.
4.5 Detection of a-Te on etched CdTe: results and analysis
Previous reports have shown that Br2+methanol treatments leave the surface of single
crystal CdTe covered with an optically identifiable amorphous Te layer
[4-7, 4-8]
.
A
similar effect is expected for polycrystalline CdTe, which can be seen clearly by the
difference in the ellipsometric spectra of Fig. 4-4.
Figure 4-4 shows two spectra for the
3 µm thick smoothened CdTe film on c-Si wafer measured at angle of incidence of 63°.
The dashed line represents the data measured before the additional Br2+methanol etching
60
steps when the surface roughness has reached its minimum, and the solid line is acquired
after the 6th additional Br2+methanol etch step after a total etching time of 18 seconds.
Because the CdTe surface is already smooth after the first set of etching steps and the
deduced surface roughness thickness is not changing over the additional etching steps,
then the ellipsometric spectra measured after the 6th etch should be similar to that
measured before the 1st etch.
Obviously, from Fig. 4-4, one concludes that changes have
occurred in the nature of the surface, even though the roughness layer thickness is not
changing significantly.
It can be proposed that the changes are characterized by the
gradual conversion of a roughness layer associated with CdTe to a roughness layer
associated with the a-Te region.
ψ (degree)
30
25
20
th
6 Br+Me etch
st
Before 1 etch
15
∆ (degree)
160
140
120
100
80
2
3
4
5
6
Photon Energy (eV)
Figure 4-4 Ellipsometric spectra for a smoothened CdTe film on a c-Si wafer measured
at angle of incidence of 63°. The broken lines represent data measured before the first
additional Br2+methanol etching step, and the solid lines represent data measured after
the 6th additional Br2+methanol etching step. The total etching time between the two is
61
18 seconds.
The starting CdTe thickness before any etching was 3 µm.
Based upon such a proposition, analysis is performed on the ellipsometric spectra
acquired in the second experiment described in Sec. 4.4 after the 36th and 37th etching
steps at an angle of incidence of 65°. The two experimental spectra are shown in Fig.
4-5 for comparison.
Figure 4-6 shows the two spectra presented separately along with
the best-fit simulations in each case.
Figures 4-7 and 4-8 also show the best-fit
structural parameters of the model along with their confidence limits.
If the real and
imaginary parts of the dielectric function (ε1, ε2) of the a-Te were extracted from the
model of Fig. 4-8 without the assumption of voids, then a relatively weak oscillator
amplitude in ε2 would be obtained, but with a shape similar to that of the previous reports
on single crystal CdTe
[4-7, 4-8]
.
The weak ε2 is attributed, in fact, to the large void
fraction in the a-Te derived from etching away the polycrystalline CdTe in comparison to
that derived from bulk single crystal CdTe.
In an initial fit of the spectra of Fig. 4-6
(left), the a-Te dielectric function from the previous reports was used as a reference along
with a variable void volume fraction.
In the final fit, an analytical model for the a-Te
dielectric function was used along with a fixed void fraction deduced from the initial fit.
The confidence limits on the void fraction in Fig. 4-8 are derived from the initial fit.
The resulting dielectric function deduced for the a-Te layer over the range of 0.75 to
6.5 eV is shown in Fig. 4-9.
In Fig. 4-10, a comparison of these best fit spectra is
provided with the corresponding results for a-Te from measurements of single crystal
62
CdTe over the range of 1.5 to 6.0 eV from the previous reports
[4-7, 4-8]
.
The analytical
expression for the real and imaginary parts of the dielectric function used in the best fit is
given as [4-9]:
ε 2 ( E ) = 0; E < Eg
ε 2 ( E ) = G ( E ) L( E ) =
ε1 ( E ) = ε1 (∞) +
(4.1)
( E − Eg ) 2
2
( E − Eg ) + E p
2
AE0ΓE
; E > Eg .
( E − E0 2 )2 + Γ 2 E 2
(4.2)
2
2 ∞ ξε 2 (ξ)
P
d ξ,
π ∫Eg ξ 2 − E 2
Here E is the photon energy and Eg is the band gap energy associated with band-to-band
transitions in the a-Te.
For E>Eg, the imaginary part of the dielectric function includes
the product of two terms, the Lorentz oscillator function L(E) and the band edge function
G(E). The latter is based on the assumption of parabolic bands and a constant dipole
matrix element.
In the Lorentz oscillator expression L(E), A is the amplitude, Γ is the
broadening parameter and E0 is the resonance energy.
In the band edge function, Ep (>
Eg) defines a second transition energy (in addition to Eg), given by Ep+Eg.
This second
transition energy separates the band edge region from the Lorentz oscillator region and
provides flexibility that is lacking in the more common Tauc–Lorentz expression [4-9].
Table 4.1 Best fit parameters and confidence limits that define Eqs. (4.1) and (4.2) for
the dielectric function of a-Te.
An
En (eV)
Γn (eV)
Eg (eV)
Ep (eV)
ε (∞)
38.79±1.97
2.949±0.053
2.867±0.075
0.909±0.103
0.497±0.154
2.819±0.143
63
1
40
ψ (degree)
35
30
25
20
th
36 etch
th
37 etch
15
∆ (degree)
180
160
140
120
100
0
1
2
3
4
5
6
7
Photon Energy (eV)
40
40
35
35
30
30
ψ (degree)
ψ (degree)
Figure 4-5 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate after
the 36th and 37th etch steps for comparison. The starting CdTe film thickness was 3500 Å.
25
20
20
th
th
37 etch
fit
15
36 etch
fit
15
180
∆ (degree)
180
∆ (degree)
25
160
140
160
140
120
120
100
100
0
1
2
3
4
5
6
0
7
Photon Energy (eV)
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 4-6 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate with a
starting thickness of 3500 Å measured after the 37th (left) and 36th (right) etching steps
(data points). Also shown are their best fits (broken lines).
64
native SiO2
20.5± 0.1 Å
c-Si
2 mm
MSE = 5.896
Figure 4-7 Model and best-fit parameters used for the analysis of the ellipsometric
spectra of Fig. 4-6 (left panel) collected after the 37th etching step applied to a CdTe film
on a crystalline Si substrate. Because the CdTe film is completely removed, this analysis
provides the structure of the c-Si substrate. MSE indicates the mean square error in the
fit.
Surface roughness
0.55 ± 0.01 / 0.45 ± 0.01
12.3 ± 0.1 Å
a-Te/void
native SiO2
20.5 Å
c-Si
2 mm
MSE = 7.669
Figure 4-8 Model and best fit parameters used for the analysis of the ellipsometric
spectra of Fig. 4-6 (right panel) collected after the 37th etching step applied to a CdTe
film on a crystalline Si substrate. This analysis yields the structure of the a-Te layer on
the c-Si substrate. The void volume fraction in the a-Te layer has been obtained by
expressing the a-Te layer in this study of polycrystalline CdTe as a mixture of the a-Te
obtained in a previous study of single crystal CdTe along with a void component.
20
ε1
15
10
5
0
15
ε2
10
5
0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 4-9 Real and Imaginary parts of the dielectric function ε1 and ε2 vs. photon
energy for a-Te generated through Br2+methanol etching of a polycrystalline CdTe film.
65
20
a-Te
reference
ε1
15
10
5
0
15
ε2
10
5
0
2
3
4
5
6
Photon Energy (eV)
Figure 4-10 A comparison of the a-Te optical properties deduced in this study (see Fig.
4-9) with the literature reference optical properties of a-Te from 1.5 to 6 eV, the latter
obtained by etching single crystal CdTe.
Now that the optical properties of a-Te have been determined, it is possible to apply
the results in a model of spectra collected in the two etching experiments in order to
improve the quality of fit to the data.
A decrease in the MSE value will provide an
indicator of the correctness of the optical properties of a-Te layer obtained in this study.
Figure 4-11 shows the ellipsometric spectra and the best fit for the same etching
experiment as that in Fig. 4-6, but after the 35th etch step (left panel).
The right panel
shows the optical model consisting of an a-Te/CdTe/c-Si structure along with the best fit
parameters, confidence limits, and MSE value.
Figure 4-12 shows the modeling results
corresponding to Fig. 4-11 but without introducing an a-Te layer and using CdTe surface
roughness in the analysis instead. Similarly, Figures 4-13 and 4-14 compare the best fit
of the ellipsometric spectra before the first additional etch for the same experiment as that
66
of Fig. 4-4, comparing the modeling results with and without the a-Te layer.
Finally in
Figs. 4-15 and 4-16 a comparison of the ellipsometric analysis after the 6th additional etch
step for the same experiment as that in Figs. 4-13 and 4-14, comparing the modeling
results with and without introducing the a-Te component into the model.
In all three
situations, introduction of the a-Te component into the model lowers the MSE and thus,
improves the quality of the fit to the spectra.
As a result, it can be concluded that the
optical properties of a-Te extracted in this study are reliable and useful.
Various features of the results of Figs. 4-11 to 4-16 are relevant for this and future
investigations.
First it can be noted that it is not necessary to include the a-Te
component in order to obtain accurate structural information when the CdTe thickness is
much larger than the a-Te surface layer thickness.
This is the case in Figs. 4-13-4-16.
In contrast when the CdTe is very thin, it becomes necessary to incorporate the a-Te
component for an accurate CdTe void volume fraction.
Figs. 4-11 and 4-12.
This can be seen by comparing
Second, for the thinner (3500 Å) as-deposited CdTe film, the a-Te
layer obtained even after many etching steps is relatively thin (14 Å) and dense as can be
seen from Fig. 4-11.
In contrast, for the thicker (3 µm) as-deposited CdTe film, the a-Te
containing layer is thicker (50-60 Å), but with a much lower volume fraction of a-Te
(0.2-0.3) as can be seen from Figs. 4-13 and 4-15.
In spite of the large physical
thickness of the layers in this case, the effective thickness is identical to that of the a-Te
layer on the thinner CdTe (13-14 Å).
This comparison suggests that etching of the very
thick film leads to greater inhomogeneity in the surface layer.
67
35
ψ (degree)
30
25
Surface roughness
0.97 ± 0.03/0.03 ± 0.03
20
14 ± 0.4 Å
a-Te / void
th
35 etch
fit
15
CdTe
0.94 ± 0.01 / 0.06 ± 0.01
∆ (degree)
180
109 ± 1 Å
CdTe / void
native SiO2
160
140
20.5 Å
c-Si
2 mm
120
MSE = 13.33
100
a-Te at surface: deff = 13.5 Å
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 4-11 Ellipsometric spectra for a CdTe thin film on a crystalline Si substrate with
a starting thickness of 3500 Å measured after the 35th etch step (left panel). Also shown
is the best fit and associated model deduced in the analysis of the ellipsometric spectra in
order to extract the a-Te/CdTe/c-Si structural parameters (right panel).
35
ψ (degree)
30
25
20
Surface roughness
78 ± 8 Å
0.11 ± 0.01/0.89 ± 0.01 CdTe / void
th
35 etch
fit
15
180
CdTe
∆ (degree)
160
1.16 ± 0.01 / −0.16 ± 0.01 CdTe / void
140
native SiO2
120
c-Si
100
80
95 ± 1 Å
20.5 Å
2 mm
MSE = 22.11
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 4-12 Experimental and best fit spectra (left panel) along with the best fit
parameters and model (right panel) for comparison with the results of Fig. 4-11, but
without introducing an a-Te component into the model. Such a model leads to a higher
MSE.
68
35
ψ (degree)
30
Surface roughness
59 ± 2 Å
0.22±0.01/0.29±0.02/0.49±0.01 a-Te/CdTe/void
25
20
st
before 1 etch
fit
15
CdTe
137 ± 12 Å
0.85±0.01/0/0.15±0.01
CdTe/a-Te/void
CdTe
semi infinite substrate
0.95 ± 0.01 / 0.05 ± 0.01
CdTe / void
∆ (degree)
160
140
MSE = 7.181
120
a-Te at surface: deff = 13 Å
a-Te at sub-surface: deff = 20.5 Å
100
2
3
4
5
6
Photon Energy (eV)
Figure 4-13 Ellipsometric spectra and the best fit (left panel) for a smoothened CdTe
film with a starting thickness of 3 µm obtained before the first additional etch after
smoothening. Also shown is the model and best fit parameters used in the analysis of
the ellipsometric spectra over the energy range of 2 to 6 eV in order to deduce the a-Te
volume fraction in the surface roughness layer (right panel).
35
ψ (degree)
30
25
20
Surface roughness
84 ± 0.7 Å
0.51 ± 0.01/0.49 ± 0.01 CdTe / void
st
before 1 etch
fit
15
CdTe
semi infinite substrate
0.94 ± 0.01 / 0.06 ± 0.01 CdTe / void
∆ (degree)
160
140
MSE = 8.168
120
100
2
3
4
5
6
Photon Energy (eV)
Figure 4-14 Experimental and best fit spectra (left panel) along with the best fit model
and parameters (right panel) for comparison with the results of Fig. 4-13, but without
introducing an a-Te component into the model. This ellipsometric analysis is associated
with a 3 µm thick smoothened CdTe film before the first additional etch after
smoothening.
69
35
ψ (degree)
30
Surface roughness
0.27 ± 0.01/0.73 ± 0.01
25
20
CdTe/a-Te
251 ± 11 Å
0.89±0.01/0.06±0.01/0.06±0.01 CdTe/a-Te/void
CdTe
semi infinite substrate
0.99 ± 0.01 / 0.01 ± 0.01 CdTe / void
th
6 etch
fit
15
160
∆ (degree)
53 ± 2 Å
a-Te / void
MSE = 8.028
140
a-Te at surface: deff = 14 Å
120
a-Te at sub-surface: deff = 15 Å
100
2
3
4
5
6
Photon Energy (eV)
Figure 4-15 Ellipsometric spectra and the best fit (left panel) for a smoothened CdTe
film with a starting thickness of 3 µm obtained after the 6th additional etch after
smoothening. Also shown is the model and best fit parameters used in the analysis of
the ellipsometric spectra over the energy range of 2 to 6 eV in order to deduce the surface
roughness thickness and the a-Te volume fraction in the CdTe structure (right panel).
35
ψ (degree)
30
25
Surface roughness
0.68 ± 0.01/0.32 ± 0.01
20
th
6 etch
fit
15
CdTe
semi infinite substrate
1.02 ± 0.01 / -0.02 ± 0.01 CdTe / void
160
∆ (degree)
100 ± 4 Å
CdTe / void
140
MSE = 39.38
120
100
80
2
3
4
5
6
Photon Energy (eV)
Figure 4-16 Experimental and best fit spectra (left panel) along with the best fit model
and parameters (right panel) for comparison with the results of Fig. 4-15 but without
introducing an a-Te component into the model. This ellipsometric analysis is associated
with a 3 µm thick smoothened CdTe film after the 6th additional etch.
70
Chapter Five
Optical Properties of Thin Film CdTe and CdS before and after CdCl2
Post-deposition Treatment
5.1 Introduction
A description of the optical properties of the TEC-15 glass substrate components has
been provided in Chapter 3.
Here in Chapter 5, the focus now shifts to the optical
properties of CdTe and CdS before and after the CdCl2 post-deposition treatment. The
optical properties of as-deposited CdTe and CdS films on single crystalline Si (c-Si)
substrates were determined as described previously [5-1].
Using this previous work on an
as-deposited CdTe film as a starting point, post-deposition CdCl2 treatment of the same
film was performed as part of this thesis, and its optical properties were obtained after the
treatment.
In the case of CdS, a polycrystalline thin film prepared in the device
configuration on a fused silica prism enables measurements of the same CdS film through
the prism before and after CdCl2 treatment, without loss of spectral range.
As a first step toward studying the layers of the full solar cell, the CdTe film
structural changes upon CdCl2 treatment were investigated using spectroscopic
ellipsometry (SE) measurements of a CdTe film on a crystalline silicon (c-Si) substrate.
71
Because the c-Si is very smooth, the complexity of the data analysis is reduced as a result
of the smooth, well-characterized interface between the film and substrate. In addition,
the absence of an underlying CdS film, which is present in the solar cell structure, avoids
the complication of alloying of the CdTe with S due to S in-diffusion.
Analyses of CdTe
films in CdCl2 treated solar cell structures are described later in Chapter 6, and in that
chapter the issue of S diffusion will be addressed.
Upon CdCl2 treatment, it was observed in this study that the optical properties of the
CdTe film on c-Si change dramatically as will be described in Sec. 5.3.
The
modification experienced by the CdS film in the SiO2/CdS/CdTe structure as a result of
the CdCl2 treatment is weaker; however, in this case, additional research needs to be
performed in the future to explore the observed substrate dependence of the CdS optical
properties and the role of the post-deposition treatment.
5.2 Optical properties of as-deposited CdTe and CdS films deposited on c-Si substrates
The polycrystalline CdTe and CdS films of this study were magnetron sputtered
under conditions similar to those yielding 14%-efficient solar cells
[5-2]
.
The CdTe
depositions were performed on native oxide-covered c-Si wafers in a system with two
chambers and a load-lock (built by AJA International, Inc.) using 60 W rf power applied
to the target, 18 mTorr Ar pressure, 23 sccm Ar flow, and a 10 ± 1 cm target-substrate
distance.
The CdS depositions were performed similarly on c-Si in the two-chamber
system to obtain reference data, and on a fused silica prism in a separate single-chamber
72
system (built at Univ. Toledo) to explore the role of CdCl2 treatments; (Dr. Victor
Plotnikov is acknowledged for assistance in the fabrication of this sample).
For both
CdS depositions, the rf power level was 50 W and the Ar pressure was 10 mTorr.
As
noted earlier, c-Si substrates and an optical quality fused silica prism were used in both
cases due to their consistent optical properties and smoothness; thus, complications in
optical analysis arising from substrate-induced surface roughness and film/substrate
interface roughness were avoided. The true substrate temperatures T for the CdTe and
CdS depositions on c-Si were 188 °C and 225 °C, respectively, as determined in an in situ
SE calibration
[5-3]
.
The nominal substrate temperature T was 200 °C for the CdS
deposition on the fused silica prism in the single-chamber system.
In this case, a
measurement of true substrate temperature was not possible since real time SE was not
used to probe the film growth process.
16
E1
14
E1+∆1
E2
E0
12
10
ε1,ε2
8
6
4
2
0
_______
-2
-4
188 °C
- - - - Single crystal CdTe
1
2
3
4
5
6
photon energy (eV)
Figure 5-1 The room temperature dielectric functions of single crystal CdTe (broken
lines) [5-4] and a CdTe film deposited at 188°C (solid lines) [5-5]. The downward arrows
point to the energy values of the four critical point transitions E0, E1, E1+∆1, and E2
identified in the band structure of Fig. 5-2.
73
Table 5.1 Fitting results for single crystal and thin film polycrystalline CdTe using an
analytical model consisting of four critical points and one T-L background oscillator.
An
Single
En (eV)
Γn (eV)
φn (degree)
µn
CP(E0)
7.283±0.407 1.491±0.004 0.041±0.006 −20.806±2.165 0.048±0.003
CP(E1)
4.871±0.137 3.310±0.002 0.300±0.011 −6.149±4.598 1.089±0.054
EG
−2.975±0.343
crystal CP(E1+∆1) 7.358±0.382 3.894±0.003 0.286±0.010 77.473±1.798 0.377±0.019
CdTe
CP(E2)
T-L
5.320±0.261 5.160±0.003 0.923±0.034 −31.056±8.876 1.560±0.094
1.710±0.034
70.853±3.536 4.790±0.067 4.773±0.380
CP(E0)
8.928±0.314 1.527±0.007 0.089±0.018 −16.791±1.194
0.048
CP(E1)
2.395±0.072 3.199±0.018 0.628±0.025 −75.501±5.900
1.089
CdTe at CP(E1+∆1) 6.458±0.131 3.981±0.019 0.516±0.022 96.117±5.746
0.377
188 °C
1.560
Film
CP(E2)
T-L
1.862±0.092 5.208±0.023 0.958±0.048
73.089±4.033
4.790
E1 E1+∆1
Figure 5-2
ε∞
9.523±6.253
1.710
3.733±0.136
E2
E0
Band structure of CdTe [5-6].
74
−4.120±0.301
E1-A
E0
8
E1-B
ε1,ε2
6
O
4
film CdS Tdep=225 C
single crystal CdS
2
0
0
1
2
3
4
5
6
photon energy (eV)
Figure 5-3 The room temperature ordinary dielectric functions of single crystal
(wurtzite) CdS (broken lines) [5-7] in comparison with the polycrystalline thin film CdS
deposited on c-Si at 225 °C (solid line) [5-1]. The three downward arrows point to the
energy values of the critical point transitions.
Table 5.2 Fitting results for single crystal and thin film polycrystalline CdS using an
analytical model consisting of three critical points and one T-L background oscillator.
An
CP(E0)
En (eV)
Γn (eV)
φn (degree)
µn
EG
ε∞
6.720±0.756 2.399±0.003 0.209±0.008 −19.056±0.836 0.103±0.013
Single
CP(E1-A) 2.581±0.190 4.802±0.004 0.349±0.021 50.393±7.018 0.777±0.085
−1.463±0.595
crystal
CP(E1-B) 5.586±0.327 5.518±0.008 0.689±0.046 101.96±4.870 0.489±0.058
CdS
T-L
90.770±5.560 6.262±0.043 3.421±0.232
3.501±0.046
6.739±0.071 2.426±0.004 0.127±0.008 −20.697±0.621
0.103
Film CdS CP(E1-A) 2.533±0.146 4.944±0.009 0.349±0.020 55.403±3.499
0.777
at 225 °C CP(E1-B) 5.458±0.112 5.400±0.018 0.620±0.026 79.673±5.466
0.489
CP(E0)
−1.597±0.103
T-L
94.931±8.038
6.262
4.602±0.282
3.501
For all dielectric functions in Figs. 5-1 and 5-3, experimental (ε1, ε2) results obtained
by inversion of (ψ, ∆) data were fit using an analytical model consisting of N critical
75
points (N=4 for CdTe and N=3 for CdS).
The locations of the four critical point features
of CdTe are identified in the band structure diagram of Fig. 5-2.
E0 represents the
fundamental band gap transition at the zone center Γ point, whereas the E1 complex is
attributed to transitions along the L line between the L6 valence and conduction bands and
between the L4,5 valence bands and the L6 conduction band.
Each critical point was
modeled using an expression derived for Lorentzian broadened transitions between
parabolic bands [5-8]:
ε =A n e(iφn ) {Γ n /2[E n − E − i(Γ n /2)]}µn
(5.1)
Thus each critical point is fit with five parameters, an amplitude An, a phase φn, a
broadening parameter or linewidth Γn, a resonance or band gap energy En, and an
exponent µn.
These parameters are given in Table 5.1 for single crystal and thin film
polycrystalline CdTe and in Table 5.2 for corresponding samples of CdS.
Also included
in the dielectric function model was a single broad background Tauc-Lorentz oscillator
which was used to fit non-parallel-band transitions in energy regions between the critical
points.
The background is modeled using the expression:
2
A 0 E 0 ΓE
E − EG
ε2 =
Θ(E − E G ) ,
2
2 2
2 2
E (E − E 0 ) +Γ 0 E
(5.2)
where Θ(E − E G ) is a unit step function, centered at E=EG such that Θ =1 when E > EG
and Θ =0 when E ≤ EG.
This expression has four variable parameters, EG, the Tauc
band gap, A0, E0, and Γ0, the Lorentz oscillator resonance amplitude, energy, and
broadening parameter, respectively.
These parameters are also included in Tables 5.1
76
and 5.2 along with ε∞, the constant contribution to the dielectric function.
may be due to transitions above the measured spectral range.
This constant
Negative values for ε∞
have been observed in similar such analyses of single crystal Si [5-9]; however, the origin
of such unphysical values is unclear.
The major difference between the polycrystalline thin film CdTe and single crystal
CdTe dielectric functions derive from the critical point broadening parameters or
linewidths, which are larger for the thin film most likely due to scattering of excited
carriers at grain boundaries which reduces the lifetimes.
For CdS, however, it is
observed that the single crystal has equal or larger critical point widths than the thin film.
This effect is likely to be due to polishing damage at the near surface of the crystal in this
case.
The differences in the critical point energies are due to in-plane strain in the thin
films, and these strain shifts are currently being quantified in order to use the dielectric
function to evaluate strain −− with the potential for on-line analysis
[5-10]
.
The strain
probed in this case lies in the plane of the film since the probing optical field, even
though impinging on the film at oblique incidence, is strongly refracted so that the optical
field lies predominantly in the plane. The differences in the critical point amplitudes
can be attributed to either voids or tensile strain which decrease the An values or
compressive strain which increase these values.
The exponents µn and the phases φn are
expected to be the same in the film and single crystal, and in fact, the µn values are fixed
for the thin films at values deduced for the single crystal. The observed differences in
the best fit φn may be attributed to changes in excitonic interactions due to grain structure
77
or defects.
5.3 Optical properties of CdCl2 post-deposition treated CdTe and CdS
In order to characterize the changes in the structure of the thin films of the solar cell
upon post-deposition treatment, the optical properties of the treated CdTe and CdS must
be obtained and compared to those of Section 5.2.
The dielectric function of the
CdCl2-treated CdTe film deposited on a c-Si substrate was extracted in order to avoid
complications of S diffusion in the completed solar cell. The optical properties of the
treated CdTe are shown in Fig. 5-4.
8
8
ε1
12
ε1
12
4
4
0
0
CdTe as-deposited
CdTe CdCl2 treated
8
8
ε2
-4
12
ε2
-4
12
CdTe single crystal
CdTe CdCl2 treated
4
4
0
0
1
2
3
4
5
6
1
Photon Energy (eV)
2
3
4
5
6
Photon Energy (eV)
Figure 5-4 (left) Best fit analytical models of the room temperature dielectric functions
for two CdTe films of thickness approximately 1000 Å, obtained from the same
deposition but with different post-deposition processing: as-deposited (no treatments;
broken line) and CdCl2-treated for 5 min at 387°C (solid line); (right) a comparison
between the CdCl2-treated CdTe film (solid line) and single crystal CdTe (broken line).
78
Table 5.3 Best fit dielectric function parameters comparing single crystal, CdCl2-treated,
and as-deposited CdTe samples.
En (eV)
An
Single
Γn (eV)
φn (degree)
µn
CP(E0)
7.283±0.407 1.491±0.004 0.041±0.006 −20.806±2.165 0.048±0.003
CP(E1)
4.871±0.137 3.310±0.002 0.300±0.011 −6.149±4.598 1.089±0.054
EG
−2.975±0.343
crystal CP(E1+∆1) 7.358±0.382 3.894±0.003 0.286±0.010 77.473±1.798 0.377±0.019
CdTe
CP(E2)
T-L
5.320±0.261 5.160±0.003 0.923±0.034 −31.056±8.876 1.560±0.094
1.710±0.034
70.853±3.536 4.790±0.067 4.773±0.380
CP(E0)
7.701±0.169 1.503±0.005 0.061±0.011 −15.625±0.449
0.048
CP(E1)
4.260±0.042 3.321±0.003 0.342±0.006 −3.146±1.034
1.089
treated CP(E1+∆1) 6.119±0.057 3.913±0.004 0.212±0.005 88.142±1.689
0.377
CdTe
1.560
CdCl2
CP(E2)
T-L
4.756±0.040 5.214±0.003 0.840±0.010 −22.885±0.821
79.254±1.208
4.790
8.928±0.314 1.527±0.007 0.089±0.018 −16.791±1.194
0.048
CP(E1)
2.395±0.072 3.199±0.018 0.628±0.025 −75.501±5.900
1.089
CdTe at CP(E1+∆1) 6.458±0.131 3.981±0.019 0.516±0.022 96.117±5.746
0.377
188 °C
1.560
CP(E2)
T-L
1.862±0.092 5.208±0.023 0.958±0.048
73.089±4.033
4.790
3.733±0.136
−3.756±0.175
1.710
4.576±0.075
CP(E0)
Film
ε∞
9.523±6.253
−4.120±0.301
1.710
As an example of the key role of the CdCl2 treatment, Fig. 5-4 and Table 5.3
compare best-fit analytical results for the room temperature dielectric functions of the
single crystal CdTe, as-deposited thin film CdTe, and thin film CdTe with the 5 min
CdCl2-treatment. The optical model used here is the same as that described in Section
5.2. The spectra in Fig. 5-4 for the treated film were obtained after a sufficient number
of etch cycles, so that its thickness matched that at which the as-deposited film was
measured (~ 1000 Å).
It is clear from Table 5.3 that the CdCl2-treatment leads to a
significant narrowing of the critical points so as to be nearly indistinguishable from the
single crystal as shown in Fig. 5-4.
This is an indication of an increase in grain size and
/or a reduction in defect density upon treatment.
79
The narrower higher energy critical points for the treated film may be due to its
higher quality surface compared to the single crystal.
Also in Table 5.3 the critical point
energies of the treated film approach those of the single crystal, indicating that strain in
the film is relaxed as a result of the CdCl2 treatment.
As a result, the primary difference
between the dielectric function of the CdCl2-treated film and the single crystal is the
presence of a small volume fraction of voids (0.01 ± 0.002) that exist preferentially near
the surface and reduce the amplitudes of the higher energy critical points.
It is more difficult to perform the corresponding experiment for CdCl2 treatment of
the CdS film.
First, the effectiveness of the etching procedure which is used to remove
the thick oxide and surface roughness layers for determination of the treated CdTe
dielectric function, in fact, has yet to be successfully demonstrated for CdS. Second, in
order for post-deposition treatment studies of the CdS to be at all relevant for device
structures, the CdS must be capped with a layer of CdTe, as this layer is likely to have a
significant impact on the structural change upon treatment.
Because an overlying CdTe
layer significantly attenuates the light irradiance entering the CdS from the CdTe/ambient
side at photon energies above the band gap of the CdTe, the use of a prism arrangement
has been explored in order to study the effect of treatment on the CdS.
In this study,
CdS with an intended thickness of 3000 Å is deposited directly onto one face of a 60°
fused silica prism held at a nominal temperature of 200°C, and then over-deposited with
CdTe to an intended thickness of 5.0 µm.
The true temperature of the surface of the
film is difficult to assess in this case and should be much lower than 200°C due to the
80
different geometry of the substrate holder necessitated by the prism and the lack of a real
time SE analysis capability on the deposition chamber (see Fig. 5-5).
Finally the sample
structure and CdS dielectric function are measured by spectroscopic ellipsometry through
the prism side before and after the CdCl2-treatment.
Sputtering chamber
Substrate holder
Heating wire
Plasma
CdTe Target
Ground Shield
Fused silica prism
substrate
CdS Target
Figure 5-5 A schematic of the sputtering chamber for CdTe/CdS deposition on a fused
silica prism (reproduced with permission from Victor Plotnikov, Ph.D. Thesis, University
of Toledo, 2009).
Figure 5-6 (left) shows the analytically derived dielectric function of CdS
as-deposited on the prism and measured from the prism side, in comparison with that of
CdS as-deposited on c-Si at 225°C and measured from the ambient side.
The dielectric
function of the CdS as-deposited on the prism is suppressed significantly in amplitude at
the higher energies, and the high energy critical point structure is very broad.
Because
the light beam does not penetrate very deeply into the CdS at these higher energies, the
results are characteristic of the CdS at the prism interface which is apparently either a low
81
density material due to incomplete space filling during nucleation or instead a physical
mixture of the substrate material with CdS due to microscopic roughness.
Alternatively,
a chemical mixture may occur at the interface due to intermixing induced by the impact
of sputtered species.
The extensive broadening of the high energy critical points E1-A
and E1-B would suggest a nanocrystalline CdS character at the interface; however other
explanations for this such as a chemical interaction and diffusion are certainly possible.
Figure 5-6 (right) shows that the CdCl2 treatment process appears to densify the CdS, but
8
8
6
6
4
4
ε1
ε1
the material exhibits a similar interface nature as the as-deposited film.
2
2
CdS as deposited on prism
CdS as deposited on c-Si
0
8
on prism
4
6
4
ε2
ε2
CdS as deposited
CdS CdCl2 treated
0
2
2
0
0
1
2
3
4
5
6
1
Photon Energy (eV)
2
3
4
5
6
Photon Energy (eV)
Figure 5-6 (left) Best fit analytical models for the room temperature dielectric functions
of a CdS film as-deposited on a fused silica prism measured from the prism side and on a
c-Si wafer measured from the ambient side; (right) best fit analytical model for the
room temperature dielectric functions of CdS measured from the prism side before and
after a 30 min CdCl2 treatment at 387°C.
82
Table 5.4 Best fit dielectric function parameters for as-deposited CdS on a fused silica
prism, CdCl2-treated CdS on the prism, and. as-deposited CdS on c-Si.
An
CP(E0)
CdS as
Γn (eV)
En (eV)
µn
φn (degree)
6.236±0.030 2.498±0.001 0.226±0.003 −24.268±0.297
EG
0.103
CP(E1-A) 0.316±0.051 4.880±0.100 1.178±0.284 −32.194±39.738 1.861±0.858
deposited
on prism
0.198±0.023
CP(E1-B) 4.474±0.129 5.286±0.026 1.084±0.090 108.21±2.472
T-L
CdCl2
CP(E0)
57.711±5.145
6.262
0.324±0.005
14.828
3.501
2.180±0.106 2.453±0.004 0.486±0.013 −34.041±3.277 0.473±0.030
treated
CP(E1-A) 1.628±0.498 4.923±0.223 2.507±0.271 −123.88±10.262 1.197±0.195
CdS on
CP(E1-B) 11.961±4.281 5.354±0.007 0.944±0.114 172.96±4.398 0.148±0.092
prism
9.153±4.829
T-L
75.427±5.834 6.810±0.262 9.145±1.009
1.804±0.021
6.739±0.071 2.426±0.004 0.127±0.008 −20.697±0.621
0.103
CP(E1-A) 2.533±0.146 4.944±0.009 0.349±0.020 55.403±3.499
0.777
CP(E1-B) 5.458±0.112 5.400±0.018 0.620±0.026 79.673±5.466
0.489
CP(E0)
CdS as
−1.597±0.103
deposited
on c-Si
ε∞
T-L
94.931±8.038
6.262
3.501
4.602±0.282
Table 5.4 shows a comparison of the parameters used in the analytical model for the
three dielectric functions of Fig. 5-6.
A comparison of the critical point energies reveals
that the CdCl2 treatment leads to a reduction in the E0 energy from the as deposited value
to a value closer to the single crystal.
This effect would appear to be characteristic of
strain relaxation, as also occurs in the case of CdCl2 treatment of CdTe.
for the E1A transition is likely to be more complicated.
The situation
A single, broad E1 peak for the
as-deposited film on the fused silica prism is an indication of a film without preferential
orientation of the crystallites.
In contrast, the clear E1A-E1B doublet for the as-deposited
film on c-Si is an indication of preferential c-axis orientation.
The observed significant
shift of oscillator strength to higher energy upon treatment could also be due to a change
83
in the grain texture that leads to preferential c-axis orientation.
Clearly further work
needs to be undertaken, in particular to understand whether the differences in the
as-deposited dielectric functions of CdS on c-Si and CdS on fused silica are due to
top-surface vs. back surface measurement method or due to differences in the nature of
deposition on the two substrates.
5.4 Etch-back profiling of CdTe thin film structure after post-deposition treatments
Analysis results are presented next that focus on the effects of post-deposition
processing on the structural depth profile of CdTe films deposited on native
oxide-covered c-Si substrates.
Sequential etching was applied to three ~ 3000 Å thick
CdTe films co-deposited on c-Si substrates held at 188°C.
These films were exposed to
the following post-deposition processing conditions: (i) as-deposited (i.e., no treatments),
(ii) thermally annealed at 387°C in an atmosphere of Ar for 30 min, and (iii) CdCl2
treated also at 387°C, but for 5 min.
etching was 0.05 vol.% in methanol.
The Br2 concentration used in this study for
For each sample, the etch-profiling method was
performed using successive immersion steps in Br2+methanol, with each etch step
leading to a ~ 300 Å reduction in the bulk layer thickness. Because of the relative
smoothness of the as-deposited CdTe on c-Si substrates (compared, for example, to
depositions on rough TEC glasses), the successive etching treatments led to very smooth
surfaces from which high accuracy dielectric function determinations were possible.
In
addition, the absence of an underlying CdS film in this case avoided the complication of
84
alloying of CdTe due to S in-diffusion that may be especially notable in the later stages of
etching as the CdTe is fully removed. This complication will be discussed further in
Chapter 6.
Figure 5-7 Resonance energies En (upper panel) and linewidths Γn (lower panel) for the
critical point transitions in single crystal CdTe (broken lines) and in db ~ 1000 Å thick
CdTe films sputter-deposited at different temperatures (points), all measured at 15°C
[5-10]
.
Figure 5-7 highlights the consistent differences between the critical point
parameters of as-deposited CdTe films and the single crystal.
85
The latter is characteristic
of CdCl2-treated CdTe as shown in Fig. 5-6 (right).
Results for five different
as-deposited films prepared at different substrate temperatures from 188 °C to 304 °C
reveal the following characteristics relative to the single crystal: (i) higher energy E0,
E1+∆1, and E2 critical point transitions, (ii) lower energy E1 transitions such that the spin
orbit splitting energy is larger than in the single crystal, and (iii) broader critical points
with the E1 transition showing the largest variation with substrate temperature.
In the
next paragraph, the focus will be on the E1 critical point in an evaluation of the effect of
the CdCl2 treatment on the structural depth profile of the CdTe film.
etching
etching
Figure 5-8 Critical point energies (upper panel) and widths (lower panel) as functions
of CdTe bulk layer thickness during etching by Br2+methanol for co-deposited CdTe
films processed in three different ways: (i) as-deposited, (ii) annealed in Ar for 30 min,
and (iii) CdCl2 treated for 5 min. The deviations at low thickness are due to the onset of
semi-transparency at the E1 critical point energy.
86
Figure 5-8 presents the depth profiles in the E1 critical point energy and width
relative to those of the single crystal values.
These results provide information on the
depth profiles in the strain and grain size, respectively, throughout the film.
experimental results
[5-11]
New
suggest that the E1 transition shifts to lower energy with
increasing strain consistent with a stress shift of (−0.2 eV/GPa).
With this new insight,
the depth profiles in the critical point energies take on greater meaning.
Similarly, Fig.
5-9 presents depth profiles in the void fraction that provide information on the structural
uniformity.
For the as-deposited CdTe film, the red-shift of E1 relative to the single crystal
value in the top panel of Fig. 5-8 suggests significant strain in this film over the studied
depth range of 1500-2000 Å; (the depth is measured relative to the substrate interface at 0
Å).
The maximum E1 energy shift of −0.12 eV at a depth of 1500 Å corresponds to a
stress level of 0.6 GPa, which is consistent with results for these as-deposited films in Fig.
5-7.
The depth profile in the void volume fraction in Fig. 5-9 provides additional
indirect evidence for this strain.
The film is observed to undergo a structural transition
near 1500 Å whereby the strain is ultimately relaxed (after 2000 Å thickness) through
generation of voids and their continued evolution with thickness as shown in Fig. 5-9.
The lower panel of Fig. 5-8 shows that the as-deposited film has a very large broadening
parameter ΓE1 ~ 0.6 ± 0.15 eV which appears to be decreasing with increasing thickness
(or distance from the substrate interface). This is indicative of a very small grain size (~
10 nm) which appears to be increasing with thickness [5-11].
87
Upon annealing of the CdTe film in Ar, the strain nearest the substrate is
significantly reduced as the grain size increases (reduced ΓE1).
Even after 30 min of
annealing in Ar, however, there is no significant reduction in the grain size within 500 Å
of the surface, and the strain in this region increases somewhat relative to the
as-deposited film (as indicated by the lower E1 energy).
Figure 5-9 shows that the void
fraction in the surface region is reduced upon annealing in Ar and thus, the structure of
the film becomes more uniform throughout the thickness.
Figure 5-9 Relative void volume fractions as functions of CdTe bulk layer thickness
during etching by Br2+methanol for co-deposited CdTe films on c-Si processed in three
different ways: (i) as-deposited, (ii) thermally annealed in Ar for 30 min at 387˚C, and
(iii) CdCl2-treated for 5 min at 387˚C. For the as-deposited and annealed films, the
void fraction is scaled relative to the observed highest density film. For the
CdCl2-treated film, the void volume fraction is scaled relative to single crystal CdTe.
88
A 5 min CdCl2 treatment leads to an E1 energy within 10 meV (i.e., within
experimental error) of the single crystal value throughout the thickness, suggesting a fully
strain-relaxed film.
In addition, ΓE1 has been reduced significantly to a constant value
of ΓE1 ~ 0.30 ± 0.02 eV throughout the bulk of the film, indicating a significant increase
in grain size.
Finally the CdCl2 treatment leads to a uniform void volume fraction
throughout most of the bulk of the film:
0.05 ± 0.02.
Considerably more scatter exists
in these CdCl2 treated data, however, compared with those of the other samples, possibly
an effect of the Br2+methanol etching of a large-grained, relatively thin film.
Figure 5-9
shows that voids have been pushed to the near-surface region of the CdCl2 treated film
which is likely to be the result of a much larger surface roughness layer thickness.
Finally, it should be noted that the void fractions for the as-deposited and Ar annealed
films in Fig. 5-9 are plotted relative to that of the as-deposited film at the minimum
thickness of ~1250 Å.
For this material, which is under significant compressive stress
(0.6 GPa), the apparent density is ~0.03 higher than that of single crystal CdTe.
For the
CdCl2 treated film, the observed void fraction of 0.05 ± 0.02 is scaled relative to the
single crystal.
89
Annealed CdTe
■ Experiment # 1
∆ Experiment # 2
Annealed CdTe
■ Experiment # 1
∆ Experiment # 2
Figure 5-10 Energy of the E1 transition (upper panel) and its width ΓE1 (lower panel) as
functions of CdTe bulk layer thickness in successive Br2+methanol etching steps for
~3000 Å thick CdTe films. The two films were processed under identical conditions
including fabrication on c-Si wafer substrates and annealing in Ar at 387°C for 30
minutes. The data for experiment #1 are the same as those depicted in Fig. 5-8.
CdCl2 treated
■ Experiment # 1
∆ Experiment # 2
CdCl2 treated
■ Experiment # 1
∆ Experiment # 2
Figure 5-11 Energy of the E1 transition (upper panel) and its width ΓE1 (lower panel) as
functions of CdTe bulk layer thickness in successive Br2-methanol etching steps for
~3000 Å thick CdTe films. The two films were processed under similar conditions
including fabrication on c-Si wafer substrates and CdCl2 treatment for 5 minutes. The
data for experiment #1 are the same as those depicted in Fig. 5-8.
90
The results of Figs. 5-8 and 5-9 can be clearly understood in terms of CdTe grain
growth and strain relaxation effects of the CdCl2 treatment, and can even identify clear
differences in behavior between the CdCl2 treatment and simply annealing in Ar.
In
further experiments, the reproducibility of the etch-profiling studies of such films has
been explored with the goal being to corroborate the above interpretation.
Figure 5-10 shows the Ar annealing behavior of thin (3000-3300 Å) CdTe films on
c-Si substrates from two independent experiments for comparison.
The solid squares
are the same results as shown in Fig. 5-8, and the open triangles denote the results of a
second experiment performed on a different sample prepared and annealed under
identical conditions. The annealing behavior is reasonably well reproduced in the two
experiments, considering that the film thickness in the second experiment is somewhat
lower.
In both experiments, the E1 energy lies ~ 20 meV lower than that of single
crystal CdTe, indicating residual stress of ~0.1 GPa, and the width ΓE1 increases toward
the surface, indicating a smaller near-surface grain size in both experiments.
Figure 5-11 shows results for E1 and ΓE1 from the two experiments on CdTe films in
which CdCl2 treatments were applied for 5 min.
been presented earlier in Fig. 5-8.
The results of the first experiment have
This first experiment was performed with a CdCl2
treatment temperature of 387°C, whereas the second was performed using a higher
temperature of 397°C.
Another difference between the two experiments -- the age of the
prepared CdCl2 sources -- was deemed insignificant.
More importantly, for both
experiments, the treatment was the first one for each of the two CdCl2 vapor sources.
91
Although the overall results of the two experiments of Fig. 5-11 are similar, certain
details in the second experiment suggest an effect of the higher temperature.
First, for
the second experiment, the grain size increases more significantly toward the surface than
in the first experiment.
In fact, the broadening parameter in the top 500 Å of the film
drops below that of single crystal CdTe. This effect may reflect a dielectric function for
the single crystal CdTe from the reference
[5-4]
that may be influenced either by
experimental errors or by greater near-surface damage.
Second, a comparison of Fig.
5-12 with Fig. 5-9 shows that the void profile in the second experiment is not nearly as
uniform as in the first.
This feature is likely due to the higher temperature which leads
to a densification of the underlying large grain crystalline material at the expense of
significant roughness with surface connected voids that extend well into the film.
A hint
of this effect appears for the CdCl2 treated film in Fig. 5-9, but the effect appears quite
strongly in Fig. 5-12.
Experiment #2
CdCl2 treated CdTe film
Experiment #2
Ar annealed CdTe film
Figure 5-12 Void volume fraction as a function of CdTe bulk layer thickness in
successive Br2-methanol etching steps for ~3000 Å thick CdTe films in a second
experiment for comparison with the results in Fig. 5-9. Two different post-deposition
92
processing procedures were used: (i) an anneal in Ar for 30 min, and (ii) a
CdCl2-treatment for 5 min. For the Ar annealed films, the void fraction is scaled
relative to the depth at which the highest density is observed. For the CdCl2-treated film,
the void volume fraction is scaled relative to single crystal CdTe. The void structure for
the film annealed in Ar is attributed to structure in the as-deposited film (as in Fig. 5-8).
In contrast, the void structure for the CdCl2 treated film is associated with extensive
near-surface roughness.
In the above studies, it is clear that a short 5 min CdCl2 treatment is observed to have
a more significant effect in relaxing strain and enhancing grain growth than a 30 min
anneal in Ar.
This reveals the reactive nature of the CdCl2 treatment.
These studies
suggest clear directions for future work which should involve the effects of treatment
time and temperature on the depth profiles of the strain and grain size.
This may enable
one to study the kinetics of grain growth and the separate roles of the surface and
substrate interface in this process.
93
Chapter Six
Optical Structure of As-deposited and CdCl2-treated CdTe Superstrate Solar Cells
6.1 Introduction
The multilayer optical structure of thin film solar cells is of interest because it
provides insights into the optical quantum efficiency as well as the optical losses that
limit the short-circuit current
[6-1]
.
The optical structure may also identify
process-property relationships that assist in process optimization. A powerful probe of
optical structure is real time spectroscopic ellipsometry (SE) [6-2] that can be performed
during the deposition of each layer of the solar cell as well as during post-deposition
processing.
In some circumstances, however, the deposition or processing geometry
precludes optical access; in this case, ex-situ SE becomes the only option.
The CdTe solar cell poses considerable challenges for analysis by ex-situ SE
[6-3]
.
First, the relatively large thickness of the as-deposited CdTe layer leads to considerable
surface roughness, and the conventional CdCl2 post-deposition treatment generates
significant additional oxidation and surface inhomogeneity.
Thus, ex-situ SE
measurements in reflection from the free CdTe surface can be very difficult if not
impossible.
Second, SE performed from the glass side of the solar cell is adversely
94
affected by the top glass surface which generates an incoherent reflection and consequent
depolarization.
In this research, the problem of the free CdTe surface is solved through the use of
Br2+methanol treatments that etch and smoothen the CdTe [6-4].
The problem of the free
glass surface is solved through the use of a 60° prism optically-contacted to the top glass
surface that eliminates the top surface reflection. In an additional approach, the top
surface reflection is eliminated through spatial filtering of the reflected beam, which is
possible due to the relatively thick glass substrate.
In this chapter, comprehensive ex-situ spectroscopic ellipsometry studies are
described that have been applied to investigate the multilayer optical structure of thin
film CdTe solar cells in the superstrate configuration before and after the CdCl2 treatment.
Dielectric functions have been obtained by SE for all layers of these cells as described in
previous chapters.
is available.
As a result, a reference library for ex-situ analysis of CdTe solar cells
The library used in this chapter is shown in Table 6.1.
With the
Br2+methanol layer-by-layer etching, it has been possible to gain a better understanding
of the underlying structure for the as-deposited CdTe film by tracking the optical
properties of the CdTe layer as a function of depth from the surface and proximity to the
CdS/CdTe interface.
In order to evaluate the role of the CdCl2 treatment, such
experiments have also been performed on the treated solar cell.
In the latter
experiments, ex-situ SE is performed from the CdTe film side in the etching process and
also from the glass side either by (i) using a 60° fused silica prism optically contacted to
95
the soda lime glass substrate with index matching fluid, or (ii) blocking the top surface
reflection using an iris.
6.2 Experimental details
In this study, the CdS and CdTe layers of the cells were prepared by rf magnetron
sputtering
[6-5]
but no back contact deposition and anneal were performed.
The CdS
depositions were performed directly on TEC-15 glass substrates at a nominal deposition
temperature of 160°C using 50 W rf power applied to the target, 10 mTorr Ar pressure, 23
sccm Ar flow, and a 10 ± 1 cm distance between the target and the substrate.
The CdTe
depositions were performed similarly on each CdS film, with the exception that the
nominal deposition temperature was 180 °C; (Dr. Jennifer Drayton is acknowledged for
deposition of these samples).
Thus, the layered structure of the cells studied here
includes TEC-15 glass coated with sputtered CdS and CdTe.
Some of these cell
structures were subjected to a 30-min. CdCl2 treatment at 387°C.
Ex-situ SE has been
applied for analysis of the CdTe-based solar cells before and after the CdCl2 treatment.
In order to perform reliable measurements from the CdTe free surface, the rough surface
region was removed through successive Br2+methanol etches, leaving a much smoother
surface suitable for SE measurements.
In a series of etches applied to the CdTe cell
structures, the CdTe layer was also removed step by step, which provided a useful
method for optical depth profiling of the structures.
96
Additional experiments were carried out in which the same CdCl2 treated solar cell
structure was probed in two ex-situ SE measurements, one from the CdTe film side and
the other from the glass substrate side.
In order to perform reliable measurements from
the glass side of the solar cell in this comparison, a 60° fused silica prism was contacted
with index-coupling fluid to the soda lime glass of the solar cell substrate, thus
suppressing the incoherent reflection from the top ambient/glass interface. These two
measurements can be compared to achieve greater confidence in the analysis of glass side
measurement, which could be adversely affected by stress in the prism and glass substrate
as well as by imperfect index-matching to the prism.
Furthermore, experimental SE
measurements from the free CdTe side performed in successive etches can be used (i) for
assessing the confidence limits on the parameters that describe the underlying structure,
as well as (ii) for depth profiling of the CdTe high energy critical point parameters as has
been described previously in Chapter 5.
6.3 Results and discussion: film side and prism side measurements
By performing numerous etching/measurement cycles, this process simulates a real
time spectroscopic ellipsometry measurement, but reversed in time. Figure 6-1 shows the
CdTe surface roughness layer thickness and bulk layer void volume fraction during
etching of the as-deposited solar cell. Each point represents an etching step that leads to
a reduction in the bulk layer thickness of the CdTe, starting from an initial value of 2.4
µm.
The thickness of the CdTe is determined from an analysis of spectroscopic data at
97
low energies (≤ 1.45 eV) where thin film interference oscillations are present.
The
surface roughness thickness and bulk layer void volume fraction are determined from the
data at high energies (≥ 3 eV) where the CdTe is opaque and high surface sensitivity is
attained.
Table 6.1 Dielectric function library used in spectroscopic ellipsometry data analyses
for CdTe solar cells.
Material
Description
File name
Soda lime
TEC-15
“SLG_pilkington_TEC15_20c_userdefined_02162006.mat”
glass
component
TEC-15
SnO2
“SnO2_pilkington_TEC15_20c_drudecppb_09172009.mat”
component
TEC-15
SiO2
“SiO2_pilkington_TEC15_20c_cauchypole_02172006.mat”
component
TEC-15
SnO2:F
“SnO2F_pilkington_TEC15_20c__inver.go_09152009.mat”
component
Sputtered
nominal 400 °C
CdS
“CdS_UT_Tser320C_20C_GO_12032005.mat”
(Figs. 6-1, 6-2,
6-7, 6-9)
Sputtered
nominal 200 °C
(Figs 6-2, 6-4);
CdTe
“CdTe_UT_Tser188C_20C_inver_06212004.mat”
Single crystal
(Figs. 6-1, 6-3,
6-5, 6-6, 6-7, 6-9)
Figure 6-1(a) shows that after about 7 etching steps the surface roughness and void
fraction stabilize with very small variations thereafter.
With successive etching steps,
the surface roughness shows random fluctuations over the range of 42-47 Å whereas the
void fraction (scaled relative to that at the etch step when the highest density CdTe is
obtained) lies in the narrow range of 0.01-0.02.
98
The void fraction for this sample is
uniform over a wide range of bulk layer thicknesses, from 0.4 to 1.6 µm.
For a CdTe
film of this starting bulk layer thickness (2.4 µm), there is also a thick region of
surface-connected microvoids that extends 0.8 µm into the film and is interpreted in the
model as a “bulk” layer.
A very high void fraction (~ 0.3) is obtained in the top 0.2 µm
of the bulk layer. This material is readily removed in the etching process.
Figure 6-2 shows the CdTe surface roughness layer thickness and bulk layer void
volume fraction during etching of the CdCl2 treated solar cell.
These results show that
after about 5 etching steps the surface roughness and void fraction stabilize with weak
variations thereafter.
In this case with successive etching steps, the surface roughness
shows random fluctuations over the range of 20-40 Å whereas the void fraction (scaled
relative to single crystal CdTe) lies in the range of −0.01-0.06, and is tentatively
attributed to a density deficit in the grain boundary regions.
proposed schematic of the film structure.
Figure 6-2 (b) shows a
The surface roughness thickness and void
fraction exhibit greater fluctuations for the CdCl2 treated solar cell possibly due to the
larger grained structure which leads to greater non-uniformity in the etching process.
void volume fraction, fv
surface roughness
thickness, ds (Å)
70
asc42 as deposited CdTe:etching process
60
low Energy for db: 1.0 ~ 1.45 eV
high E range for (ds, fv): 3.0 ~ 6.5 eV
50
40
0.3
depth resolution
1/α (3 eV) ~ 400 Å
0.2
0.1
0.0
4000
8000
12000
16000
20000
24000
CdTe bulk thickness, db (Å)
Figure 6-1
Evolution of the surface roughness thickness and a depth profile of the void
99
volume fraction plotted versus bulk layer thickness obtained in successive Br2+methanol
etching steps that reduce the bulk layer thickness of an as-deposited CdTe component of a
solar cell.
0.5 void fraction
void volume fraction, fv
surface roughness
thickness, ds (Å)
120
asc34 treated
etching process
100
0.20-0.30 void fraction
0.01 µm
0.3 µm
low E range for db : 1.0 ~ 1.45 eV
80
high E range for (ds, fv) : 3.0 ~ 6.0 eV
60
1.8 µm
40
2.1 µm
20
0.30
depth resolution
1/α(3 eV) ~ 400 Å
0.15
0.02-0.05 void fraction
0.00
5000
10000
15000
20000
bulk layer thickness, db (Å)
(b)
(a)
Figure 6-2 (a, left) Evolution of the surface roughness thickness and a depth profile of
the void volume fraction plotted versus bulk layer thickness obtained in successive
Br2+methanol etching steps that reduce the bulk layer thickness of the CdCl2-treated
CdTe component of a solar cell; (b, right) a schematic structure suggested from (a).
Additional information on the depth profile of the structure can be deduced from
analyses of the energies and widths of the critical point transitions.
The results of such
analyses when applied to the as-deposited CdTe solar cell, are depicted in Fig. 6-3. The
critical point energies show a systematic variation with etching to a bulk layer depth of
0.5 µm.
This can be attributed to an increase in compressive strain with increasing
thickness for the as-deposited CdTe film.
Figure 6-3 also shows that the broadening
parameter values (or linewidths) are relatively constant, but are quite large compared with
those of the CdCl2 treated CdTe of Fig. 6-4.
100
For the E1, E1+∆1, and E2 transitions, the
broadening values for the as-deposited film at 0.8 µm are 0.45, 0.48, and 1.08 eV,
respectively.
The corresponding values for the CdCl2-treated films are 0.30, 0.30, and
0.98 eV, respectively.
These results suggest that the as-deposited CdTe film has a
smaller grain size.
For the corresponding Br2+methanol etching results shown in Fig. 6-4(a), obtained
on the CdCl2-treated solar cell, the energies remain essentially constant with etching from
below the surface region to a depth of 0.8 µm.
This result shows that the effects of the
CdCl2 treatment in the CdTe solar cell are not only to increase the grain size, but also to
relax strain in the film.
A second experiment performed with higher depth resolution,
however, shows that as the CdS interface is approached, detectable shifts occur that may
be attributed either to residual interface strain and/or to the presence of S in the CdTe.
Unfortunately, it is not possible to probe through the CdS/CdTe interface since etching
studies of a single CdS film show that it is severely roughened and ultimately
delaminated by the Br2+methanol etch.
Figure 6-4(b) shows results for the depth profile of the linewidths of the prominent
E1, E1+∆1, and E2 critical points.
The widths associated with the surface layer are
typically broader possibly due to the presence of an inhomogeneous region generated by
the CdCl2 treatment.
The widths reach a minimum once the surface layer is removed
and the bulk film void fraction stabilizes below 0.06.
It should be noted that the E1
linewidth shows behavior opposite to this possibly due to correlation with the nearby
E1+∆1 linewidth. As etching of the CdTe progresses toward the CdS interface, all three
101
broadening parameters increase.
This effect may be attributed to CdTe crystallite
growth in the CdCl2 treatment that progresses from the surface to the CdS interface,
leaving a structure such as that shown in the schematic of Fig. 6-4(c).
Alternatively, an
alloying effect of S with CdTe may be a possible explanation; however; this explanation
is not favored due to the lack of systematic variations in the critical point energies.
The results in Fig. 6-4(b) for the critical point widths can be understood using a
simple model of independent line broadening mechanisms each described by h∆νi ~ h/τi
(in photon energy), whereby the resulting transition lifetime is given as: 1/τ = 1/τ1 +
1/τ2 + 1/τ3 +…
[6-6]
Here ‘1’, ‘2’, and ‘3’ indicate, for example, the processes of phonon
scattering, impurity scattering, and grain boundary scattering.
The latter process can be
written as 1/τ3 = υ/R, where υ is the electron group velocity and R is the average grain
radius.
For a polycrystalline material in which grain boundary scattering controls the
variation in linewidth, the result h∆ν ≡ Γ = Γb + (hυ/R) is obtained, where (hυ/R) is the
grain boundary scattering term and Γb is the single crystal width
[6-6]
.
In fact Γb is
typically controlled by phonon scattering, which leads to a dependence of Γb on the
measurement temperature.
Thus, the simple schematic of the sample structure in Fig.
6-4(c), could account for the increase in transition widths with increasing depth as shown
in Fig. 6-4(b).
When impurity scattering is the dominant mechanism, for example,
considering S atoms in a random CdTe1-xSx (x < 0.1) alloy, then R can be considered as
proportional to the average distance between atoms.
102
Figure 6-3 (left) Depth profiles of the critical point energies of the E1, E1+∆1 and E2
transitions in the as-deposited CdTe layer of a solar cell, plotted versus bulk layer
thickness obtained in successive Br2+methanol etching steps that reduce the bulk
thickness; (right) depth profiles of the linewidths of the E1, E1+∆1 and E2 transitions
obtained in the same experiment.
103
asc34 treated: etching process
low E range for db : 1.0 ~1.45 eV
0.40
3.36
3.34
high E range for (ΓE1, ΓE1+∆1, ΓE2) : 3.0 ~ 6.0 eV
0.35
energy of E1 transition (3.310 eV)
3.32
ΓE1 (eV)
E1 (eV)
3.38
3.900
0.25
0.35
ΓE1+∆1 (eV)
E1+∆1 (eV)
energy of E1+∆1 transition (5.894 eV)
3.896
3.892
high E range for (ΓE1, ΓE1+∆1, ΓE2) : 3.0 ~ 6.0 eV
width of E1 transition (3.310 eV)
width of E1+∆1 transition (5.894 eV)
0.30
1.05
energy of E2 transition (5.160 eV)
ΓE2 (eV)
E2 (eV)
5.20
5.19
0.30
asc34 treated: etching process
low E range for db : 1.0 ~1.45 eV
1.00
5.18
width of E2 transition (5.160 eV)
0.95
5.17
8000
12000
16000
20000
8000
12000
16000
20000
bulk layer thickness, db (Å)
bulk layer thickness, db (Å)
(a)
0.5 void fraction
0.01 µm
(b)
0.20-0.30 void fraction
1.5 µm
2.1 µm
0.02-0.03 void fraction
(c)
Figure 6-4 (a, top left) Depth profiles of the critical point energies of the E1, E1+∆1 and
E2 transitions in the CdCl2-treated CdTe layer of a solar cell, plotted versus the bulk layer
thickness obtained in successive Br2+methanol etching steps that reduce the bulk
thickness; (b, top right) depth profiles of the linewidths of the E1, E1+∆1 and E2
transitions obtained in the same experiment; (c, bottom) a schematic structure suggested
from (b).
A similar analysis was applied to a second CdCl2-treated solar cell structure
co-deposited with the structure of Figs. 6-2 and 6-4 again using the high energy range of
104
the spectra collected during etching to determine depth profiles in the energies and
linewidths of the CPs. The purpose of this analysis is to assess the reproducibility of the
observed behavior in Fig. 6-4 while achieving a greater depth resolution and approaching
closer to the CdS/CdTe interface.
Figure 6-5 shows the energies of the E1, E1 + ∆1, and E2 transitions versus CdTe
thickness all from successive etches. The data in the energies in Fig. 6-5 show relatively
weak variations; however, as the CdS interface region is approached, E2 -- which appears
to be a more sensitive indicator of structural deviations from the single crystal -increases systematically, possibly due to interface compressive strain or to in-diffusion of
S. The very weak shifts in E1, E1 + ∆1, which appear only at the end of etching below a
bulk layer thickness of 2000 Å, are not consistent with compressive strain; thus,
in-diffusion of S seems to be a more likely possibility to explain the behavior of the E2
transition.
The broadening parameters corresponding to each critical point have also been
investigated.
Figure 6-6 shows that ΓE1, ΓE1 + ∆1, and ΓE2 all increase gradually, an effect
which corroborates similar results obtained from the co-deposited solar cell presented
previously in Fig. 6-4(b).
This effect may be due to grain size reductions and/or to the
effects of S alloying when the interface is approached, as in the case of the energy E2
described in the previous paragraph.
Given the small differences in the energies in Fig.
6-5 compared to crystal CdTe, strain is unlikely to cause the significant broadening
effects in Fig. 6-6.
105
c-CdTe E1+∆1 = 3.894 eV
Figure 6-5 Energies of the E1, E1+∆1, and E2 transitions as functions of CdTe bulk layer
thickness in successive etches of a CdCl2 treated CdTe solar cell that reach within 0.1 µm
of the CdS/CdTe interface.
Figure 6-6 Broadening parameters ΓE1 , ΓE1+∆1, and ΓE2 as functions of CdTe bulk layer
thickness in successive etches of a CdCl2 treated CdTe solar cell that reach within 0.1 µm
of the CdS/CdTe interface.
In order to understand better the variation of the critical point parameters in CdTe
solar cell structures, the results of Figs. 6-5 and 6-6 can be considered in view of similar
106
results on thin films in Chapter 5.
First, the Chapter 5 results (Fig. 5-8, top) suggest that
interface strain is not a significant factor for the CdCl2-treated sample structure, and thus,
any weak variations in the energies in Fig. 6-5 may be more likely due to alloying.
Second, considering the depth profile of the broadening parameters in Fig. 5-8 (bottom),
such results for the Ar annealed film are interpreted to suggest that grain boundaries
remain fixed at the surface but grain growth occurs sub-surface.
In contrast, the CdCl2
treatment is shown to generate a more rapid grain growth effect not only well into the
bulk but also on the surface.
Thus, a key role of the CdCl2 treatment is to generate
uniform grain growth throughout the thickness.
The results in Figs. 6-4 (b) and 6-6
suggest that grain growth does not extend all the way to the CdS interface in the solar cell,
possibly due to the role of the CdS in pinning the grain boundaries or the diffusion of S
which may suppress grain growth. Alternatively, the CdCl2 treatment for the cells of
Figs. 6-4 (b) and 6-6 may not be fully optimized.
In addition to the structural analyses of Figs. 6-1 – 6-6 that focus on the high energy
SE data for the CdTe surface roughness and structural depth profiles, it is also possible to
extract characteristics of the underlying CdS and its layered structure from the low energy
data. This information is obtained from the same low energy data range that provides
the CdTe bulk layer thicknesses, plotted along the abscissas in Figs. 6-1 – 6-6.
Figure
6-7 shows the deduced pseudo-dielectric function (solid lines) from SE measurement
after the 15th etch step for the CdCl2 treated sample of Figs. 6-2 and 6-4.
Also shown in
Fig. 6-7 is the least-squares regression analysis best fit (broken lines).
107
A simple
multilayer model that leads to this best fit with a relatively small number of free
parameters, seven in all, is shown in Fig. 6-8. It incorporates the glass substrate,
including (i) a fixed optical structure as obtained in a previous analysis of uncoated
TEC-15 consisting of SnO2 (267 Å); SiO2 (215 Å); and SnO2:F (3178 Å); (ii) an
interfacial roughness layer of fixed thickness between the TEC-15 and the CdS whose
fixed thickness is chosen to match the surface roughness thickness measured from the
uncoated TEC-15 (296 Å) and whose composition is a fixed 0.5/0.5 effective medium
mixture of the overlying and underlying materials; (iii) a CdS layer of variable thickness
and void volume fraction; (iv) a single interface layer of variable thickness between the
CdS and CdTe modeled as an effective medium of the two materials with variable
composition; and (v) the bulk CdTe and its 0.5/0.5 CdTe + void surface roughness layer,
both of variable thickness.
The amorphous Te layer on the etched surface is neglected
in this study since it has little effect on the deduced parameters when the CdTe layer is
thick.
Because the starting TEC-15 transparent conducting oxide exhibits a surface
roughness layer with a thickness of approximately 300 Å, roughness is sure to propagate
throughout the structure and thus occurs at each interface.
As a result, any layers that
are generated at the critical CdS/CdTe interface by the chemical interaction between the
CdS and CdTe are modulated by roughness.
Thus, as a first approximation, a single
effective medium layer of CdS+CdTe of variable composition is used to represent the
layer at the CdS/CdTe interface.
108
10
5
Fit
asc34 etch 15th
5
0
-5
10
<ε2>
5
<ε2>
Fit
th
asc34
step
asc34 15
etch
15th
<ε1>
<ε1>
10
0
5
0
-5
1.0
1.5
2.0
-5
2
3
4
5
6
PHOTON ENERGY (eV)
PHOTON ENERGY (eV)
Low energy data provide:
CdTe, CdS, and interface
thicknesses, along with their
compositions
High energy data provide:
CdTe surface roughness
CdTe composition and grain size or
defect density
Figure 6-7 Experimental pseudo-dielectric function spectra for the CdTe solar cell of
Figs. 6-2 and 6-4 after the 15th etching step; also shown is the best fit using the structural
model of Fig. 6-8.
CdTe surface
roughness
CdTe/void = 0.5/0.5
35 ± 0.4 Å
CdTe bulk
CdTe/void=0.96±0.01/0.04±0.01
CdTe/CdS
interface
CdTe/CdS = 0.51±0.02/0.49±0.02
935± 10 Å
CdS bulk
CdS/void = 0.93±0.01/0.07±0.01
1247± 25 Å
CdS/SnO2:F
interface
SnO2:F
CdS/SnO2:F = 0.5/0.5
SnO2:F = 1.00
14529± 32 Å
296 Å
3178 Å
SiO2
SiO2 = 1.00
215 Å
SnO2
SnO2 = 1.00
267 Å
Soda lime glass
glass = 1.00
semi-inf.
Fixed
TEC15
structure
Figure 6-8
Structural model for the CdTe solar cell after the 15th etch step that
provides the best fit in Fig. 6-7.
109
After the structural analysis of Figs. 6-7 and 6-8 was performed, confidence in the
method was sought by performing two more analyses on a second CdCl2 treated solar cell
structure deposited under the same conditions, one analysis from the film side and the
other from the glass substrate side through a prism in optical contact with the free surface
of the glass.
Separate sample pieces from the same solar cell deposition were studied in
these two analyses.
The previously deduced dielectric function library in Table 6.1 was
used in the analysis of the ex-situ SE data in Fig. 6-9 acquired from the CdTe free surface
after 8 etching steps and from the prism/glass substrate side without CdTe etching. The
structural models of Figs. 6-10 and 6-11 used in the analysis of both data sets shown in
Fig. 6-9 are the same as that of Fig. 6-8; simple models with six free parameters yielded
the fits in Fig. 6-9.
16
(b)
(a)
60
ψ (degree)
ψ (degree)
12
8
4
th
Data: 8 etch
Fit
0
Data: glass side
Fit
300
∆ (degree)
∆ (degree)
30
15
300
150
0
-150
0.9
45
150
0
1.0
1.1
1.2
1.3
1.4
1.5
Photon Energy (eV)
-150
0.9
1.0
1.1
1.2
1.3
1.4
1.5
Photon Energy (eV)
Figure 6-9 Ex situ SE spectra in (ψ, ∆) (symbols) (a) from the free CdTe surface after 8
Br2+methanol etching steps and (b) from the prism/glass side without etching. The best
fit results (solid lines) yield the structural parameters in Figs. 6-10 and 6-11, including the
thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS interface, and CdS bulk layers,
as well as the volume fractions of CdS/CdTe in the interface layer and void in the CdS
bulk layer.
110
Figure 6-10 The best fit results from the free CdTe surface after 8 Br2+methanol
etching steps yielding the thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS
interface, and CdS bulk layers, as well as the volume fractions of CdS/CdTe in the
interface layer and void in the CdS bulk layer.
Figure 6-11 The best fit results from the prism/glass side without etching yielding the
thicknesses of the CdTe roughness, CdTe bulk, CdTe/CdS interface, and CdS bulk layers,
as well as the volume fractions of CdS/CdTe in the interface layer and void in the CdS
layer.
Figures 6-10 and 6-11 show the multilayer models of the sample structures, the latter
depicting the placement of a 60o fused silica prism on top of the free surface of the glass
substrate.
In this configuration, an index-matching fluid proves vital in eliminating
unwanted incoherent reflections. Considering the assumptions and simplifications of the
model, the agreement in the structural parameters listed in the two figures is excellent.
Good agreement is obtained even in the CdTe/CdS interface layer composition although
111
this layer should require a more complex model that includes not only interface
roughness modeled using an effective medium approximation, but also interdiffusion [6-7]
modeled using stable phase CdTe1-xSx and CdS1-xTex alloy dielectric functions.
As final supporting results for the overall approach, the CdS layer and CdTe/CdS
interface thicknesses have been deduced from spectra collected at the CdTe free surface
in 24 successive etches as shown in Fig. 6-12.
In the 25 analyses, the same model was
used for all the spectra, now obtained as a function of CdTe bulk thickness before and
after each etching step.
These fits should provide independent values for CdS/CdTe
interface layer thickness and CdS bulk layer thickness since the unprocessed (ψ, ∆)
spectra data vary rapidly with CdTe bulk layer thickness due to variations in the
interference pattern as shown in Fig. 6-9 (left panel).
In fact, these independent values
should be constant since the etching does not affect the sub-surface material, and any
variations provide a measure of the uncertainty in these values.
For the selected optical
model to be justified, the confidence limits for the interface thickness and composition
must be smaller than the values themselves.
In fact maximum deviations of ±2-3%
from the average values are obtained and the average values lie within the confidence
limits of the analyses performed on spectra collected through the prism/glass.
112
Figure 6-12 CdS and CdTe/CdS interface layer thicknesses deduced from spectra
collected through the prism/glass (solid line) and from spectra collected from the CdTe
surface in successive etches (points, dotted line extrema).
Among the key final results of Fig. 6-12 include a 1000 Å thick interface region of
(CdS+CdTe) and a 1030 Å thick layer of CdS.
The (CdS+CdTe) interface layer for this
sample is found to be a 0.7/0.3 vol. fraction mixture of CdTe/CdS; however, this mixture
merely provides a dielectric function that approximates that of the interface region and
should not be interpreted physically.
Further studies of the optical properties of the
interface are in progress [6-8].
6.4 Results and discussion: through the glass measurements
SE measurements directly through the top glass have been performed using a method
in which the reflection from the glass/film-stack interface is collected whereas the
reflection from the ambient/glass interface is blocked.
Invasive prism attachment is
avoided by eliminating the top glass surface reflection, an approach that is more practical
for off-line or on-line cell and module mapping applications. An example of such SE
113
data analysis for a magnetron sputtered CdTe solar cell is demonstrated here in a
step-by-step process in which additional fitting parameters are introduced while
observing a measure of the quality of the fit.
The goal is to increase the complexity of
the optical model systematically over the six and seven parameter models of Figs. 6-8,
6-10, and 6-11 by incorporating additional thicknesses and volume fractions and
determining the parameters that are most important in ensuring a good fit. The most
complicated optical model used for analysis of through-the-glass SE spectra collected on
CdTe solar cells on glass superstrates shown in Fig. 6-13 includes a total of 12 variable
parameters with satisfactory confidence limits on all parameters.
(ψ, ∆)
(ambient)
Soda lime glass
SnO2 (d)
TEC-15
SiO2 (d)
structure
SnO2:F + CdS (d & fCdS)
CdS +void (d & fv)
CdTe/CdS interface (d & fCdTe )
CdTe (d, ∆d/d)
Surface roughness
(d, fv)
Figure 6-13 Multilayer stack used to model the thicknesses and compositions of the
individual layers of the CdTe solar cell. The SE beam enters through the glass, and the
reflection from the top surface is blocked since it is incoherent with respect to the
reflection from the glass/film interface.
114
0.22 1
2
3
4
5
6
7
8 9 10 11 12
number of fitting parameters
0.20
MSE
0.18
0.16
0.14
0.12
0.10
Step-by-step MSE reduction
0
10
20
30
40
50
60
Steps
Figure 6-14 Step-by-step MSE reduction by adding one fitting parameter at a time.
Starting with the CdTe thickness as a variable, each additional parameter was
subsequently fitted. It was found that fitting the SnO2:F thickness provided the greatest
improvement in MSE among all 2-parameter attempts. Similar methodology was used
for all 12 parameters. Circular points indicate the best n-parameter fit with n given at
the top and the added parameter given in Table 6.2.
The SE spectra collected at one spot on the 3 x 3 cm2 CdTe solar cell and at angles of
incidence of 60° and 65° were modeled over the spectral range from 0.75 to 3.0 eV,
below the glass absorption onset.
Figure 6-14 shows the step-by-step reduction in mean
square error (MSE), expressed in terms of the deviations in the real and imaginary parts
of ρ ≡ tanψ exp(i∆), obtained by adding best fitting parameters one at a time.
Table 6.2
shows the sequence of fitting parameters that are introduced in order of importance to
obtain the best n-parameter fit (n = 2, 3, 4…12).
best final 12-parameter fit.
Figure 6-15 shows the SE data and the
The best fit parameters are shown in Table 6.3 along with
115
their confidence limits.
Table 6.2 Best fitting parameters added step by step to improve the mean square error
(MSE) in modeling through-the-glass SE measurements of a CdTe solar cell.
Best fitting parameter to add to
# of fitting parameters
MSE
improve MSE
1
CdTe thickness
0.2208
2
SnO2:F thickness
0.1735
3
CdTe non-uniformity
0.1565
4
CdS thickness
0.1448
CdTe surface
5
0.1363
roughness (50/50)
6
Void fraction in CdTe roughness
0.1134
7
CdS volume fraction in SnO2:F
0.1056
8
SiO2 thickness
0.1010
9
Void volume fraction in CdS
0.0968
CdS/CdTe interface
10
0.0883
thickness (50/50)
11
SnO2 thickness
0.0881
CdTe volume fraction in CdS/CdTe
12
0.0860
interface
The fitting parameter sequence in Table 6.2 is understandable in that the thickest
layers have the greatest impact in the step-by-step fitting procedure. As a result this
new analysis approach also provides information on the starting TEC-15, namely, the
thicknesses of the SnO2 at the glass interface, the intermediate SiO2 layer, and the doped
116
conducting SnO2:F layer without assuming fixed values.
For the solar cell, the analysis
provides additional information on possible modification of the SnO2:F by
over-deposition of CdS, as well as the standard parameters of CdS thickness, its void
fraction, the combined CdS/CdTe interface roughness-interaction thickness, and the CdTe
thickness.
As shown in Fig. 6-13, a mixture of SnO2:F and CdS is used to model the
interaction between the two materials when CdS is deposited on the TEC-15; however,
the exact nature of this interaction will be the subject of more detailed future studies.
It
is not clear if the modification is simulating the effect of CdS penetrating the roughness
and near surface void structure or, alternatively, if there is a uniform modification of the
SnO2:F properties. Other approaches to describe the interaction, e.g., a modification in
the free electron density or mobility in SnO2:F has not yet achieved success.
The
interaction layer between the CdS and the CdTe has been described as a simple effective
medium of the two materials that simulates interface roughness, although a more realistic
approach may be to use alloy layers in addition to the effective medium layer as
described previously.
It should be noted that the deduced CdS thickness of 1225 ± 14 Å
is in good agreement with the intended CdS thickness of 1300 Å.
117
Figure 6-15 Ellipsometric spectra (points) in ψ (top) and ∆ (bottom) at an angle of
incidence of 60° as measured through the glass at a single point on a 3 x 3 cm2 CdTe solar
cell sample. The solar cell was treated with CdCl2 but no back contact processing was
performed. Also shown is a best fit (lines) using the model structure of Fig. 6-13 with
the parameters listed in Table 6.3.
Table 6.3 Multilayer stack thicknesses, non-uniformity, and compositions, the latter
expressed in terms of volume fractions, along with parameter confidence limits for the
best fit to SE data obtained through the glass.
Soda lime glass
SnO2 thickness
339 ± 13 Å
SiO2 thickness
157 ± 5 Å
SnO2:F thickness
3123 ± 24 Å
CdS volume fraction
2.3% ± 0.5%
CdS thickness
1225 ± 14 Å
Void volume fraction
13.5% ± 0.6%
CdTe/CdS interface thickness
868 ± 32 Å
CdTe volume fraction
73.3% ± 1.6%
CdTe bulk thickness
19702 ± 43 Å
CdTe thickness nonuniformity
3.1% ± 0.1%
CdTe surface
851 ± 16 Å
roughness thickness
Surface void fraction
28.4% ± 0.7%
Ambient
The new analysis procedure, through-the-glass SE, is useful in determining the
118
optical structure of CdTe solar cells for off-line or on-line analysis in a mapping mode.
This method is useful because it is non-destructive, and the large roughness layer
thickness of the CdTe does not present a problem. Analysis of the SE data using a
step-by-step analysis methodology identifies the important thicknesses and compositional
parameters for successful optical characterization of the solar cell.
6.5 Summary
Ex-situ spectroscopic ellipsometry has been applied to perform multilayer analyses
of CdTe solar cell structures.
This capability exploits a database obtained from both
ex-situ and in-situ measurements that includes the dielectric functions of all component
layers of the cell. As a supplementary tool, Br2+methanol etching was used to reduce
the CdTe bulk layer thickness in a layer-by-layer fashion for depth profiling purposes.
The SE measurements made after a variable number of etching steps enables tracking of
changes in the critical point energies and broadening parameters near the surface, in the
bulk CdTe, and near the CdS/CdTe interface.
This capability was used to smoothen the
CdTe free surface so that measurements of the multilayer stack can be performed for
correlation with through-the-glass measurements of the solar cell.
Good agreement is
obtained between the CdS thickness and CdS/CdTe interface layer thickness between the
two measurement approaches.
Thus, the validity of the through-the-glass method of
solar cell analysis has been supported through this study.
119
Chapter Seven
RTSE Analysis of CdTe Solar Cell Structures in the Substrate Configuration
7.1 Introduction
In the conventional configuration for thin film CdTe solar cells used in both research
and manufacturing, one starts with a glass superstrate which is coated with a transparent
conducting oxide top contact
[7-1]
. In this configuration, the CdS window layer of the
heterojunction is deposited on the transparent conductor first and the CdTe active
photovoltaic layer is deposited on top of the CdS. In this sequence, the heterojunction is
protected from the ambient by the much thicker layer of CdTe.
Furthermore, since the
CdTe surface is exposed to the ambient in this configuration, the CdTe layer can be
treated with CdCl2 just prior to p+ back contact formation.
In the reverse or substrate configuration, one cannot apply the same sequence of
operations.
In this case, because the CdTe layer is deposited first, the back contact is
formed simultaneously with the deposition process, rather than as a separate step.
Furthermore the CdCl2 treatment must then be performed either after the CdTe deposition,
which is not likely to leave an optimum surface for subsequent heterojunction formation,
or after the CdS deposition, which is not likely to produce a favorable effect on the CdS
120
surface for subsequent top contact formation.
As a result CdTe solar cells in the
substrate configuration have not reached the level of performance of cells in the
superstrate configuration [7-2].
In this Chapter, results of a real time spectroscopic ellipsometry (SE) study of CdTe
film growth on Mo, which is a standard back contact metal used in the substrate
configuration, are presented, and the information content of such SE measurements will
be discussed in detail.
Ex situ SE results for a completed solar cell in the same substrate
configuration will also be presented and discussed.
7.2 Analysis of CdTe deposition on rough molybdenum
Figure 7-1 shows the time evolution of (ψ, ∆) at 5 photon energies selected from the 706
spectral positions acquired during CdTe sputter deposition at 50 W target power and 18
mTorr Ar pressure; (Dr. Anthony Vasko is acknowledged for deposition of this sample,
and additional measurement assistance of Dr. Jian Li is acknowledged).
The substrate
was a glass slide coated with thin film Mo, held at a nominal temperature of 200°C.
This corresponds to a true temperature of 237°C when a crystalline Si wafer substrate is
used.
The full spectral acquisition time was 2 s and the angle of incidence of the
measurement was 65.68°.
This was the first real time experiment performed during
CdTe deposition using the SE system described in Chapter 1.
The Mo film was also
prepared by magnetron sputtering and was found to exhibit a surface roughness ~80 Å
121
300
80
0.743 eV
ψ (degree)
1.166eV
200
2.637 eV
1.653 eV
40
6.500 eV
100
6.500 eV
1.653 eV
20
∆ (degree)
1.166 eV
60
0
2.637 eV
0.743 eV
-100
0
0
10
20
30
40
50
0
10
Time (min)
20
30
40
50
Time (min)
Figure 7-1 Time evolution of (ψ, ∆) at 5 photon energies selected from 706-point
spectra acquired during sputter deposition of CdTe on a Mo coated glass slide. The full
spectral acquisition time was 2 s and the angle of incidence was 65.68°.
thick. The analysis results obtained here for the Mo optical properties can be applied in
future studies of solar cells in the substrate configuration.
7.2.1 MSE minimization for the analysis of CdTe/Mo
The analysis of the spectra for the experiment of Fig. 7-1 used a two-variable,
time-averaged, mean-square error (MSE) minimization procedure over selected time
intervals.
The time averaged MSE serves as a criterion to obtain the correct structural
evolution as well as the optical properties of the deposited CdTe film, as a multilayer
within the selected time intervals. Even the structure and optical properties of the Mo
thin film can be determined in the procedure.
Each selected time interval during
deposition has been separated into two components in the average MSE minimization
122
procedure.
In one component, film growth occurs as a bulk/roughness structure with
variables db and ds which are the bulk and surface roughness layer thicknesses,
respectively.
In the other component, film growth occurs through the filling of the
rough interface between the underlying and growing materials as an interface/roughness
structure with variables fi and ds, where fi is the volume fraction of new material filling
the roughness of the underlying material and ds is the surface roughness layer thickness
on the growing film. Additional details on the two analysis components will be given in
the following paragraphs.
In order to achieve the desired results, one seeks to maintain
the MSE value below ~5 throughout the time range of the deposition.
In this analysis
procedure, four time intervals have been selected in all, leading to four individual layers
in the CdTe film growth process, and the average MSE minimization procedure has been
applied to the growth of each layer.
For the first CdTe layer, the time interval for the average MSE minimization
procedure was 3.303~13.936 min and the energy range in the MSE calculation was
0.74~6.5 eV.
Minimization of the average MSE for this layer requires the following
nine steps, grouped in three iterations.
Iteration A: Estimate Mo roughness thickness
Step 1.
A value di is estimated for the surface roughness thickness on the Mo
substrate.
Step 2.
Numerical inversion software is applied to the in-situ experimental data (ψ,
∆) collected before initiation of the deposition to deduce the dielectric function (ε1, ε2) of
123
the bulk Mo based on the value di from Step 1.
Step 3.
A least-squares regression model is created, using a previously-determined
reference dielectric function for CdTe
[7-3]
as an initial approximation.
A dynamic
growth analysis is performed over the 3.303~13.936 min time range and the
time-averaged MSE, denoted <MSE> is determined.
The starting time is selected to
ensure that filling of the substrate/film interface roughness has occurred as described later,
and the ending point is selected to maintain an acceptable MSE versus time, typically less
than 5.
Step 4.
The di value is adjusted and Steps 2 and 3 are repeated. The two results for
the <MSE> are compared and continued iterative adjustments in di are made until the
minimum <MSE> is found.
Finally, di is fixed at the value that minimizes <MSE>.
Iteration B: Estimate CdTe structure and optical properties
Step 5.
With the optimum di value fixed from Iteration A, further estimates are
made for a pair of values for the CdTe bulk and surface roughness layer thicknesses (db,
ds).
Step 6.
Inversion of the experimental data (ψ, ∆) is performed next to deduce the
dielectric function (ε1, ε2) of CdTe at the ending time 13.936 min.
Step 7.
Using the inverted CdTe dielectric function, dynamic growth analysis is
performed over the time range 3.303~13.936 min, in order to determine <MSE>.
Step 8.
Steps 6 and 7 are iterated using successive adjustments in (db, ds) within a
two-dimensional grid, until the minimum <MSE> is found.
124
The minimum <MSE> then
yields the best fit results for {db(t), ds(t)} and for the inverted CdTe dielectric function.
Iteration C:
Refine Mo roughness thickness as well as the CdTe structure and
optical properties
Step 9.
Next, rather than using the CdTe reference dielectric function of Step 3, the
inverted dielectric function of Step 8 is used in a repetition of Steps 1, 2, 3, and 4.
refinement provides an improved value of di.
with the refined di value.
This
Steps 5, 6, 7, 8, and 9 are then repeated
A final iteration is performed for internal consistency.
The final results of Steps 1−9 are as follows.
The minimum average MSE and the
interface roughness thickness are given by: <MSE>min = 2.98 and di = 79.6 Å. The best
fit bulk and surface roughness layer thicknesses at the ending time of 13.936 min are db =
423 Å; ds = 56.7 Å.
These results are summarized in the first entry of Table 7.1.
The average MSE minimization procedure has also been applied for the other three
CdTe layers.
For each of these three layers, the minimization steps were performed in a
similar way as those in the analysis of the first CdTe growth layer; however, now di can
be fixed at the final result from the first layer analysis and only Iteration B is needed.
Thus, one only need to estimate the pair of (db, ds) values and invert the experimental (ψ,
∆) data to obtain the dielectric function (ε1, ε2) of the CdTe at the ending time.
With the
resulting CdTe dielectric function, dynamic growth analysis is performed, the <MSE> is
extracted, and the process is iterated through adjustments of (db, ds) until <MSE>min is
found.
For the growth analysis with <MSE> = <MSE>min the db and ds values are
correct and the associated inverted dielectric function for CdTe is also correct.
125
These three successive <MSE> minimizations based on Iteration B have yielded the
following results.
The second CdTe layer covered the time range of 15.229~23.024 min,
and analysis employed the energy range 1.2~6.5 eV; <MSE>min was obtained at db = 313
Å and ds = 54.5 Å. The third CdTe layer analysis used the ranges 24.246~34.480 min
and 1.0 ~ 6.5 eV; <MSE>min was obtained at db = 404 Å; ds = 66.0 Å.
The fourth and
topmost CdTe layer analysis used the ranges 36.002~40.948 min and 1.5~6.5 eV;
<MSE>min was obtained at db = 108 Å; ds = 67.0 Å.
These results for all four layers are
summarized in Table 7.1.
Table 7.1
layers.
CdTe bulk and surface roughness layer thicknesses for the top four CdTe bulk
Time range (min)
db (Å)
ds (Å)
Data analysis energy range (eV)
<MSE>min
3.303~13.936
423
56.7
0.74~6.5
2.98
15.229~23.024
313
54.5
1.20~6.5
2.60
24.246~34.480
404
66.0
1.00~6.5
8.71
36.002~40.948
108
67.0
1.50~6.5
1.30
The overall <MSE> minimization can be described by a flow chart as shown in Fig.
7-2 for the most complicated case of the first CdTe layer. The schematic structure of the
full CdTe layer stack is shown in Fig. 7-3.
126
Iterations
Step 1. Estimate di, the Mo substrate
surface roughness layer thickness.
Step 2. Apply inversion routine to the exp. data (ψ, ∆) obtained just prior
to deposition to deduce the dielectric function (ε1, ε2) of the bulk Mo.
Step 3. Apply reference dielectric function for CdTe, and
A
perform dynamic growth analysis over the time range
3.303~13.936 min, to extract the average MSE.
Step 4. Minimum <MSE> ?
N
Adjust the di value.
Y
Minimum <MSE>; best fit di.
Step 5. Fix di at the best fit value, and estimate
C
CdTe (db, ds) at the ending time t = 13.936.
Step 6. Perform inversion of the exp. data (ψ, ∆) to
deduce the dielectric function (ε1, ε2) of CdTe.
Step 7. Perform dynamic CdTe growth
B
analysis over the time range 3.303~13.936
min, to determine the average MSE.
Step 8. Minimum <MSE> ?
N
Adjust the (db, ds) values.
Y
Minimum <MSE>; best fit di, (db, ds)
Y
Minimum <MSE> ?
N
Use the CdTe dielectric function deduced in Step 7 instead
Step 9.
of the reference CdTe dielectric function, and return to Step
1 in order to refine di value; minimum.
Minimum <MSE>; best Mo, CdTe
structures and dielectric functions
Final results :
<MSE>=2.98; di=79.6 Å; db=423 Å;
ds=56.7 Å.
Figure 7-2 Flow chart of the three-iteration <MSE> minimization procedure for CdTe
film growth on a rough Mo film substrate.
127
Ambient
ds
surface roughness
db4
fourth CdTe bulk layer
d2j+1 = d2j,end + (0.5−fi)d2j,end + 0.5ds
d2j = d2j-1,end + 0.5(ds−d2j-1,end) + db
di4
db3
di3
fourth interface roughness
d4 = d3,end + 0.5(ds−ds3,end) + db
d3 = d2,end + (0.5−fi)ds2,end + 0.5ds
d2 = d1,end + 0.5(ds−ds1,end) + db
d1 = (0.5−fi)di + 0.5ds
db2
third CdTe bulk layer
third interface roughness
second CdTe bulk layer
second interface roughness
di2
db1
di1
first CdTe bulk layer
first interface roughness
Mo
Figure 7-3 The schematic structure describing the final optical model for deposition on
rough Mo.
Each of the four CdTe bulk layers will be associated with an interface layer adjacent
to the underlying material which is filled in upon deposition of the overlying material.
During each interface filling time, db is set to zero. The Bruggeman EMA has been used
to model the dielectric function of the interface layer.
The volume percent void in the
interface layer is one fitting parameter which is varied in the interface filling analysis for
modeling purposes.
Therefore, during each interface filling time, the void volume
percent should decrease from 50% to 0% as the overlying material volume percent
increases from 0% to 50%.
The first interface layer is a three-component composite of
materials including Mo (50%), (void + CdTe) (50%), and the other three topmost
interface layers are three-component composites of materials including the lower CdTe
128
film (50%), and (void + the upper CdTe film) (50%).
For the interface layer filling
analysis, the first interface layer (0~3.277 min) used the energy range (0.74~6.5 eV).
The second, third, and fourth interface layers spanned the time ranges of (14.012~15.229
min), (23.024~24.246 min), and (34.480~36.002 min), respectively, using the energy
ranges of 1.5~6.5 eV in all cases.
A detail of the structural evolution of the first
interface and bulk CdTe layers is shown in Fig. 7-4.
Mo
ds (0.5/0.5): (CdTe #1/void)
di (0.5/0.5−fi/fi): (Mo/CdTe #1/void)
ds (0.5/0.5): (CdTe #1/void)
db
di
(0.5/0.5): (Mo/CdTe #1)
Mo
Figure 7-4 The schematic structures describing the interface filling (left) and bulk layer
growth (right) models for the first interface layer.
7.2.2 Structural evolution
The fit quality is given by the magnitude of the MSE which degrades rapidly for
deposition times t > 15.5 min. This result is shown in Fig. 7-5 (left) (broken line). The
goal of the multilayer model is to determine if this MSE degradation can be attributed to
the evolution of the dielectric function with accumulated bulk layer thickness.
Such an
effect can be modeled using a succession of layers, each having a dielectric function
determined independently, whereas the same model for the surface roughness layer can
be used throughout.
Figure 7-5 (right) includes the results for the MSE from the four-layer model (solid
line) on an expanded scale in which case the quality of the fit remains very good (MSE<5)
129
for the full ~40 min deposition process. In the final film structure, the effective
thicknesses of the four layers including the interface filling regions are (interface to
surface) 491, 369, 465, and 174 Å, for a total effective thickness of 1499 Å.
The sharp
minima at 14, 23, 34.4, and 37.5 min in the four-layer MSE indicate the times at which
the dielectric functions of the four layers were determined.
Figure 7-5 (Left) MSE, which is a measure of the quality of the fit to RTSE data, for the
complete CdTe deposition using optical models for the CdTe film consisting of one bulk
layer (broken line) and four bulk layers (solid line). In both cases a one-layer model for
surface roughness was employed; (right) the MSE for the model with four bulk layers is
shown on an expanded scale.
Figures 7-6, 7-7 and 7-8 show the final results of such modeling, in which case four
separate bulk layers are used along with a single evolving surface roughness layer.
Although the best fit leads to an improvement in MSE as shown in Fig. 7-5 (left) (i.e., by
a factor of ~40), the exact origin of the improvement requires further study as will be
seen from an inspection of the four dielectric functions in Sec. 7.2.3. The evolution of
the surface roughness layer thickness (Fig. 7-6), the overlying material volume fraction
during the interface filling region (Fig. 7-7), the bulk layer thickness for all four CdTe
130
component layers (Fig. 7-8, left), and the effective thickness or mass per unit area (Fig.
7-8, right) versus deposition time have all been deduced using the four-layer model for
the CdTe film.
The broken line jumps in the surface roughness thickness ds result from
the consideration of each bulk layer individually with an independent surface roughness
layer thickness.
At the jumps, the surface roughness on the underlying layer is
instantaneously transformed into an interface roughness layer with the subsequent
development of roughness on the overlying layer starting from ds = 0 Å.
The continuity
of ds before generation and after filling of the interface layers is an indication of the
internal consistency of the analysis.
The evolution of the accumulated effective
thickness versus deposition time is determined from adding the following components:
(i) First interface filling layer: d1 = (0.5−fi) *di+0.5*ds, where db=0, 0 ≤ fi ≤ 0.5, t0 ≤ t < t1;
(ii) First bulk layer: d2 = d1,end + 0.5*(ds−ds1,end) + db, where t1 ≤ t < t2;
(iii) Second interface filling layer: d3 = d2,end + (0.5−fi)*ds2,end + 0.5*ds, where t2 ≤ t < t3;
(iv) Second bulk layer: d4 = d3,end + 0.5*(ds−ds3,end) + db, where t3 ≤ t < t4
………
In these equations, dj,end and dsj,end are the values of the effective thickness and the
surface roughness thickness at the end of the time range for layer j.
The end of the time
range for the jth bulk layer t2j is defined somewhat arbitrarily in order to maintain an MSE
that is acceptably low (e.g. less than ~ 5), and the end of the time range for the jth
interface filling layer t2j+1 is defined such that the void fraction fi reaches zero.
In
addition, the interface void fraction fi is related to the overlying material volume fraction
131
fm=0.5−fi.
Figure 7-6 Evolution of the surface roughness thickness versus deposition time
determined using a four-layer model for CdTe film growth on rough Mo. The spikes in
the surface roughness thickness result from the consideration of each bulk layer
individually with an independent surface roughness layer. In this case, the surface
roughness layer on the underlying layer is instantaneously transformed into an interface
layer at the vertical broken lines upon initial growth of the overlying layer, whose
roughness layer starts from zero thickness.
50
fmfm(%)
40
30
20
10
0
0
10
20
30
Time (min)
Figure 7-7 Time evolution of the CdTe overlayer volume percent during interface
filling of the underlying CdTe roughness layer for CdTe growth on Mo.
132
Figure 7-8 (Left) Evolution of the individual bulk layer thicknesses versus deposition
time determined using a four-layer model for CdTe film growth on Mo; (right) evolution
of effective thickness of CdTe, including all bulk, interface, and surface layer
components.
7.2.3 Optical properties
In Fig. 7-9, the Mo dielectric function is shown, valid for the nominal deposition
temperature of 200 °C. These results were deduced by inversion after determination of
the Mo surface roughness thickness value of 79.6 Å, through the 3D <MSE>
minimization procedure.
In this <MSE> minimization procedure, an independent
dynamic analysis provides interface, bulk, and surface roughness thicknesses, di, db, ds,
respectively, that describe the structural evolution of CdTe growth on the rough Mo film.
133
5
ε1
0
-5
inversion di = 79.6 Å at nom. 200 °C
-10
inversion di = 79.6 Å at R.T.
50
ε2
40
30
20
10
0
0
1
2
3
4
5
6
7
Photon energy (eV)
Figure 7-9 Mo dielectric function at a nominal temperature of 200 °C acquired by
inversion assuming a Mo substrate roughness thickness of 79.6 Å (solid line). For the
overlying CdTe, four bulk layers and a roughness layer are used to describe the best fit
model. For the first bulk layer, the Mo/CdTe interface roughness, the CdTe bulk, and
CdTe surface roughness layer thicknesses di, db, ds, respectively, are determined in a
dynamic analysis, in which case the criterion is the minimum average MSE. The
Mo/CdTe interface roughness thickness di is taken to be the same as the Mo substrate
film roughness thickness. Also shown is the Mo dielectric function at room temperature
before heating to the deposition temperature as determined by inversion, again assuming
a Mo surface roughness layer thickness of 79.6 Å (broken line).
In an attempt to corroborate the surface roughness thickness on Mo from the starting
room temperature (ψ, ∆) spectra, reference dielectric functions for Mo [7-4], MoO3 [7-5] and
MoOx [7-5] were applied in conjunction with the models of Table 7.2.
Table 7.2 Five models used to evaluate the Mo overlayer thickness using reference
dielectric functions from the literature.
ds
Ambient
Ambient
Ambient
Ambient
Mo/void
MoO3/void
MoOx/void
MoO3/Mo
Mo
Mo
Mo
Mo
Mo
Model 1
Model 2
Model 3
Model 4
Model 5
81.9 ± 2.9 Å
50%/50%
273.1
86.4 ± 2.9 Å
50%/50%
267.6
117.4 ± 2.3 Å
50%/50%
190.9
121.6 ± 2.3 Å
50%/50%
188.4
ds
91.2 ± 2.5 Å
[fi/(1−fi)] 50%/50%
MSE
238.5
134
Ambient
MoOx/Mo [fi/(1−fi)]
The results of Table 7.2 show that the Mo substrate roughness thickness of 79.6 Å
deduced through the 3D <MSE> minimization procedure is close (within 12 Å) to the
corresponding model that applies reference dielectric function results (Model 1).
Figure 7-10 (a-d) shows the real (top) and imaginary (bottom) parts of the dielectric
functions of the four CdTe layers that comprise the film deposited on the Mo covered
glass substrate.
Each of these CdTe dielectric functions has been determined by
numerical inversion, incorporating the full underlying film structure and after
determining the surface roughness and bulk layer thicknesses through minimization of
the <MSE> in a dynamic analysis of the growing layer.
Figures 7-10(b-d) include
comparisons of the dielectric functions of two successive layers.
successive dielectric functions leads to two observations.
Comparison of the
First, using the range of
energies greater than ~2.5 eV, no significant differences in the void fraction, critical point
energies, and critical point widths are observed. This suggests a relatively uniform layer
throughout the thickness.
Second, for the range of energies below 2.5 eV, artifacts are
observed, and these increase in amplitude with the increase in layer number.
These two
observations lead to the conclusion that the increase in MSE in Fig. 7-5 for the one bulk
layer model is not due to non-uniformity with depth, but rather other features of the
measurement or model such as a spatial distribution of thicknesses over the surface of the
beam or inadequacies of the model for the Mo substrate interface.
Such features do not
affect the data quality in the opaque regime above 2.5 eV for each layer.
135
For comparison in Fig. 7-10(e), the solid line is the dielectric function deduced in a
study of CdTe deposited on a c-Si substrate as reported by Li
[7-6]
. The sample on c-Si
was deposited at a substrate temperature of 188 °C, an rf power of 60 W, and an Ar
pressure of 18 mTorr.
The ε1 spectra at low energies suggest that the void volume
fraction is consistently higher for the component layers of the CdTe film on Mo.
In fact,
the first layer CdTe film material on Mo can be described as an effective medium of the
CdTe film material on Si plus voids; the deduced volume fraction of voids is 0.10.
This
result indicates that the void volume fraction in thin film CdTe may depend on the
substrate and in particular its surface roughness thickness.
Because the comparison in
Fig. 7-10(e) is for the first CdTe bulk layer on Mo, it appears that in the case of the Mo
substrate, voids develop immediately in the deposition process, likely an effect of
shadowing by the roughness on the Mo.
Later in this chapter, an ex situ spectroscopic
ellipsometry analysis of a CdTe solar cell on ZnTe:Cu/Mo will be performed to evaluate
the ability to characterize the full solar cell in the substrate configuration.
12
12
10
8
10
6
6
4
4
2
0
0
-2
10
-2
10
8
8
6
6
ε2
ε2
2
CdTe/Mo layer # 2
CdTe/Mo layer # 1
(b)
8
ε1
ε1
CdTe/Mo layer # 1
(a)
4
2
4
2
0
0
0
1
2
3
4
5
6
7
Photon energy (eV)
0
1
2
3
4
Photon energy (eV)
136
5
6
7
12
12
10
8
10
6
6
4
4
2
2
0
0
-2
-2
10
10
8
8
6
6
ε2
ε2
CdTe/Mo layer # 4
CdTe/Mo layer # 3
(d)
8
ε1
ε1
CdTe/Mo layer # 3
CdTe/Mo layer # 2
(c)
4
4
2
2
0
0
0
1
2
3
4
5
6
7
0
1
2
12
4
5
6
7
CdTe/Mo layer #1
CdTe/c-Si
(e)
10
3
Photon energy (eV)
Photon energy (eV)
8
6
ε1
4
2
0
-2
12
10
ε2
8
6
4
2
0
0
1
2
3
4
5
6
7
Photon energy (eV)
Figure 7-10 Real (top panel) and imaginary (bottom panel) parts of the dielectric
functions of the four layers [(a)-(d)] of a CdTe thin film deposited on rough Mo. These
results are determined from inversion, after determining the CdTe roughness and bulk
layer thicknesses through minimization of the average MSE obtained throughout the
layer analysis; (e) also shown is a comparison of the first layer dielectric function of
CdTe deduced in this study with that of CdTe deposited on a smooth c-Si substrate at a
nominal temperature of 200 °C [7-6]. In (b)-(d) comparisons are provided between the
dielectric function of a given layer and that of the layer underneath it.
7.2.4 Comparison of RTSE and AFM
Lastly, it is of interest to compare the roughness thickness for the final CdTe film on
the Mo coated glass substrate as deduced by RTSE with that obtained by atomic force
microscopy (AFM) using a 1 x 1 µm2 image area.
The RTSE/AFM comparison results
are in reasonable agreement as shown in Fig. 7-11, where the root-mean-square (rms)
137
value from AFM is indicated. Considering the surface height distribution from AFM, it
is clear that spectroscopic ellipsometry is not sensitive to area fractions of surface
asperities and depressions at the level of ~ 0.3 or less.
Roughness ds = 67.1 Å
depressions
asperities
Figure 7-11 Comparison of the surface roughness thickness at the end of the deposition
for a 1496.5 Å thick CdTe film on Mo as deduced by RTSE with the relative surface
height distribution and rms roughness from AFM.
7.3
Ex situ spectroscopic ellipsometry analysis of a CdTe solar cell in the substrate
configuration
The goal of ex situ spectroscopic ellipsometry (SE) studies of solar cells in the
substrate configuration is two-fold.
First, the substrate configuration precludes
transmission measurements for optical analysis of the solar cells.
Thus, a reflection
measurement is the only choice for optical analysis, and ellipsometry is the most
powerful reflection measurement available.
With such a measurement, it may be
possible to establish a sufficient in-depth understanding of the growth processes so that
they can be optimized for highest efficiency solar cells.
138
Second, SE may be developed
as an in-line monitor of substrate-type solar cells, for example in a roll-to-roll process.
7.3.1 Metallic back contact for CdTe solar cells in the substrate configuration
Figure 7-12 shows a comparison of measured pseudo-dielectric functions (solid
lines) for the Mo thin films deposited (a) on glass and (b) on Kapton, two possible
substrates used for rigid and flexible solar modules, respectively.
Thus, these Mo films
serve as the first deposited or back contact layer of the CdTe solar cells in the substrate
configuration. The pseudo-dielectric functions of Fig. 7-12 are calculated directly from
the ellipsometry spectra using an optical model consisting of a single perfect interface
between the ambient and the film.
Thus, the pseudo-dielectric function approaches the
true dielectric function of the film only when the film is opaque and its surface is
atomically smooth and oxide-free. Both oxides and surface roughness on an opaque
film lead to deviations of the pseudo-dielectric function from the true dielectric function.
In addition, semitransparent films also generate strong differences between the two due to
the presence of back reflected light and associated interference fringes.
In the real time SE studies described earlier in this Chapter, the dielectric function
for Mo at room temperature has been established and this has been used in the optical
modeling of the pseudo-dielectric functions in Fig. 7-12.
In fact, the Mo sample used as
a reference was fabricated on a smooth glass substrate and was analyzed using a (surface
roughness)/(semi-infinite bulk) optical model.
Using this reference, the ex situ
ellipsometric spectra of Fig. 7-12 can be analyzed by applying the corresponding optical
model to deduce the microscopic structure of these samples.
139
The results at the left in
Fig. 7-12 are for the Mo film deposited on glass, and reveal an excellent fit to the data
5
Exp. data
fit data
-5
<ε1>
<ε1>
0
-10
Exp. data
fit data
-15
0
84 ± 3 Å Mo/void = 0.54 ± 0.02/0.46 ± 0.02 ds
db
semi-inf
Mo
MSE = 17.9
20
10
608 ± 3 Å Mo/void = 0.49 ± 0.01/0.51 ± 0.01
ds
semi-inf
db
Mo/void = 0.32 ± 0.01/0.69 ± 0.01
MSE = 28.2
<ε2>
<ε2>
30
10
(b)
(a)
0
0
1
2
3
4
5
6
7
PHOTON ENERGY (eV)
0
0
1
2
3
4
5
6
7
PHOTON ENERGY (eV)
Figure 7-12 A comparison of measured pseudo-dielectric functions (solid lines) for Mo
thin films deposited by sputtering (a) on glass and (b) on Kapton. Also shown are the
fits (broken lines) using a reference dielectric function for dense Mo determined
separately, and the multilayer models depicted in the insets.
(broken lines) using a model of bulk Mo (no density deficit relative to the reference) with
a 84 Å thick surface roughness layer on top.
This is consistent with the results for the
reference Mo film which was prepared under similar conditions and exhibited a
roughness thickness of ~ 80 Å, as determined in the analysis of CdTe over-deposition.
The results at the right in Fig. 7-12 are for Mo deposited on Kapton; these results are
considerably different, particularly in terms of the overall magnitude of the dielectric
function at low energies.
energies.
In addition, ε1 does not become negative for this film at low
This suggests that the film lacks conducting channels in the plane of the film
and so the electrons behave as bound electrons at the frequencies of the optical field.
In
fact, a fit to the ellipsometric data suggests a very thick roughness layer (~ 600 Å) which
140
is nearly opaque.
Beneath this layer there appears to be a region of even larger density
deficit (~70%) in the bulk Mo film.
It is not clear whether this density deficit is due to
true voids or to roughness at the interface to the Kapton substrate.
The impact of the
resulting microstructure on solar cell performance is unclear; however, it is evident that
ex situ SE can be used to characterize the substrate metal optical properties and structure
and the effects of the substrate on overlying film optical properties and structure.
7.3.2 Transparent p+ back contact for CdTe solar cells
In order to develop a complete optical model for the CdTe solar cell in the substrate
configuration, it is also necessary to extract the optical properties of the ZnTe p+
semiconductor back contact in a structure that is easier to analyze than the completed
solar cell.
As a result, these studies were performed using ZnTe deposited by magnetron
sputtering directly onto a glass substrate; (Dr. Viral Parikh is acknowledged for the
deposition of this sample).
Doping ZnTe p-type was possible using Cu, and the Cu
content in the ZnTe sputtering target is 1 wt.%.
Figure 7-13 shows the raw ellipsometric
spectra and the best fit that provides the bulk and surface roughness layer thicknesses
(5237 Å and 90 Å, respectively).
In this analysis the dielectric function is modeled as a
sum of four critical point structures, three to match the peaks observed most clearly in ψ
in Fig. 7-13 and the fourth to simulate the gradual absorption onset.
A fifth
Tauc-Lorentz oscillator simulates a broad background absorption centered above 6 eV.
The best fit result is shown in Fig. 7-14 as the solid lines.
141
Handbook data [7-7] for single
crystal ZnTe are also shown in Fig. 7-14 as the points.
Significant differences can be
observed between the results for the doped thin film sample presented here and the
ψ
Ψ (degree)
30
20
10
exp.data
fit
exp.data
fit
0
300
∆ (degree)
200
100
ZnTe:Cusurface roughness 90.4
0
-100
0
1
2
3
PHOTON ENERGY (eV)
±0.9 Å
±1.1 Å
ZnTe:Cu
5236.6
glass
1 mm
4
5
6
PHOTON ENERGY (eV)
Figure 7-13 Ellipsometric spectra (solid lines) and best fit (broken lines) using the
structural model and best fit parameters shown in the inset. The dielectric function is
determined simultaneously using a model assuming a sum of critical point structures.
The resulting dielectric function is shown in Fig. 7-14.
handbook results for the single crystal.
The differences are likely to be due to the heavy
Cu doping as well as the fine-grained polycrystallinity of the thin film.
The three higher
energy critical points at 3.61, 4.15, and 5.27 eV mirror those in the single crystal, but are
consistently broader; however, the band gap critical point expected at ~ 2.2 eV in the thin
film could not be detected in spite of considerable efforts to incorporate it into the model.
Instead, an oscillator at ~ 1.8 eV with a very large width of ~ 1 eV is needed to fit the low
energy spectra.
Thus, the disappearance of the 2.2 eV band gap structure is interpreted
as a true effect, not an artifact of the analysis, since the band gap critical points in
142
7
undoped CdTe-based alloys of similar thickness on glass are easily observable using the
same method of analysis.
As a result, the severely broadened band edge absorption is
interpreted as an effect of the Cu incorporation in the film which is likely to be
significantly greater than typical doping levels in semiconductors.
Tables 7.3 and 7.4
provide the parameterized results for polycrystalline ZnTe:Cu and single crystal ZnTe
assuming four critical point oscillators and one Tauc-Lorentz oscillator, the latter serving
as the broad back ground.
The critical point oscillator expression is given by:
ε = Σn [Anexp(iφn)] [Γn/(2En−2E−iΓn)]µn.
(7.1)
The free parameters include amplitudes, resonance energies, broadening parameters,
phases, and exponents, respectively: (An, En, Γn, φn, µn); n = 1, 2, 3, 4.
For the thin
film ZnTe:Cu, the exponents µn are fixed at the best fit values from the analysis of single
crystal ZnTe data.
Table 7.3 Best fit critical point and Tauc-Lorentz oscillator parameters describing the
inverted dielectric function of polycrystalline ZnTe:Cu. The exponents µn are fixed at
the single crystal values of Table 7.4.
CPPB
An
En(eV)
Γn(eV)
φn
µn
1
4.10 ± 0.42
1.78 ± 0.14
0.96 ± 0.22
−124.34 ± 10.76
0.08
2
2.69 ± 0.06
3.61 ± 0.01
0.72 ± 0.01
−112.82 ± 3.34
2.09
3
11.86 ± 0.67
4.14 ± 0.01
0.55 ± 0.02
45.02 ± 2.92
0.38
4
6.64 ± 0.23
5.27 ± 0.01
1.28 ± 0.02
−19.90 ± 3.10
1.52
oscillator
T-L oscillator
An
En (eV)
1
36.24 ± 11.89
6.35 ± 0.18
Γn (eV)
4.28 ± 0.46
143
Eg (eV)
1.49 ± 0.49
ε1 offset
2.79 ± 0.61
Table 7.4 Best fit critical point and Tauc-Lorentz oscillator parameters for single crystal
ZnTe [7-7].
CPPB
An
En(eV)
Γn(eV)
φn
µn
1
8.05 ± 0.45
2.21 ± 0.03
0.20 ± 0.03
−37.31 ± 9.34
0.08
2
4.85 ± 0.09
3.58 ± 0.01
0.50 ± 0.01
−78.30 ± 3.48
2.09
3
16.09 ± 1.01
4.25 ± 0.02
0.51 ± 0.03
137.85 ± 9.29
0.38
4
3.43 ± 0.20
5.22 ± 0.02
0.77 ± 0.05
−35.46 ± 7.67
1.52
oscillator
T-L oscillator
An
En (eV)
1
219.76 ± 19.98
4.74 ± 0.06
Γn (eV)
2.86 ± 0.06
ε1 offset
-3.64 ± 0.75
Eg (eV)
2.48 ± 0.06
Figure 7-14 Dielectric function of thin film ZnTe:Cu prepared by magnetron sputtering
with 1 wt.% Cu in the ZnTe target (solid lines). A model consisting of four critical
points in the band structure has been used in this analysis. The data points are literature
results for single crystal ZnTe [7-7].
7.3.3 Optical analysis of the CdTe solar cell in the substrate configuration
In the optical analysis of the CdTe solar cell in the substrate configuration, a device
is studied here that is complete with the exception of the top contact; (Dr. Anthony Vasko
is acknowledged for deposition of this sample, and Dr. Jian Li is acknowledged for
144
measurement assistance).
Thus, the top surface from which the light beam reflects is
CdS, which is relatively rough. After the CdS deposition, this solar cell was treated
with CdCl2 at a temperature of 387 °C for a duration of 30 min. In order to analyze the
structure of the solar cell, a reference set of dielectric functions is adopted including CdS
obtained at room temperature using in situ SE for a film prepared in the same sputtering
chamber at a substrate temperature of 310 °C, a rf power level at the target of 50 W, an Ar
pressure of 10 mTorr, and an Ar flow of 23 sccm.
The spacing between the target and
the substrate was ~ 10 cm and the substrate was a smooth c-Si substrate for ease of
analysis.
Single crystal CdTe was also used as a reference, as obtained from real time
SE of a film prepared by molecular beam epitaxy in a literature study
[7-8]
. The two
other dielectric functions include that of ZnTe:Cu, obtained as described above (see Figs.
7-13 and 7-14), and Mo, obtained in the real time SE study of CdTe deposition on
Mo/glass.
In the latter analysis, correction was made for the surface roughness layer on
the Mo film.
A step-by-step analysis, in which one free parameter is introduced at a time, was
developed specifically for ex situ studies of CdTe solar cells.
As each parameter is
introduced, a measure of the quality of the fit is observed, with the goal being to increase
the complexity of the optical model by incorporating additional thicknesses and
compositions, and in this way, determine which parameters are most important for a high
quality fit and which parameters are unnecessary.
Figure 7-15 shows the step-by-step reduction in the mean square error (MSE)
145
obtained by adding best fitting parameters one at a time. The MSE is expressed in terms
of the standard deviations of the real and imaginary parts of ρ ≡ tanψ exp(i∆).
Figure
7-16 shows the experimental spectroscopic ellipsometry data for the CdTe solar cell on
the Mo surface in the substrate configuration.
The data from 0.75 to 2.5 eV are shown
on an expanded scale relative to those over the range from 2.5 to 6.5 eV.
The fringe
pattern over the range from 0.75 to 1.5 eV provides information on the CdTe thickness
and the modulation of these fringes provides information on the optical properties and
thicknesses of the materials underneath the CdTe.
The single fringe over the range from
1.5 to 2.3 eV provides information on the CdS thickness and the modulation of this fringe
provides information on the underlying (opaque) CdTe.
information on the critical points of the CdS.
The data above 2.5 eV provides
Even very small details of the cell
structure can be inferred from the raw data, as described next.
First, the critical points of the CdS are broadened compared to the film deposited on
crystal Si from which the reference data were obtained.
This broadening may be due to
oxidation at the surface as a result of the CdCl2 treatment. Alternatively, the effect may be
due to a smaller grain size in the CdS when it is deposited on a CdTe thin film as
compared to when it is deposited on bulk crystal Si.
interdiffusion of the CdS and CdTe.
Finally, the effect may be due to
A second observation is that the CdS band gap
appearing in the data is lower than that in the model, suggesting either interdiffusion or
differences in strain between the reference film deposited on crystal Si and the solar cell
of Fig. 7-16 deposited on CdTe. In spite of these small deviations, a reasonably good fit
146
(solid line) can be obtained using the model shown in Fig. 7-17.
It should be
emphasized that this represents a first attempt at analyzing solar cells in the substrate
configuration and improved results are expected by breaking the structure down and
developing optical properties more relevant to the structure.
For example, the optical
properties of the CdS can be obtained from a single-layered sample that has been exposed
to the same CdCl2 treatment as the solar cell.
0.8 1
2
3
4
6
5
7
9 10 11 1213 14
8
number of fitting parameters
MSE/1000
0.6
0.4
0.2
Step-by-step MSE reduction
0
10
20
30
40
Steps
50
60
70
80
Figure 7-15 Step-by-step MSE reduction by adding one fitting parameter at a time.
Starting with the CdTe thickness as a variable, each additional parameter was
subsequently fitted. It was found that fitting the CdS thickness provided the greatest
improvement in MSE among all 2-parameter attempts. Similar methodology was used for
all 14 parameters. Circles connected by the solid line indicate the best n-parameter fit
with n given at the top and the added parameter given in Table 7.5.
147
Exp. data
fit
Ψ (degree)
40
30
20
10
Exp. data
fit
0
∆ (degree)
300
200
100
0
-100
0.5
1.0
1.5
2.0
3
4
5
6
7
PHOTON ENERGY (eV)
PHOTON ENERGY (eV)
Figure 7-16 Ellipsometric spectra for a CdTe solar cell deposited on Mo in the substrate
configuration (points). The cell was exposed to a CdCl2 treatment before this
measurement. The top contact of the solar cell is not incorporated over the area probed,
leading to the structure: ambient/CdS/CdTe/ZnTe:Cu/Mo. The solid line depicts the
optical model shown in Fig. 7-17.
The fitting parameter sequence in Table 7.5 is understandable in that the thickest
layers have the greatest impact in the step-by-step fitting procedure.
When the initial
estimate of a fitting parameter is close to its best fitting result, this fitting parameter will
be added into the model at a lower priority in the sequence, an example being the ZnTe
thickness.
The results provide information on the ZnTe thickness and void fraction, the
ZnTe/Mo interface thickness, its Mo volume fraction, as well as the CdS thickness, and
148
void fraction, the combined CdS/CdTe interface roughness-interaction thickness, its CdTe
fraction, and the CdTe thickness, and void fraction, all shown in Fig. 7-17.
Also
determined is the percent non-uniformity, which describes the thickness distribution over
the area of the probe beam.
The following comments can be made regarding the deduced structure of Fig. 7-17.
The high void fraction in the Mo substrate is consistent with the study of individual such
layers as noted above.
Apparently these voids are not completely filled in by the
overlying deposition of ZnTe:Cu. The model is not able to discern roughness at the
CdTe/ZnTe:Cu interface, not because it does not exist, but rather, because the optical
properties of the two materials do not exhibit sufficient contrast to detect it.
The ~1300
Å thick interface layer between the CdS and CdTe is attributed to a combination of
interface roughness and alloying.
The volume fractions in the layers of the structure
also convey some information; however, some results require closer scrutiny.
The CdTe
(3% void) of this substrate solar cell structure appears to be similar in density to that
typically observed in CdCl2 treated superstrate cells (typically 1-3% voids). The CdS in
the substrate cell appears to be denser than that in superstrate cells.
Finally, the low
volume fraction of Mo in the Mo/ZnTe interface layer seems incongruous in view of the
higher Mo fraction in the surface layers of the bare Mo film as shown in Fig. 7-12.
result needs to be studied in greater detail in future analyses.
This
Generally such difficult
analyses are a work in progress -- like the cell itself -- and will improve as more is
learned about the optical properties and microstructure.
149
For example, a three
component effective medium approximation may be needed to describe the ZnTe:Cu/Mo
interface, consisting of Mo, ZnTe:Cu, and finally void.
In this way, voids trapped at the
substrate interface can be quantified.
Table 7.5 Best fitting parameters added step by step to improve the standard mean
square error (MSE) in the ellipsometric analysis of a CdTe solar cell in the substrate
configuration.
# of fitting parameters
Best fitting parameter added to
improve MSE
Standard MSE
1
CdTe thickness
764.2
2
CdS thickness
475.7
3
Mo void volume fraction
214
4
CdS roughness thickness
(50/50)
113.3
5
ZnTe/Mo interface thickness
(50/50)
86.05
6
CdTe void volume fraction
67.84
7
Mo volume fraction in ZnTe
53.02
8
CdS roughness void volume
fraction
40.05
9
CdTe non-uniformity
36.4
10
CdS/CdTe interface thickness
(50/50)
35.54
11
CdS void volume fraction
34.93
12
CdTe volume fraction in
CdS/CdTe interface
34.33
13
ZnTe void volume fraction
33.95
14
ZnTe thickness
33.87
150
CdS surface roughness thickness
Surface void fraction
159 ± 5 Å
0.25 ± 0.01
CdS thickness
Void volume fraction
2433 ± 9 Å
0.007 ± 0.003
CdS/CdTe interface thickness
CdTe volume fraction
1348 ± 20 Å
0.835 ± 0.009
CdTe thickness
Void volume fraction
CdTe thickness non-uniformity
13715 ± 66 Å
0.030 ± 0.003
3.8% ± 0.2%
CdTe/ZnTe interface thickness
0
ZnTe thickness
Void volume fraction
1120 ± 114 Å
−0.026 ± 0.011
ZnTe/Mo interface thickness
Mo volume fraction
293 ± 63 Å
0.195 ± 0.061
Mo void volume fraction
0.395 ± 0.027
Figure 7-17 Optical model for a CdTe solar cell in the substrate configuration
(excluding the top contact) deposited on a Mo film surface. This model and the best fit
parameters provide the solid line results in Fig. 7-16.
151
Chapter Eight
Spectroscopic Ellipsometry Studies of II-VI Alloy Films
8.1 Introduction
In the single junction superstrate solar cell configuration, the highest efficiency for a
CdTe device prepared by sputtering is 14%
[8-1]
and the corresponding result for
close-spaced sublimation is 16.5% [8-2]. The latter champion solar cell has the following
characteristics: VOC = 845 mV, JSC = 25.9 mA/cm2, and FF (fill factor) = 75.5%.
The
current-voltage (J-V) and quantum efficiency (QE) curves of the champion cell are
shown in Figure 8-1.
Figure 8-1 Current-voltage and normalized quantum efficiency spectra for a champion
16.5% efficient CdTe/CdS thin-film solar cell [8-2].
152
In order to improve on these cell efficiencies, materials and devices for tandem cell
structures have been investigated in the research laboratory
[8-3]
.
Instead of using a
single junction device based on CdTe with a near-optimum band gap of 1.5 eV, the top
cell absorber material of the tandem must have a wider band gap, and the bottom cell
absorber material must have narrower band gap.
A practical conversion efficiency of
25% has been predicted for a two-junction two-terminal polycrystalline thin-film tandem
cell with energy band gaps of 1.14 eV for the bottom cell and 1.72 eV for the top cell [8-4].
CdTe-based ternary alloy materials, such as Cd1-xMgxTe and Cd1-xMnxTe are attractive
due to the flexibility of controlling their band gaps through the molar composition x, and
as a result, these materials have been considered as wide band gap top cell candidates for
such tandem cells as shown in Fig. 8-2.
In addition to the II-VI alloys with wide band
gaps, the alloy Cd1-xHgxTe is a possible narrow band gap bottom cell candidate.
Cd1-xHgxTe is flexible enough to tailor the band gap from −0.15 eV for x = 1, i.e.
semimetallic, to 1.5 eV for x = 0 [8-5].
Spectroscopic ellipsometry is an excellent non-contacting technique for investigating
thin-film semiconductor optical properties, electronic structure, and surface and bulk
microstructure. This technique has been applied for materials evaluation in order to
explore the opportunities and identify the potential difficulties in the fabrication of II-VI
materials for top cells in two-junction devices with either monolithic two-terminal (see
Fig. 8-2) or mechanically stacked four-terminal structures.
In this research, two top cell
materials, Cd1-xMnxTe and Cd1-xMgxTe, the latter with a band gap as wide as 1.98 eV,
153
were measured.
These films were obtained by sputtering in order to assess their
suitability in tandem PV devices with a Cd1-xHgxTe bottom cell.
The widest band gap
Cd1-xMgxTe was obtained from a target of 60 wt.% CdTe and 40 wt.% MgTe.
Also in
this research, Cd1-xHgxTe thin films with a band gap variation from 0.81 eV to 1.58 eV
were measured.
These films were obtained by sputtering using a target of 60 wt.%
CdTe and 40 wt.% HgTe through a variation of the deposition temperature.
glass
ZnO
CdS
Cd1-xMgxTe
ZnTe
ZnO
CdS
Cd1-xHgxTe
Cu/Au
Figure 8-2
Two-terminal tandem cell based on Cd1-xMgxTe and Cd1-xHgxTe absorbers.
8.2 Top cell material candidates: Cd1-xMnxTe and Cd1-xMgxTe
8.2.1 Cd1-xMnxTe and Cd1-xMgxTe preparation
The Cd1-xMnxTe and Cd1-xMgxTe films described in this section were magnetron
sputtered on soda-lime glass substrates from targets fabricated from 87 wt. % CdTe and
13 wt. % MnTe, and from 80 wt. % CdTe and 20 wt. % MgTe, respectively; (Viral Parikh
is acknowledged for the deposition of these samples).
A first estimate of the
composition of the as-deposited alloys films was made on the basis of the optical
154
absorption edge determined from the transmission spectra.
The Cd1-xMnxTe film
thickness was typically about 1 µm; Cd1-xMgxTe films were thinner -- about 0.2 µm.
The deposition conditions for CdxMg1-xTe are shown as the first two entries of Table 8.1.
Table 8.1
films.
Deposition parameters used to prepare the CdxMg1-xTe and CdxHg1-xTe thin
CdTe
CdxMg1-xTe
(20 wt.% MgTe)
CdxMg1-xTe
(40 wt.% MgTe)
CdxHg1-xTe
CdxHg1-xTe
CdxHg1-xTe
CdxHg1-xTe
CdxHg1-xTe
CdxHg1-xTe
Rf power (W)
20
Pressure (mTorr)
18
Substrate temp (°C)
250
50
20
200
50
5
290
27
27
27
27
27
27
10
10
10
10
10
10
23
44
70
85
97
153
A CdCl2 post-deposition treatment was performed as an important step in fabricating
solar cells using the alloys as the active layers.
Several effects of the CdCl2 treatment
are believed to enhance the solar cell performance of the alloy films, including relaxing
the strain, increasing the grain size, improving the alloy/CdS interface, and reducing the
lattice mismatch there [8-6].
For evaluation purposes, the CdCl2 treatment was performed
on a 2 cm × 3 cm piece of each sample placed in a 2.5 cm diameter quartz tube. The
CdCl2 source was fabricated by forming a saturated methanol solution of the chloride and
evaporating it from the surface of a heated glass plate.
155
The sample was placed above
the source plate with the film side facing the plate and a 1 mm gap between the two.
A
typical 30 minute CdCl2 treatment was performed on the Cd1-xMgxTe films at a
temperature of 387°C.
were evaluated.
To treat the Cd1-xMnxTe films, two post-deposition approaches
In one approach, the CdCl2 vapor treatment was carried out on the
films under the same conditions as for Cd1-xMgxTe.
In the other, a two-step process was
applied in which a high temperature annealing step was carried out at 520°C for 10
minutes under 2% H2/Ar, followed by the standard CdCl2 vapor treatment at 385°C for 30
minutes in dry air.
In order to compare the optical results before and after CdCl2 treatment, pure CdTe
films of more than 1 µm in thickness were magnetron sputtered onto soda-lime glass at
250°C.
For these CdTe films, a CdCl2 treatment in dry air ambient was performed at a
temperature of 387°C with different times optimized depending on the film thicknesses.
8.2.2 Data analysis and results
A rotating compensator multichannel spectroscopic ellipsometer with a 0.75 - 6.5 eV
photon energy range was used to investigate the optical properties of the as-deposited and
annealed films.
The information extracted from SE measurements is very useful for
assessing the surface and bulk characteristics of the samples.
As-deposited Cd1-xMgxTe and Cd1-xMnxTe optical properties
Figure 8-3(a-b) shows the pseudo-dielectric functions of RF magnetron sputtered
CdTe, Cd1-xMnxTe, and Cd1-xMgxTe films in the as-deposited state. The thicknesses of
156
CdTe, Cd1-xMnxTe and Cd1-xMgxTe films are 1.41 µm, 1.0 µm and 0.18 µm, respectively.
The band gaps of 1.63 eV for the Cd1-xMnxTe film and 1.61 eV for the Cd1-xMgxTe film
were estimated from optical transmission measurements.
These two alloy films as well
as the CdTe film are transparent below their band gaps, and the spectral density of
interference fringes in the lower energy range of ~ 0.75 eV to 2.0 eV scales with the film
thickness.
In the high energy range of 2.0 eV to 6.5 eV, features are observed
corresponding to the higher energy band gaps at the critical points (CPs) in the joint
density of states.
The nature of these CPs and the information that can be extracted
from them will be discussed shortly.
A comparison of pseudo-dielectric functions over the high energy range for the
Cd1-xMnxTe film of Fig. 8-3(a-b) after Br2/methanol etch and after selected times of long
term laboratory storage is given in Fig. 8-3(c).
The freshly-deposited sample exhibits
higher amplitudes in < ε > than a sample that has been stored for a period of time. The
data for the Cd1-xMnxTe sample in Figure 8-3(a-b) were taken one week after film
deposition.
Thus, it is reasonable to interpret the relatively low dielectric function
amplitudes of the stored samples to surface oxidation.
By tracking <ε2 > values during
Br2/methanol chemical etching processes, one can develop an optimum procedure to
remove the oxide, and achieve the most abrupt interface to the ambient as this leads to
maximum <ε2 >.
For the results in Figure 8-3(c), the E1, E1 + ∆1, and E2 critical-point
structures can be seen clearly in all spectra. The sample measured immediately after
etching by Br2/methanol chemical solution showed the highest amplitudes of the
157
pseudo-dielectric function in the higher energy region.
Fig. 8-3(c) are in accord with those of epitaxial films
In fact, the maximum values in
[8-7]
and bulk crystals
[8-8]
.
Thus,
the pseudo-dielectric function after etching is expected to be very close to true dielectric
function with only small deviations due to residual surface roughness or a thin Te-rich
surface layer generated by the etching process.
16
8
CdTe
CdTe
12
8
0
4
-4
0
1
2
3
4
5
1
6
16
CdMnTe
Cd
1-xMnxTe
8
4
<ε<2e>2 >
<ε
<e
>
1>
1
2
3
4
5
6
8
12
4
Cd1-x
CdMnTe
MnxTe
0
-4
0
16
CdTe
CdTe
4
1
2
3
4
5
1
6
2
3
4
5
6
8
12
Cd
CdMgTe
1-xMgx Te
4
8
CdCdMgTe
1-xMgxTe
0
4
-4
0
1
2
3
4
5
6
1
2
3
4
(a)
12
12
3
4
E2
2
6
4
1
2
2
3
0
0
-2
E2
8
E 1+ ∆ 1
6
E 1 E 1+ ∆ 1
10
< ε 2>
< ε 1>
8
6
(b)
E1
1
2
10
5
Energy (eV)
Energy (eV)
-2
2
3
4
5
6
Energy (eV)
2
3
4
5
Energy (eV)
6
(C)
Figure 8-3 Real (a) and imaginary (b) parts of the pseudo-dielectric functions of RF
sputtered CdTe (Eg = 1.50 eV), Cd1-xMnxTe (Eg = 1.63 eV) and Cd1-xMgxTe (Eg =
1.61 eV) films all in the as-deposited state; (c) Pseudo-dielectric function of as deposited
Cd1-xMnxTe samples after different storage times in laboratory ambient: (1) immediately
after Br2/methanol etch; (2) 3 weeks after deposition; and (3) 1.5 years after deposition.
158
Once the maximum value of <ε2> is obtained through etching, the resulting spectra as in
Fig. 8-3(c) (immediately after etch), interpreted as an approximation to the true dielectric
function, can be further interpreted through CP analysis. Although the imaginary part of
the dielectric function is most closely related to the absorptive behavior of films and thus
the joint density of electronic states, however, both real and imaginary parts encode this
information due to the Kramers-Kronig relationships.
The fundamental and higher band
gap energies determined from the CP features provide information on the alloy
composition and strain whereas the broadening energies of the CP features provide
information on the crystalline grain size arising from the polycrystalline structure. The
band structure parameters of a single CP, including the band gap and broadening energies
can be deduced from both parts of ε(E) by fitting to a standard analytic line shape [8-9]
ε ( E ) = Aeiφ Γ µ / [(2 E − 2 E0 + iΓ) µ ] ,
(8-1)
where A is the CP amplitude, Γ and φ are the Lorentzian broadening energy and phase
angle, and E0 and µ are the threshold energy and exponent, the latter defined by the
nature of the singularity in the electronic joint density of states. These parameters are
readily determined by fitting second-derivative spectra d2ε(E)/dE2.
For the ternary alloy
system, once relationships have been established between the molar composition x and
the CP energies in the band structure, as determined from the dielectric function ε(E),
usually from studies of single crystals, such relationships can be used to estimate the
composition of any unknown alloy
[8-9,8-10]
.
Figure 8-4 shows the experimental
second-derivative spectra in the pseudo-dielectric function <ε(E)> of an as-deposited
159
Cd1-xMnxTe sample, along with best fit results obtained using Eq. (8-1).
2
2
2
2
d < ε 1 >/d ω
100
0
2
d < ε >/ d ω
2
d < ε 2 >/d ω
E 2 (5.033 eV)
E 1 + ∆ 1 (3.884 eV)
E 1 (3.352 eV)
-100
2
3
4
5
Energy (eV)
6
Figure 8-4 Best fit (lines) to the second derivative of the experimental pseudo-dielectric
function (points) for the as-deposited Cd1−xMnxTe film of Fig. 8-3 (c: immediately after
etch). The three CP transitions, E1, E1 + ∆1, and E2, are indicated by arrows with best fit
energies of 3.352, 3.884, and 5.033 eV, respectively. The composition of x=0.06 can be
estimated by the empirical relationship between E1, the strongest CP in this case, and the
composition [8-7].
Returning to the results of Fig. 8-3(c), very large changes in the pseudo-dielectric
function induced by sample exposure to laboratory ambient can be observed by SE.
This effect can be explored in greater detail by RTSE.
Figure 8-5 shows the continuous
variation in the pseudo-dielectric function of the 3-week-old Cd0.94Mn0.06Te sample
immediately after Br2/methanol etching and upon exposure to air.
For such
measurements, the native oxide layer was removed by 0.01-0.02% Br2/methanol in a few
seconds of etching time.
Upon exposure of the resulting clean sample to air, the
pseudo-dielectric function does not change much during the initial several minutes;
however, with increasing time both real and imaginary parts of the pseudo-dielectric
function gradually decrease.
In fact, the imaginary part of the pseudo-dielectric function
near the E1 + ∆1 CP energy (~ 3.88 eV) decreased by 5% over a one hour period.
160
Time increasing
14
< ε 1>
12
10
8
6
4
min
min
min
min
min
min
min
12
10
Tim e increasing
0.5
3.5
6.5
9.5
18
38
58
16
8
0.5
3.5
6.5
9.5
18
38
58
6
4
< ε 2>
18
2
0
-2
2
m in
m in
m in
m in
m in
m in
m in
-4
0
-6
-2
-8
1
2
3
4
Energy (eV)
5
6
1
2
3
4
Energy (eV)
5
6
Figure 8-5 Variation of the pseudo-dielectric function of as deposited Cd0.94Mn0.06Te
with time after Br2/methanol etching, measured in situ at room temperature during
exposure to laboratory ambient.
20
16
As-deposited
One-step
o
385 C 30 min with CdCl2
12
then 385 C 30 min with CdCl2
Two-step
o
first 520 C 10 min in H2,
o
16
As-deposited
One-step
o
385 C 30 min with CdCl2
12
Two-step
o
first 520 C 10 min in H2,
o
then 385 C 30 min with CdCl2
< ε2 >
20
<ε1>
8
8
4
4
0
0
-4
1
2
3
4
5
6
1
Energy (eV)
2
3
4
Energy (eV)
5
6
Figure 8-6 Pseudo-dielectric functions of as-deposited and one-step and two-step CdCl2
treated Cd0.94Mn0.06Te samples.
Optical properties of CdCl2-treated and Cd1-xMnxTe and Cd1-xMgxTe
Figure 8-6 shows the pseudo-dielectric function of as-deposited (3-week-old) and
annealed Cd0.94Mn0.06Te samples.
Using the dielectric functions obtained from the
Br2/methanol-etched Cd0.94Mn0.06Te sample and assuming Te oxide (TeO2) on the surface,
the latter shown in Fig. 8-7
[8-11]
, a simple 3-layer model of oxide/Cd0.94Mn0.06Te/glass
could be employed to fit the experimental results for the as-deposited sample. The
161
thickness of TeO2 layer was found to be ~ 35 Å.
For the annealed sample, however, it
was difficult to fit the experimental data due to lack of reference dielectric functions for
the surface layer components.
In particular, for the sample that was vapor treated with
CdCl2 at 385°C, the pseudo-dielectric function was much different from that of the
as-deposited samples.
It was further observed that even after the treated films were
etched using several etching steps of 0.04 volume % Br2 in methanol, the
pseudo-dielectric function showed much different spectral behavior from that of the
as-deposited sample.
This suggests that the top layer is substantially modified during
treatment, both chemically and morphologically; however, there remains the possibility
that the behavior is due to a thick metallic oxide generated during treatment that does not
respond to the Br2 methanol etch in the same way as the native oxide.
Figure 8-7
Index of refraction and extinction coefficient of amorphous TeO2.
162
20
20
As-deposited
o
387 C 30 m in CdCl2 treated
16
As-deposited
o
387 C 30 m in CdCl2 treated
16
12
< ε 2>
< ε 1>
12
8
8
4
4
0
0
-4
1
Figure 8-8
samples.
2
3
4
Energy (eV)
5
6
1
2
3
4
Energy (eV)
5
6
Pseudo-dielectric functions of as-deposited and CdCl2 treated Cd1-xMgxTe
Figure 8-8 shows the pseudo-dielectric function of as-deposited and CdCl2-treated
Cd1-xMgxTe samples. For the CdCl2-treated Cd1-xMgxTe sample, much less deterioration
in the bulk optical characteristics is observed compared with CdCl2 treated Cd1-xMnxTe,
suggesting that the CdCl2 treatment is effective for Cd1-xMgxTe PV devices.
Most
importantly, the critical point structure of the Cd1-xMgxTe is retained upon treatment with
a clear narrowing of the widths.
Dielectric functions of as-deposited and CdCl2 treated samples over the energy range
of 3.0 ~ 6.0 eV are accumulated in Fig. 8-9 (a-c).
For these measurements, the films
were previously etched using one or more steps, each consisting of brief immersion in a
0.04 volume % Br2 in methanol solution.
The original intent of this process was to
remove oxides that are observed to develop on as-deposited and CdCl2-treated CdTe
films due to their exposure to the laboratory and treatment ambients.
Interestingly, for
CdTe it has been found that successive etching steps lead to a significant step-wise
smoothening of the film surface simultaneously with decreasing bulk layer thickness due
163
to step-wise film dissolution. In fact, a roughness layer of thickness of up to a micron or
more can be eliminated in several successive etching steps, and ultimate stabilization of
the roughness thickness at ~20-40 Å can be observed.
It is under stable, smooth-surface
conditions that the measurements on CdTe of Fig. 8-9(a) are made.
Under these
conditions, the dielectric function deduced from the measured ellipsometry spectra is
reasonably representative of the true dielectric function, enabling determination of the
critical point energies and widths by dielectric function fitting.
etching treatments lead to a Te-rich surface layer (~10 Å)
It is known that the
[8-12]
; however, its effect is
expected to be smaller than that of the residual roughness and has been neglected in this
study.
Figure 8-9(c) compares the approximate dielectric function of Br2/methanol-etched
Cd1-xMgxTe in the as-deposited (left) and CdCl2-treated (right) states. This Cd1-xMgxTe
film was sputter-deposited to a thickness of 0.18 µm on a soda-lime glass slide.
The
results in Fig. 8-9(c) may differ somewhat from the true dielectric function due to the
presence of the residual roughness and a Te-rich surface layer. The key observation in
the comparison of the panels of Fig. 8-9(c) is that noted earlier in Fig. 8-8, namely, the
critical points E1, E1+∆1, and E2 are clearly observed at similar energy positions in both
sample states.
effects.
Any observed shifts may be due to incomplete accounting of surface
In fact, all critical points become sharper upon CdCl2 treatment. This is an
indication that the composition of the film is retained upon treatment and that the
crystalline grain size increases significantly, as well.
164
For comparison, in Fig. 8-9(a),
which depicts the corresponding results for CdTe (i.e., with no alloying), similar behavior
is observed.
In contrast, for Cd1-xMnxTe in Fig. 8-9(b), the CdCl2 treatment leads to a
complete loss of the critical point structures and these cannot be recovered by continued
etching.
This demonstrates that the treatment leads to a significant chemical
modification of the Cd1-xMnxTe film, most likely phase segregation and oxidation of the
Mn, which can account for its poor performance when incorporated into actual devices.
The fact that the Cd1-xMgxTe does not experience such a modification upon CdCl2
treatment and retains the band structure characteristics of the as-deposited film (along
with a significant increase in grain size) has demonstrated its promise for the
development of devices from this material.
Quantitative information can be obtained from fits to the approximate dielectric
functions of Figs. 8-9(a) and 8-9(c) which provide the energy positions and widths of the
dielectric function peaks.
These results are given in Fig. 8-9 as the solid lines for all
films except for the CdCl2-treated Cd1-xMnxTe in which case the critical point structure is
lost.
The critical point parameters including the energy positions Ej and widths Γj are
presented in Table 8.2.
Among the key observations of Table 8.2 include:
(i) retention of the critical point structure for Cd1-xMgxTe upon CdCl2 treatment
without a significant change in the energy positions (in consideration of surface
variations), indicating success of alloying in the CdCl2-treated films;
(ii) reduction of the critical point transition widths upon CdCl2 treatment for the
CdTe and Cd1-xMgxTe, an indication of an increase in grain size or a reduction in defect
165
density;
(a)
(b)
(c)
Figure 8-9 Approximate dielectric functions, i.e., optical properties deduced with a best
attempt to eliminate surface effects, for as-deposited films and CdCl2-treated films
obtained by SE after Br2+methanol etching that improves the surface quality (points); (a)
CdTe; (b) Cd1-xMnxTe; (c) Cd1-xMgxTe; the solid lines show the results of fits to extract
critical point energies and widths. The result for the CdCl2-treated Cd1-xMnxTe could
not be fit with a critical point parabolic band model.
166
(iii) increase of the critical point transition widths for the as-deposited alloys
compared with the as-deposited CdTe, possibly due to a smaller grain size in the
as-deposited alloy films; and
(iv) similar critical point widths for the CdCl2-treated CdTe and Cd1-xMgxTe,
indicating the effectiveness of the treatment in improving the alloy.
Table 8.2 Critical point parameters of transition energy and width obtained in the fits to
the dielectric functions of Fig. 8-9.
E0 (eV)
E1 (eV)
Γ(Ε1) (eV)
E1+∆1 (eV)
Γ(E1+∆1) (eV)
E2 (eV)
Γ(E2) (eV)
CdTe
as-dep.
1.497
3.274
0.411
3.844
0.484
5.193
0.993
CdTe
CdCl2-treat.
1.499
3.331
0.200
3.883
0.368
5.208
0.796
Cd1-xMgxTe
as-dep.
1.615
3.354
0.480
3.901
0.520
5.179
1.252
Cd1-xMgxTe
CdCl2-treat.
1.633
3.303
0.216
3.878
0.309
5.197
0.879
Cd1-xMnxTe
as-dep.
1.548
3.363
0.430
3.914
0.483
5.182
1.215
Cd1-xMnxTe
CdCl2-treat.
---------------
Furthermore, two additional Cd1-xMgxTe samples prepared by magnetron sputtering
from alloy targets were studied in detail by ex situ SE.
The goal of this study was to
compare the previously-described as-deposited film prepared with low Mg content and
band gap of Eg = 1.615 eV with those prepared under conditions leading to higher Mg
content. This comparison was performed on as-deposited films without CdCl2 treatment.
For the sample labeled CGT42, the target was CdTe (80 wt.%) + MgTe (20 wt.%), and
Cd1-xMgxTe deposition was performed on a soda lime glass substrate at a temperature of
200°C using 50 W rf power at the target, 20 mTorr Ar pressure, and 30 sccm Ar flow.
167
The deposition time was 2 hours, and the final Cd1-xMgxTe film thickness was ~ 0.3 µm.
For the much higher Mg content sample labeled CGT92, the target was CdTe (60 wt.%) +
MgTe (40 wt.%), and Cd1-xMgxTe deposition was performed on an aluminosilicate glass
substrate at a temperature of 290°C using 50 W rf power at the target, 5 mTorr Ar
pressure, and 30 sccm Ar flow.
In this case, the deposition time was 6 hours, and the
Cd1-xMgxTe film thickness was ~ 2.9 µm.
Spectroscopic ellipsometry was performed on these two samples at angles of
incidence of 60° and 65°, respectively.
0.74~6.50 eV.
The photon energy range was standard:
A two-layer surface-roughness/bulk model for the film was used to
analyze the experimental data (ψ, ∆). The dielectric function of the bulk layer was
modeled using a sum of critical point oscillator terms, each of the form of Eq. (8-1),
given by
ε = εTL + Σ 4j=1 [Ajexp(iφj)][Γj/(2Ej − 2E − iΓj)]µj.
(8-1)
Here oscillators labeled j = 1, 2, 3, 4 correspond to the E0 (band gap), E1, E1+∆1, and E2
critical point transitions. An additional oscillator, denoted εTL, is modeled using the
Tauc-Lorentz expression in order to simulate a broad background in ε.
Each of the
critical point oscillators has five free parameters: amplitude Aj, energy Ej, width Γj, phase
φj, and exponent µj. In this analysis, the focus is on the critical point energies Ej and the
width Γ0 of the lowest band gap critical point.
168
12
CdMgTe
42
Cd
1-xMgxTe
<ε1>
CGT42
8
<ε1, ε2>
<ε2>
4
ambient
0
roughness
CdMgTe
-4
ds = 94 Å
db = 2908 Å
glass
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 8-10 Pseudo-dielectric function obtained directly from experimental
(ψ, ∆) data using a single interface conversion formula for a Cd1-xMgxTe sample
prepared from a target of CdTe (80 wt.%) + MgTe (20 wt.%) (CGT42). The
solid line describes experimental data and the dashed line describes the best fit
result. The deduced bulk and surface roughness layer thicknesses are shown.
E0 = 1.71 eV , ΓE0 = 0.13 eV
8
CdMgTe
42
Cd
1-xMgxTe
ε1
CGT42
ε1, ε2
ε2
4
0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 8-11 Best fit analytical dielectric function obtained from an analysis of
the experimental (ψ, ∆) data for the Cd1-xMgxTe sample of Fig. 8-10 prepared
from a target of CdTe (80 wt.%) + MgTe (20 wt.%) (CGT42).
The energies can provide information on the band gaps, alloying, and strain, whereas
the width of the E0 critical point provides information on defects, grain size, and disorder.
169
Figure 8-10 shows the pseudo-dielectric function data for the lower Mg content
Cd1-xMgxTe sample (CGT42) and the best fit to these data. Figure 8-11 shows the true
dielectric function of this Cd1-xMgxTe film which is extracted in the best fit.
deduced band gap, 1.71 eV, and the broadening parameter is 0.13 eV.
The
This band gap
corresponds to a molar composition of x = 0.15 using the relationship established
previously
[8-7]
.
Optical transmission spectroscopy yielded a band gap value in
agreement with the SE result.
8
CdMgTe 92
Cd1-xMgxTe
< ε1, ε2 >
<ε1>
<ε2>
CGT92
4
ambient
ds = 137 Å
roughness
0
CdMgTe
db = 28887 Å
glass
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 8-12 Pseudo-dielectric function obtained directly from experimental
(ψ, ∆) data using a single interface conversion formula for a Cd1-xMgxTe sample
prepared from a target of CdTe (60 wt.%) + MgTe (40 wt.%) (CGT92). The solid
line describes experimental data and the dashed line describes the best fit result.
The deduced bulk and surface roughness layer thicknesses are shown.
170
CdMgTe 92
Cd1-xMgxTe
E0 = 1.98 eV, ΓE0 = 0.16 eV
ε1
ε1, ε2
8
ε2
CGT92
4
0
0
1
2
3
4
5
6
7
Photon Energy (eV)
Figure 8-13 Best fit analytical dielectric function obtained from an analysis of
the experimental (ψ, ∆) data for the Cd1-xMgxTe sample of Fig. 8-12 prepared
from a target of CdTe (60 wt.%) + MgTe (40 wt.%) (CGT92).
Figures 8-12 and 8-13 show the corresponding results for the higher Mg content
sample (CGT92).
0.16 eV.
The band gap of this film is 1.98 eV, and the broadening parameter is
The band gap corresponds to a composition [8-7] of x = 0.30, indicating a linear
relationship between film molar and target wt. % composition for CdTe and the two alloy
samples with a slope of 0.0075/wt.% MgTe.
Table 8.3 shows the values of the fundamental gap energy E0 and its width, as well
as the energies of the higher energy critical points E1, E1+∆1, E2, for the two Cd1-xMgxTe
samples.
Also shown for comparison are the corresponding results for pure CdTe before
and after the CdCl2 treatment. The shifts in the energies of critical points upon CdCl2
treatment for CdTe in this case are due to relaxation of strain and the narrowing of the E0
peak is due to an increase in grain size.
It is clear that the E0 band gap increases as the
target alloy composition increases; the use of 20 wt.% MgTe in the target does not lead to
171
significant broadening of the E0 transition relative to pure untreated CdTe.
However, 40
wt.% MgTe leads to a significant broadening effect as a result of either a smaller grain
size or increased disorder in the film due to alloying. The non-monotonic behavior
observed in the E1 and E1+∆1 higher energy gaps with alloying are likely to be due to
changes in electronic structure as well as to strain in the films.
Table 8.3 Critical point energies and E0 broadening parameters for two
as-deposited Cd1-xMgxTe alloys from spectroscopic ellipsometry. Also shown
are corresponding results for as-deposited and CdCl2-treated CdTe.
E0 (eV)
Γ(E0) (eV)
E1 (eV)
E1+∆1 (eV)
E2 (eV)
CdTe
CdCl2-treat.
CdTe
Untreated
1.503
0.061
3.321
3.913
5.214
1.527
0.089
3.199
3.981
5.208
Cd1-xMgxTe
42
(20 wt.% MgTe)
1.710
0.128
3.567
3.725
5.175
Cd1-xMgxTe
92
(40 wt.% MgTe)
1.983
0.161
3.479
3.816
5.143
8.3 Bottom cell material: Cd1-xHgxTe
8.3.1 Cd1-xHgxTe film preparation
Efforts have also focused on the optical characterization of as-deposited Cd1-xHgxTe
films grown at different substrate temperatures for use as a bottom cell absorber material.
The Cd1-xHgxTe films were deposited by rf magnetron sputtering as described in Table
8.1 on 1 mm thick soda-lime glass, using a sputtering target containing CdTe (60 wt.%) +
HgTe (40 wt.%).
Individual films were grown at substrate temperatures of 23°C, 44°C,
70°C, 85°C, 97°C, and 153°C.
This substrate deposition temperature range is of
172
greatest interest in order to avoid metallic Hg inclusions
[8-13]
.
All such films were
deposited at an Ar pressure of 10 mTorr and an RF power of 27 W; (Dr. Viral Parikh is
acknowledged for deposition of these samples).
In addition, CdCl2 post-deposition
treatments were performed on the Cd1-xHgxTe films.
The higher temperature of the
post-deposition process in CdTe has been shown to increase the grain size and thus
improve the efficiency of the cells.
A two stage process was explored for the
Cd1-xHgxTe consisting of an anneal in an inert gas at 387°C and then a CdCl2 vapor
treatment at the same temperature.
Optical characterization was performed in order to
determine the band gap variation with substrate temperature.
Thus, ex situ
spectroscopic ellipsometry data were acquired on as-deposited and CdCl2-treated
Cd1-xHgxTe films before and after Br2/methanol etching.
8.3.2 Results and discussion
Figure 8-14 shows the band gaps of the Cd1-xHgxTe films in the as-deposited state for
different substrate temperatures, whereas Fig. 8-15 shows comparisons of the dielectric
functions from the inversion process and from the corresponding analytical model fit.
Table 8.4 also shows the energy and width of the critical point with the strongest peak in
ε2 as described in Fig. 8-15.
173
1.6
Bandgap (eV)
1.4
1.2
1.0
0.8
20
40
60
80
100
120
140
160
Substrate Temperature (C)
Figure 8-14 Band gap of as-deposited thin film Cd1-xHgxTe as a function of the
substrate temperatures over the range from 23°C to 153°C.
Table 8.4 Energy position and width of the critical point generating the strongest peak in
ε2 for as-deposited thin film Cd1-xHgxTe.
Temperature (°C)
23
44
70
85
97
153
E (eV)
3.30
3.11
3.44
3.47
3.49
3.51
174
Γ (eV)
1.97
2.50
1.64
1.89
1.77
0.85
14
14
g.o. fit inversion
inversion
12
Ts = 23°C
Eg = 1.58 eV
10
Ts = 44°C
Eg = 1.57 eV
10
8
e1,e2
8
e1,e2
g.o. fit inversion
inversion
12
6
6
4
4
2
2
0
0
-2
-2
0
1
2
3
4
5
6
0
7
1
2
3
eV
14
6
7
g.o. fit inversion
inversion
12
Ts = 70°C
Eg = 1.51 eV
10
Ts = 85°C
Eg = 1.15 eV
10
8
8
e1,e2
e1,e2
5
14
g.o. fit inversion
inversion
12
6
6
4
4
2
2
0
0
-2
-2
0
1
2
3
4
5
6
0
7
1
2
3
4
5
6
7
eV
eV
14
14
g.o. fit inversion
inversion
12
g.o. fit inversion
inversion
12
Ts = 97°C
Eg = 1.37 eV
10
Ts = 153°C
Eg = 0.81 eV
10
8
e1,e2
8
e1,e2
4
eV
6
6
4
4
2
2
0
0
-2
-2
0
1
2
3
4
5
6
7
0
1
2
3
4
5
6
7
eV
eV
Figure 8-15 Dielectric functions from mathematical inversion and from the
corresponding analytical model fit for as-deposited Cd1-xHgxTe films prepared with
different substrate temperatures.
The agreement between the two methods for dielectric function determination
supports the validity of the functional form of the analytical model.
175
Figure 8-14 shows
that the band gap decreases abruptly at a substrate temperature of about 75°C.
At low
temperatures the band gaps are close to that of CdTe, however, the dielectric function
shape is inconsistent with polycrystalline CdTe.
It is possible that nanoscale Hg
inclusions exist, giving rise to a broad plasmon resonance centered near 3.5 eV.
The
semiconductor component of all films except that grown at the highest temperature must
have a very small grain size or high defect density in accordance with the large
broadening values.
In the film prepared at the highest temperature of 153°C,
semiconducting Cd1-xHgxTe with x ~ 0.4 appears to have been obtained with a larger
grain size
[8-14]
.
These trends are confirmed by XRD measurements which reveal that
the films grown at 85°C and 97°C consist of small grains coalesced to form larger grains
with diffuse grain boundaries.
Figure 8-16 presents a comparison of the pseudo-dielectric functions of as-deposited
and CdCl2 treated Cd1-xHgxTe films, including the results for each of the two samples
before and after a single Br2+methanol etching step.
The key observations of Fig. 8-16
include (i) the blue shift of the critical point energies for Cd1-xHgxTe upon CdCl2
treatment, indicating the loss of Hg content in the alloy film after the treatment; and (ii)
reduction of the critical point transition widths for the Cd1-xHgxTe upon CdCl2 treatment,
an indication of an increase in grain size or a reduction in defect density.
This
post-deposition treatment has proven to be the most challenging aspect of Cd1-xHgxTe and
other alloy film preparation and further work is needed to optimize these processes
specifically for the alloys.
176
12
16
As developed
st
As developed 1 etch step
CdCl2 treated
14
8
st
12
CdCl2 treated 1 etch step
6
<ε 2 >
10
<ε1>
Cd1-xHgxTe critical points blue shift after CdCl2 treatment
E1+∆1
E2
E1
10
8
6
4
2
2
-2
0
-4
-2
-6
0
1
2
3
4
5
6
7
Photon energy (eV)
E2
E1 E1+∆1
0
4
As developed
st
As developed 1 etch step
CdCl2 treated
st
CdCl2 treated 1 etch step
0
1
2
3
4
5
6
7
Photon energy (eV)
Figure 8-16 Comparison of the real (left) and imaginary (right) parts of the
pseudo-dielectric function of as-deposited and CdCl2 treated CdxHg1-xTe films, including
results (a) before and (b) after a single Br2/methanol etching step.
177
Chapter Nine
Summary and Future Directions
9.1
Summary
The most significant accomplishment of this thesis research is the first demonstration
of ex-situ spectroscopic ellipsometry (SE) performed through the glass superstrate for
non-destructive evaluation of CdTe solar cells in the configuration used widely by
industry.
This measurement approach avoids the problem of the very rough free surface
of CdTe, which makes quantitative optical measurements from the film side extremely
difficult, if not impossible.
The validity of such through-the-glass measurements has
been corroborated by ex-situ SE measurements from the film side of the solar cell
performed destructively after smoothening the CdTe film surface with a succession of
Br2+methanol etching steps.
The measurement approach in which the CdTe solar cell is
probed non-destructively through the glass has a wide variety of applications including:
(i) off-line mapping of large area coated glass plates, (ii) on-line monitoring of such
plates, as well as (iii) interpreting quantum efficiency measurements in terms of optical
and electronic losses.
178
In Chapter 3 of this thesis, ex-situ SE was shown to provide the four sets of optical
properties of the materials that comprise the Pilkington TEC-15 glass superstrate of the
CdTe solar cell.
These materials include the soda lime glass superstrate material, and
the SnO2, SiO2, and SnO2:F thin film materials deposited in on-line coating processes.
The dielectric functions of these four materials were measured from the film side in each
case, and they serve as a database that enables analysis of CdCl2-treated and untreated
CdTe solar cells in measurements through the glass or from the film side.
Both
ellipsometry and transmittance experiments have been performed on the set of TEC-15
samples, and the optical models have been selected for each material so as to fit the
measured ellipsometry and transmittance spectra simultaneously and thereby provide
accurate dielectric functions of the component materials of the superstrate.
In the thesis research described in Chapter 4, a Br2+methanol step-wise chemical
etching process has been developed that both reduces the bulk thickness of the CdTe and
smoothens its surface, but without introducing measurable changes in the underlying thin
film structure.
In studies that demonstrate this capability, in-situ real time SE and
ex-situ SE measurements have been compared, the latter in conjunction with repetitive
chemical etching.
By comparing the CdTe thin film structure during deposition as
monitored by real time SE with that during the etching process using ex-situ SE, it has
been shown that the etching process is the reverse of the deposition process.
This means
that for each CdTe bulk layer thickness during growth or etching, the underlying bulk
layer of the sample has the same void fraction profile.
179
In Chapter 5, the Br2+methanol etching procedure has been demonstrated for the
analysis of CdTe structural modifications by CdCl2 treatments.
This approach is
preferred over in-situ real time SE studies of CdCl2 treatment because in the real time
studies many aspects of the sample structure are likely to change simultaneously, making
interpretation extremely difficult.
In Chapter 5, the power of Br2+methanol etch
profiling was demonstrated first in studies of ~3000 Å thick CdTe films on crystalline Si
substrates processed in three different ways: (i) as-deposited, (ii) thermally annealed in Ar
gas for 30 min, and (iii) CdCl2 treated for 5 min.
For such films, depth profiles in the
relative void volume fraction and the critical point energies and widths as functions of
CdTe bulk layer thickness during etching by Br2+methanol have provided information on
microstructure, grain size, and strain.
As a result, it has been demonstrated that the
CdCl2 treatment relaxes the strain in the CdTe network and generates a relatively uniform
grain structure throughout the thickness of the thin films.
Differences in the depth
profiles between Ar annealed and CdCl2 treated films suggest that in the former case, a
fine grained structure is retained at the surface.
Also in Chapter 5, the optical properties of CdCl2 treated CdTe and CdS were
presented, and comparisons made with the optical properties of the as-deposited films.
The changes that occur in the optical properties of CdTe and CdS upon CdCl2 treatment
are significant and demonstrate the necessity of expressing these optical properties in
terms of photon-energy independent parameters that describe the critical point features.
These parameters in turn can be expressed in terms of the physical properties such as
180
mean free path or grain size and stress.
With such a database, it then becomes possible
to use the physical properties as free parameters in the optical analysis of CdCl2-treated
solar cells.
This more advanced approach will be undertaken in future work.
In the research effort described in Chapter 6, the database consisting of optical
properties of the TEC-15 and semiconductor components of the CdTe solar cell has been
applied to analyze the CdS/CdTe solar cell structure obtained after CdCl2 post-deposition
treatment.
The measurements of the solar cell structure were performed
non-destructively from the glass superstrate side using a 60° prism contacted to the glass
superstrate with index matching fluid.
As described earlier in this summary, the
metrological capability developed here is the most significant accomplishment of this
thesis research due to its potential application for on-line monitoring and off-line
mapping of complete modules.
Also in Chapter 6, the application of ex-situ spectroscopic ellipsometry was
described for analyzing the structure of the thin film CdTe solar cell destructively using a
succession of Br2+methanol etching treatments.
In this way, the optical properties of the
CdTe component of the cell could be determined as a function of depth from the surface
and proximity to the CdS/CdTe interface.
Using this method of depth profiling, a better
understanding of the overall film structure could be obtained in comparison with the
non-destructive method.
In addition to providing a depth profiling capability, the
destructive method has also been useful in corroborating the structure measured
non-destructively through the glass superstrate.
181
In Chapters 7 and 8, the application of ex-situ spectroscopic ellipsometry to more
advanced problems in the development of CdTe-based solar cell materials and devices
was described.
First, in the research described in Chapter 7, ex-situ SE has provided the thicknesses
and structural properties of CdTe solar cells in the substrate configuration in which case
the CdTe is deposited directly on opaque Mo metal.
Although ex-situ SE in the
substrate configuration has the advantage that there is no overlying glass to limit the
spectral range that reaches the semiconductors, the basic problem in this configuration is
the surface roughness on the top-most transparent conductor, or CdS layer in the full or
partial device configuration, respectively.
There is less motivation at this time to
address these issues since all current manufacturing processes for CdTe solar cells exploit
the superstrate configuration.
Second, in the research described in Chapter 8, ex-situ SE has been applied to study
the CdTe based ternary alloys Cd1-xMnxTe, Cd1-xMgxTe, and Cd1-xHgxTe, which are
potential absorber layer components of sputtered II-VI tandem solar cells.
In this study
it was shown that ex-situ SE could provide the critical point band gaps and broadening
parameters from which the success of alloying could be assessed.
By repeating such
measurements after the CdCl2 treatments, the ability of such treatments to generate the
desired increase in grain size while maintaining the desired alloy composition and band
gap could also be assessed. As a result, it was shown that ex-situ SE could be used in
182
conjunction with absorber materials fabrication for the development of sputtering and
post-deposition processes to be adopted in device structures.
9.2
Future directions
The most pressing goal of future research is to identify a robust optical model that
can be used for all CdTe based solar cells such that small changes in materials properties
such as CdS and CdTe layer thicknesses, void fractions, mean free paths or grain sizes,
strain, and uniformity can be determined non-invasively in the actual solar cell
configuration. Possible modifications of the TCO layers of the superstrate by CdS
over-deposition is also of interest.
sensitivity to changes is a key goal.
Although accuracy is not necessarily needed,
If the optical model can be optimized, then it may
be possible to achieve accuracy and sensitivity simultaneously. Along the way, it will
be helpful to correlate spectroscopic ellipsometry (SE) results with other methods such as
cross-sectional transmission electron microscopy (XTEM) and secondary ion mass
spectrometry (SIMS) in order to better understand the nature of the interactions at the
CdS/CdTe and SnO2:F/CdS interfaces in the solar cells.
In particular, it is important to
improve the SnO2:F/CdS interface model and understand how the SnO2:F may be
modified by CdS over-deposition and post-deposition treatments.
In this section, more
detailed suggestions for future studies to continue this thesis research will be reviewed in
order of the relevant chapter.
183
Additional improvement in the analysis of TCO-coated glass superstrates, an
extension of the TEC glass studies of Chapter 3, is an important direction for future
research.
For the complete TCO stack, the simulated spectra are not in close agreement
with experimental data over two regions of the photon energy range.
The largest
deviations occur at the lowest energies (< 1.0 eV) where the Drude behavior dominates.
Four different complexities may be evaluated in greater detail in the future so as to
improve the fitting in this region: (i) additional layers in the optical model, such as
interface roughness between pairs of bulk layers; (ii) additional terms in the analytical
model for the top-most SnO2:F, which is the most strongly absorbing layer; (iii)
non-uniformity over the area of the beam due to macroscopic scale roughness; and (iv)
non-uniformity with depth due, for example, to a void volume fraction or free carrier
concentration gradient.
Although some of these approaches have been attempted
individually, they have not been evaluated in combination.
Also in the region near
4.50-4.75 eV, where a transition from SnO2:F semi-transparency to opacity is observed,
improved modeling results will require a closer match between the regimes where an
analytical function is assumed for the dielectric function and where exact inversion is
applied.
This may require (i) an improved analytical model for the absorption onset
and/or (ii) more advanced inversion methods that incorporate not only the surface
roughness thickness, but also the bulk layer thickness.
The focus in this thesis has been on the development of an optical database for
TEC-15, the most common coated superstrate used for CdTe solar cells.
184
Other types of
coated TEC glass deserve similar attention, such as TEC-7 and TEC-8, as well as coated
glass from other manufacturers. TEC-7 and TEC-8 may be used for solar cells in which
light scattering or "haze" is desirable, e.g., cells with thin (< 1 µm) CdTe layers or with
thin film silicon absorber layers.
Results reported in Chapter 3 suggest that the optical
properties of the top-most SnO2:F of TEC-7 and TEC-8, differ from those of TEC-15, and
these TEC glass types should be reanalyzed on the basis of this assumption.
Of recent
interest are the high resistivity transparent top layers typically undoped or weakly doped
SnO2, called "HRT" or "buffer" layers, which are incorporated as a fourth layer of
experimental TEC glass stacks. The ultimate challenge is to characterize the optical
properties of these layers in the full multilayer configuration consisting of
glass/SnO2/SiO2/SnO2:F/HRT.
In summary, future research must involve standardizing
the analysis procedure for TEC-15 so that it is applicable for other TEC glasses.
Furthermore, standardized procedures are also needed for the analysis of the four-layer
stacks, applied to cases in which samples of the HRT layer on simpler substrates are
unavailable.
In Chapter 4, step-wise Br2+methanol etching was evaluated for depth-profiling of
CdCl2 treated and untreated CdTe thin film materials and solar cells while retaining a
smooth surface for high quality optical measurements throughout the process.
In this
chapter, it was shown how a residual amorphous tellurium (a-Te) layer ~13-14 Å in
effective thickness could be detected and characterized under certain circumstances.
The presence of this layer was neglected in the depth-profiling studies of CdTe thin film
185
materials in Chapter 5 and solar cells in Chapter 6. This simplification was based on
modeling performed for very thick CdTe layers, namely that the impact of the a-Te layer
on the deduced film structure is minimal.
Further work needs to be done, however, in
order to evaluate the validity of this simplification under all circumstances.
For
example, when the surface roughness region of a CdTe solar cell is completely etched
away, the void fraction in the CdTe reaches a sharp minimum.
Future work must be
performed to determine if this minimum continues to be observed when a-Te is included
in the optical model, or if it is an artifact of neglecting the a-Te.
In addition, the effect
on critical point analyses that results from incorporating a-Te in the optical model must
be evaluated.
A more detailed analysis involving analytical removal of a known
thickness of a-Te may provide a better understanding of the CdTe solar cell depth profiles
in the mean free path or grain size and film strain. Although very good consistency has
been observed in the depth profiles for the CdTe material in Chapter 5, the depth profiles
for the CdTe solar cells in Chapter 6, in particular the critical point energies, were not
easily interpretable, possibly as a result of simultaneous variations in film strain and
sulfur in-diffusion.
Very accurate critical point analyses will be needed in the future to
separate out these effects.
In Chapter 5, clear differences between the optical properties of CdCl2 treated and
untreated CdTe have been characterized and understood in terms of differences in strain,
which shifts the critical point energies, and differences in defect density or grain size,
which changes the broadening parameters.
For CdS, however, the dielectric function of
186
a thin film as-deposited on a fused silica prism and measured through the prism differs
considerably from those of thin films as-deposited on crystalline Si substrates and
measured from the film side at a thickness of 500 Å.
The motivation for such a study
was to characterize the change in dielectric function of CdS upon CdCl2 treatment using a
configuration that corresponds more closely to the actual device, i.e., an underlying oxide
and an overlying CdTe layer such that the free surface of the CdS is not exposed during
the treatment.
In the prism deposition experiment described in Chapter 6, the origin of
the differences in the as-deposited materials must be studied in greater detail in the future
through additional experimentation.
The low amplitude of the dielectric function in the
high energy range is of particular interest.
Even the CdCl2 treatment does not restore
this amplitude to the level observed for CdS deposited on crystalline Si and measured
from the film side.
Future experiments may determine if this effect is (i) due to extrinsic
differences in the deposition process, e.g., higher interface contamination levels or lower
substrate temperature associated with deposition on the prism, (ii) specific to the interface
structure of CdS on fused SiO2, or (iii) intrinsic to the CdS interface in general which
becomes evident using light from the substrate side with a very short penetration depth
(photon energies > 4 eV).
The future directions designed to extend studies of the step-wise etching for
materials and solar cells decribed in Chapters 5 and 6, respectively, must focus on the role
of temperature and time in the Ar annealing and CdCl2 treatment processes.
For the
thinner CdTe materials on crystalline substrates, one may be able to establish the nature
187
of the grain growth processes.
For example, in the case of annealing in Ar gas, grain
growth appears to start from the substrate interface with pinned grain boundaries at the
surface, whereas for CdCl2 treatment, the mechanism is clearly different with both
near-surface and sub-surface grain growth occurring.
In the case of the solar cell
structures, by exploring the kinetics versus temperature and time one may be able to
separate out effects of grain growth in CdTe from those of CdS in-diffusion.
In the research described Chapter 7, a novel approach for real time spectroscopic
ellipsometry (RTSE) analysis has been applied to a CdTe deposition in the substrate
configuration on Mo.
In this analysis, which involves a synthesis of exact inversion and
least squares regression analysis methods, excellent fits to the data have been obtained
with a low mean square error.
In spite of these excellent fits, the resulting inverted
dielectric functions show artifacts indicating that improvements in the multilayer model
for the substrate/film are required.
These improvements may include (i) incorporation
of thickness non-uniformity over the cross-section of the optical beam or (ii) application
of a multilayer or graded model to describe the CdTe/Mo interface or even an alternative
effective medium theory.
Because of the uncertainty involved in modeling rough
interfaces between the CdTe and its substrate, a case in point being the CdTe/Mo
interface, subsequent studies to extract high accuracy dielectric functions of the CdTe
have used the smoothest possible substrates -- single crystal silicon wafers.
In the future,
if complete solar cells are to be studied successfully by real time SE, such issues
involving rough interfaces must addressed comprehensively.
188
As described in the inroductory pararaph of this section, the key studies in Chapters 6
and 7 on ex situ SE of CdTe solar cells in the superstrate and substrate configurations
represent works in progress, and significant improvements are to be expected through
additional future studies. Promising future directions will be outlined in the following
paragraphs.
This discussion provides a roadmap that future research should follow,
continuing from the foundation established through this initial thesis research.
It is also
recommended, because of the complexity of the models being developed, that a
step-by-step procedure be undertaken as described in Chapters 6 and 7 in which case the
number of free parameters of the model is increased successively.
With this approach,
the success or failure of the suggestions for improvement made below can be
quantitatively evaluated.
Tin side characteristics of the glass superstrate
Analysis of through-the-glass spectroscopic ellipsometry on superstrate CdTe solar
cells may benefit from a detailed analysis of the reflection from the Sn side of the glass,
i.e., the side through which the light enters.
The modeling of the bare soda lime glass as
described in Chapter 3 was performed using uncoated glass from the side opposite to the
Sn side.
The focus of an improved analysis will be on characterization of the optical
properties of the Sn side using the dielectric function of the opposite side from Chapter 3
as the underlying base material.
One or two layers may be required to characterize the
residual Sn clusters on the glass surface as well as atomic Sn diffused into the glass.
189
The Sn side reflection must be taken into account carefully when considering the
polarization changes that occur when the incident and specularly reflected light beams,
the latter from the glass/film interface, crosses the Sn side interface.
In addition to the
more detailed analysis of the Sn side of the glass for through-the-glass SE, it will also be
important to characterize the strain in the glass.
The effect of strain in the superstrate
glass is similar to that of strained windows in real time ellipsometry, and the successful
correction procedures are expected to consist of zone averaged measurements as well as
an offset correction in the ellipsometric angle ∆.
SnO2:F/CdS interface
The surface roughness on the TEC-15 SnO2:F is up to ~300 Å thick, and when high
resistivity transparent layers are added to the top of the TEC glass, the roughness can
increase even further.
This surface roughness is likely to appear as interface roughness
when the CdS is deposited on the superstrate surface.
As the superstrate of the CdTe
solar cell becomes rougher, it becomes more difficult to determine all the characteristics
of the solar cell because one must use thicker effective medium layers at the rough
interfaces, and effective medium theories generally involve considerable uncertainty.
In
the analysis of the superstrate solar cell, however, when one attempts to incorporate a
SnO2:F/CdS effective medium mixture as an interface roughness layer, the thickness of
this layer expands to the entire thickness of the SnO2:F, and the CdS volume fraction
decreases to relatively small values.
The interpretation of this observation is that the
dominant effect of the interaction between the SnO2:F and CdS is not interface roughness,
190
but rather modification of the SnO2:F layer either in the CdS deposition process or in the
CdCl2 treatment.
Future experiments for modeling improvement include characterizing
TEC-15 glass not only before solar cell deposition, but also after all cell processing, in
the latter case after removing both the CdTe and CdS layers by etching.
In such
experiments, one may be able to identify the optical characteristics of the SnO2:F layer
that change upon cell processing, e.g., free carrier concentration, optical absorption onset,
and/or void fraction, as well as the key processing parameters that lead to the change in
optical properties.
Once this information is available, then it may be possible to include
both interface roughness and SnO2:F modification in the ex situ SE modeling of the solar
cell.
Optical properties of CdS and CdTe
The poorer fits to the ex situ ellipsometric spectra for the superstrate and substrate
solar cells in the neighborhood of the CdTe and CdS fundamental band gaps and high
energy critical points may be attributed to mean free paths, grain sizes, or strain levels in
the solar cell layers that may be different than those in the layers used to extract the
reference dielectric functions.
differences.
Layer interdiffusion may also contribute to the
As a result, improvements in fitting can be expected if such effects can be
incorporated in the modeling.
For example, for the superstrate solar cell of Chapter 6,
the fringe pattern in ψ just below the band gap is more pronounced in the data than in the
model, an indication of a longer mean free path or larger grain size in the solar cell than
in the CdTe material used for reference data.
191
Furthermore, for the substrate solar cell
analysis of Chapter 7, the CdS layer band gap is noticeably lower and the CdTe band gap
is higher in the experimental data than in the model, possibly indicating that the stress in
the CdS is lower and that in the CdTe is higher than in the reference materials. These
simple observations made on the basis of (ψ, ∆) comparisons could be made quantitative
through a parameterization of the CdS and CdTe reference dielectric functions explicitly
in terms of mean free path, stress, and alloying generated by inter-diffusion.
CdS/CdTe interface thickness
The thickness of the CdS/CdTe interface layer for both superstrate and substrate solar
cells has been observed to lie in the range of 800-1400 Å.
In the case of the superstrate
solar cells, this thickness is significantly greater than the expected interface roughness
layer if the thin CdS layer were to conformally cover the SnO2:F of the TEC-15 without
generation of additional roughness.
As an alternative approach to using a single layer
model for the CdS/CdTe, a three layer model may be implemented in future research.
The layers at the interfaces to the bulk CdS and CdTe layers would be CdS-rich and
CdTe-rich layers, respectively, representing the interdifusion regions, and the intervening
layer would represent the interface roughness.
It would be reasonable to fix the
composition of the latter at ~0.5/0.5 CdS/CdTe for stability in the modeling when using
three interface layers.
Two different approaches could be used for modeling the optical functions of the two
interface layers in the three layer model.
The first method is the simplest, involving the
use of effective medium theories that incorporate small volume fractions of CdTe in CdS
192
and CdS in CdTe.
This approach may be applicable if interdiffusion occurs through the
grain boundaries, and thin regions at the boundary regions then assume the composition
of the adjoining layer.
Alternatively, ternary alloys of CdS1-x Tex with a small Te content
in CdS, and CdTe1-xSx with a small S content in CdTe could be assumed based on a
model of uniform bulk interdiffusion. .For this latter approach, the dielectric functions of
metastable ternary alloys are available; however. future efforts must focus on determining
the dielectric functions of the stable alloy phases that form at the elevated temperatures of
solar cell processing.
CdTe properties and roughness thickness
For future improvement of the ex situ analysis of both superstrate and substrate CdTe
solar cells, the ability to incorporate void profiles of various functional forms must be
included in the modeling.
As detailed in Chapters 4-6, real time SE measurements have
shown clearly that complicated void profiles can exist in the as-deposited CdTe films and
solar cells, and that these profiles are suppressed in the CdCl2 treatment processes.
The
results of Chapter 6 do suggest small deviations in density for the CdCl2 treated CdTe,
however, which may lead to significant effects in ex situ modeling when accumulated
throughout the thickness of 2-3 µm films. Also for the superstrate solar cell analyzed
through the glass in Chapter 6, the surface roughness on the CdTe at the back of the solar
plays an important role due to its effect on the back-reflected light beam.
In fact, the
CdTe roughness parameters are the fifth and sixth most important ones in the
12-parameter model.
Improvements may be possible in this case through the use of
193
multiple layers to describe the roughness, or even a graded layer.
It is also of interest to
test effective medium approximations other than that of Bruggeman for comparisons of
the fitting quality.
The p+ doped back contact
For the CdTe solar cell in the superstrate configuration studied by ex situ SE analysis
(Chapter 6), the p+ back contact treatment consisting of in-diffusion of a 30 Å layer of Cu
has not been applied. It is of interest to explore the optical properties of this back
contact material to determine if changes can be detected in the mean free path as derived
from the widths of the critical points. Such an experimentation is best performed after
CdCl2 treatment followed by a Br2+methanol etch that leads to a smooth and
well-characterized surface, from which it is easiest to detect changes upon Cu
in-diffusion.
For the CdTe solar cell in the substrate configuration, as described in Chapter 7, the
p+ back contact ZnTe:Cu has been studied in detail.
This thin film material shows clear
evidence of heavy doping in the form of a significantly broadened absorption onset.
In
fact, if this material is to be used as a transparent back contact, improvements in optical
properties will be needed since a significant absorption tail exists even below 2 eV.
Reduction in low energy absorption may be possible through a reduction in the doping
level.
Finally, analysis of the p+ back contacts used in superstrate and substrate solar
cells may benefit from infrared spectroscopic ellipsometry which may be able to detect
the free carrier absorption in the contact regions or in discrete heavily doped layers.
194
A final area of research that deserves additional future study follows from the alloy
research of Chapter 8.
In general for future progress in this area, a closer correlation
between the ex situ SE measurements of the alloys before and after CdCl2 treatment and
the device measurements would be helpful in order to establish the relationships between
fundamental materials properties and device performance.
Both real time SE
measurements and Br2+methanol etching experiments would also be helpful to establish
the role of the CdCl2 treatment in modifying the structure of the film throughout the
depth.
Such experiments may help to guide future improvements in the post-depositon
treatment processes.
Finally, solar cell depositions using individual binary targets would
enable continuous compositional ranges to be obtained in a single deposition across the
superstrate surface, and such materials and cells can then be characterized by mapping SE
in terms of their band gaps.
This approach would enable expeditious study and
subsequent optimization of alloy composition not possible with discrete ternary targets.
195
References
[1-1] P. Drude, Ann. d. Phys. Chem. (Leipzig) 39. 481-552, (1890).
[1-2] E.D. Palik, Handbook of Optical Constants, Academic Press, Orlando, Vol. 1, 1985;
Vol. 2, 1991.
[1-3] A. Rothen, “The ellipsometer, an apparatus to measure thicknesses of thin surface
films”, Rev. Sci. Instrum. 16, 26-30, (1945).
[1-4] R. M. A. Azzam and N. M. Bashara, Ellipsometry and Polarized Light,
(North-Holland, Amsterdam, 1977), Page 421.
[1-5] B. D. Cahan and R.F. Spanier, “A high speed precision automatic ellipsometer”, Surf.
Sci. 16, 166-176, (1969).
[1-6] S. N. Jasperson and S. E. Schnatterly, “An improved method for high reflectivity
ellipsometry based on a new polarization modulation technique”, Rev. Sci. Instrum. 40,
761-767, (1969).
[1-7] D. E. Aspnes and A. A. Studna, “High precision scanning ellipsometer”, Appl. Opt.
14, 220-228, (1975).
[1-8] R. W. Collins, “Automatic rotating element ellipsometers: Calibration, operation,
and real-time applications,” Rev. Sci. Instrum. 61, 2029–2062, (1990).
196
[1-9] H. G. Tompkins and E. A. Irene (Editors), Handbook of Ellipsometry, William
Andrews Publications, Norwich, (2005).
[1-10] D. E. Aspnes, A. A. Studna, and E. Kinsbron, “Dielectric properties of heavily
doped crystalline and amorphous silicon from 1.5 to 6.0 eV”, Phys. Rev. B 29, 768-779,
(1984).
[1-11] H. Arwin and D. E. Aspnes, “Unambiguous determination of thickness and
dielectric function of thin films by spectroscopic ellipsometry”, Thin Solid Films 113,
101-103, (1984).
[1-12] J. Li, J. Chen, J. Zapien, N. Podraza, C. Chen, J. Drayton, A. Vasko, A. Gupta, S.
Wang, R.W. Collins, and A. Compaan, “Real time analysis of magnetron-sputtered
thin-film CdTe by multichannel spectroscopic ellipsometry”, Mater. Res. Soc. Symp. Proc.
865, 9-14, (2005).
[1-13] R. M. A. Azzam and N. M. Bashara, Ellipsometry and Polarized Light,
(North-Holland, Amsterdam 1977), Page 14.
[1-14] Max Born, and Emil Wolf, Principles of Optics. Cambridge University Press,
Cambridge, (1999).
[1-15] J. D. Jackson, Classical Electrodynamics, John Wiley & Sons, New York (1975).
[1-16] B. Johs, J. Hale, N. J. Ianno, C. Herzinger, T. Tiwald, and J. A. Woollam, “Recent
developments in spectroscopic ellipsometry for in situ applications”, SPIE Proceedings
4449, 41-57, (2001).
[1-17] G. E. Jellison, Jr., “Spectroscopic ellipsometry data analysis: measured versus
197
calculated quantities”, Thin Solid Films, 313–314, 33-39, (1998).
[2-1] A. Luque and S. Hegedus, Handbook of Photovoltaic Science and Engineering,
John Wiley & Sons, Ltd. West Sussex, (2003).
[2-2] A. Mondal, B.E. McCandless and R.W. Birkmire, “Electrochemical deposition of
thin ZnTe films as a contact for CdTe solar cells”, Sol. Energy Mater. Sol. Cells 26
181–187, (1992).
[2-3] Ilvydas Matulionis, Sijin Han, Jennifer A. Drayton, Kent J. Price, and Alvin D.
Compaan, “Cadmium telluride solar cells on molybdenum substrates. In: Proceedings of
the Materials Research Society Symposium on II–VI Compound Semiconductor
photovoltaic materials”, Mat. Res. Soc. Symp. Proc. 668, H8.23.1-6, (2001).
[2-4] D. A. Jenny, J. J. Loferski, and P. Rappaport , “Photovoltaic effect in GaAs p-n
junctions and solar energy conversion”, Phys. Rev. 101, 1208-1209, (1956).
[2-5] A. D. Compaan, U.S. Patent No. 5,393,675 (Feb. 28, 1995).
[2-6] M. Shao, A. Fischer, D. Grecu, U. Jayamaha, E. Bykov, G. Contreras-Puente, R.G.
Bohn, and A.D. Compaan, “Radio-frequency-magnetron-sputtered CdS/CdTe solar cells
on soda-lime glass”, Appl. Phys. Lett., 69, 3045-3047, (1996).
[2-7] B. E. McCandless, I. Youm, and R. W. Birkmire, “Optimization of vapor
post-deposition processing for evaporated CdS/CdTe solar cells”, Prog. Photovolt. Res.
Appl., 7, 21-30, (1999).
[2-8] A. Gupta, I. Matulionis, J. Drayton, and A.D. Compaan, “Effect of CdTe thickness
198
reduction in high efficiency CdS/CdTe solar cells”, Mat. Res. Soc. Symp. Proc. 668,
H6.4.1, (2001).
[2-9] B. E. McCandless, Y. Qu and R. W. Birkmire, “A treatment to allow contacting
CdTe with different conductors”, Proc. 1st World Conference on Photovoltaic Energy
Conversion, 107-110, (1994).
[2-10] J. Li, J. Chen, J. Zapien, N. Podraza, C. Chen, J. Drayton, A. Vasko, A. Gupta, S.
Wang, R.W. Collins, and A. Compaan, “Real time analysis of magnetron-sputtered
thin-film CdTe by multichannel spectroscopic ellipsometry”, Mater. Res. Soc. Symp. Proc.
865, F1.2.1, (2005).
[2-11] J. Li, J. Chen, and R. W. Collins, “Analysis of the dielectric functions of CdS and
CdTe for grain size, stress, and temperature: potentialities for on-line monitoring”,
Conference Record of the 33rd IEEE Photovoltaic Specialists Conference, 529-534,
(IEEE, Piscataway NJ, 2008).
[2-12] R. W. Collins, A.D. Compaan, Jian Li, and Jie Chen, “RTSE Studies of the
Fabrication of High Efficiency CdTe PV,” DOE Solar Energy Technologies Program
Review/Peer Review Meeting, Denver, Apr 17-19, 2007. (Manuscript available on-line at:
http://www1.eere.energy.gov/solar/review_meeting/pdfs/p_15_collins_univ_toledo.pdf)
[3-1] J. Chen, J. Li, D. Sainju, K.D. Wells, N.J. Podraza, and R.W. Collins, “Multilayer
analysis of the CdTe solar cell structure by spectroscopic ellipsometry”, Conference
Record of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conversion,
475-478, (2006).
199
[3-2] C. M. Herzinger, B. Johs, W. A. McGahan, J. A. Woollam, and W. Paulson,
“Ellipsometric determination of optical constants for silicon and thermally grown silicon
dioxide via a multi-sample, multi-wavelength, multi-angle investigation”, J. Appl. Phys.
83, 3323-3336, (1998).
[4-1] P. V. Meyers, C. H. Liu, and T. J. Frey, U.S. Patent 4,710,589 (1987).
[4-2] R. W Birkmire, B. E. McCandless, and S. S. Hegedus, “Effects of processing on
CdTe/CdS materials and devices”, Int. J. Sol. Energy 12, 145-154, (1992).
[4-3] H. Arwin, D.E. Aspnes, and D.R. Rhiger, “Properties of Hg0.71Cd0.29Te and some
native oxides by spectroscopic ellipsometry”, J. Appl. Phys. 54, 7132-7138, (1983).
[4-4] A. D. Compaan, “The status of and challenges in CdTe thin-film solar-cell
technology”, Mater. Res. Soc. Symp. Proc. 808, 545-555, (2004).
[4-5] J. Lee, P. I. Rovira, I. An, and R. W. Collins, “Rotating-compensator multichannel
ellipsometry: Applications for real time Stokes vector spectroscopy of thin film growth”,
Rev. Sci. Instrum. 69, 1800-1810, (1998).
[4-6] R.W. Collins, A. Ferlauto, G. Ferreira, C. Chen, J. Koh, R. Koval, Y. Lee, J. Pearce,
and C.R. Wronski, “Evolution of microstructure and phase in amorphous, protocrystalline,
and microcrystalline silicon studied by real time spectroscopic ellipsometry”, Solar
Energy Mater. Solar Cells 78, 143-180, (2003).
[4-7] D. E. Aspnes and H. Arwin, “Nondestructive analysis of Hg1-xCdxTe (x=0.00, 0.20,
0.29, and 1.00) by spectroscopic ellipsometry. I. Chemical oxidation and etching”, J. Vac.
Sci. Technol. A 2, 1309-1315, (1984).
200
[4-8] H. Arwin and D. E. Aspnes, “Nondestructive analysis of Hg1-xCdxTe (x=0.00, 0.20,
0.29, and 1.00) by spectroscopic ellipsometry. II. Substrate, oxide, and interface
properties”, J. Vac. Sci. Technol. A 2, 1316-1323, (1984).
[4-9] A. S. Ferlauto, G. M. Ferreira, J. M. Pearce, C. R. Wronski, R. W. Collins, X. Deng,
and G. Ganguly, “Analytical model for the optical functions of amorphous
semiconductors from the near-infrared to ultraviolet: Applications in thin film
photovoltaics”, J. Appl. Phys. 92, 2424-2436, (2002).
[5-1] J. Li, N. J. Podraza, R. W. Collins, “Real time spectroscopic ellipsometry of
sputtered CdTe, CdS, and CdTe1-xSx thin films for photovoltaic applications”, Physica
Status Solidi (a) 205, 901-904, (2008).
[5-2] A. Gupta and A.D. Compaan, “All-sputtered 14% CdS/CdTe thin-film solar cell
with ZnO:Al transparent conducting oxide”, Appl. Phys. Lett. 85, 684-686, (2004).
[5-3] A. Compaan, V. G. Karpov, R. W. Collins, and D. Giolando, NREL Subcontract
RXL-5-44205-01, University of Toledo, Annual Report, p.70
[5-4] J. A. Woollam Company, Optical Property Database.
[5-5] J. A. Zapien, J. Chen, J. Li, J. Inks, N. J. Podraza, C. Chen, J. Drayton, A. Vasko, A.
Gupta, S. L. Wang, R. W. Collins, and A. D. Compaan, “Real time spectroscopic
ellipsometry of thin film CdTe deposition by magnetron sputtering for photovoltaic
applications”, Conference Record of the Thirty-First IEEE Photovoltaic Specialists
Conference -2005, 461-464, (2005).
[5-6] J. R. Chelikowsky and M. L. Cohen, “Nonlocal pseudopotential calculations for the
201
electronic structure of eleven diamond and zinc-blende semiconductors”, Phys. Rev. B 14,
556-582, (1976).
[5-7] S. Ninomiya and S. Adachi, “Optical properties of wurtzite CdS”, J. Appl. Phys. 78,
1183-1190, (1995).
[5-8] H. G. Tompkins and E. A. Irene (Editors), Handbook of Ellipsometry, William
Andrews Publications, Norwich, P. 229, (2005).
[5-9] J. Leng, J. Opsal, H. Chu, M. Senko, and D. E. Aspnes, “Analytic representations of
the dielectric functions of materials for device and structural modeling”, Thin Solid Films
313–314, 132-136, (1998).
[5-10] Jian Li, Jie Chen, N.J.Podraza, and R.W. Collins, “Real time spectroscopic
ellipsometry of sputtered CdTe: Effect of growth temperature on structural and optical
properties”, Conference Record of the 2006 IEEE 4th World Conference on Photovoltaic
Energy Conversion, 392-395, (2006).
[5-11] J. Li, J. Chen, and R. W. Collins, “Analysis of the dielectric functions of CdS and
CdTe for grain size, stress, and temperature: potentialities for on-line monitoring”,
Conference Record of the 33rd IEEE Photovoltaic Specialists Conference, 529-534,
(2008).
[6-1] A. Luque and S. Hegedus, Handbook of Photovoltaic Science and Engineering,
(John Wiley & Sons, Ltd. 2003).
[6-2] H.G. Tompkins and E.A. Irene (Editors), Handbook of Ellipsometry, William
Andrew Publications, Norwich, (2005).
202
[6-3] J. Chen, J. Li, D. Sainju, K.D. Wells, N.J. Podraza, and R.W. Collins, “Multilayer
analysis of the CdTe solar cell structure by spectroscopic ellipsometry”, Conference
Record of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conversion,
475-478, (2006).
[6-4] H. Arwin, D.E. Aspnes, and D.R. Rhiger, “Properties of Hg0.71Cd0.29Te and some
native oxides by spectroscopic ellipsometry”, J. Appl. Phys. 54, 7132-7138, (1983).
[6-5] A. D. Compaan, “The status of and challenges in CdTe thin-film solar-cell
technology”, Mater. Res. Soc. Symp. Proc. 808, 545-555, (2004).
[6-6] G. F. Feng and R. Zallen, “Optical properties of ion-implanted GaAs: The
observation of finite-size effects in GaAs microcrystals”, Phys. Rev. B 40, 1064-1073,
(1989).
[6-7] B. E. McCandless, G. M. Hanket, D. G. Jensen, and R. W. Birkmire, “Phase
behavior in the CdTe—CdS pseudobinary system”, J. Vac. Sci. Technol. A 20, 1462-1467,
(2002).
[6-8] J. Li, N. J. Podraza, and R. W. Collins, “Real time spectroscopic ellipsometry of
sputtered CdTe, CdS, and CdTe1-xSx thin films for photovoltaic applications”, Physica
Status Solidi (a) 205, 901-904, (2008).
[7-1] A. D. Compaan and R. G. Bohn, Thin-film Cadmium Telluride Photovoltaic Cells,
Final Subcontract Report, 1 November 1992 to 1 January 1994 (available NTIS
publication NREL/TP-451-7162; DE 94011886).
[7-2] V. P. Singh, J. C. McClure, G. B. Lush, W. Wang, X. Wang, G. W. Thompson, and
203
E. Clark, “Thin film CdTe-CdS heterojunction solar cells on lightweight metal substrates”,
Solar Energy Materials and Solar Cells 59, 145-161, (1999).
[7-3] J. A. Zapien, J. Chen, J. Li, J. Inks, N. J. Podraza, C. Chen, J. Drayton, A. Vasko, A.
Gupta, S. L. Wang, R. W. Collins, and A. D. Compaan, “Real time spectroscopic
ellipsometry of thin film CdTe deposition by magnetron sputtering for photovoltaic
applications”, Photovoltaic Specialists Conference, 461-464, (2005).
[7-4] B. W. Veal and A. P. Paulikas, “Optical properties of molybdenum. I. Experiment
and Kramers-Kronig analysis”, Phys. Rev. B 10, 1280-1289, (1974).
[7-5] S. H. Mohamed, O. Kappertz, J. M. Ngaruiya, T. P. L. Pedersen, R. Drese, and M.
Wuttig, “Correlation between structure, stress and optical properties in direct current
sputtered molybdenum oxide films”, Thin Solid Films 429, 135-143, (2003).
[7-6] Jian Li, Ph.D. Thesis, The University of Toledo, 2009 (unpublished).
[7-7] E. D. Palik, Handbook of Optical Constants, (Academic Press, Orlando, 1991), P.
750.
[7-8] B. Johs, C. Herzinger, J. Dinan, A. Cornfeld, and J. Benson, “Development of a
parametric optical constant model for Hg1−xCdxTe for control of composition by
spectroscopic ellipsometry during MBE growth”, Thin Solid Films 313, 137-142, (1998).
[8-1] A. Gupta and A. D. Compaan, “All-sputtered 14% CdS/CdTe thin-film solar cell
with ZnO:Al transparent conducting oxide”, Applied Physics Letters 85, 684-686, (2004).
[8-2] X. Wu, J. C. Keane, R. G. Dhere, C. DeHart, A. Duda, T. A. Gessert, S. Asher, D.
H. Levi, P. Sheldon. “16.5%-efficient CdS/CdTe polycrystalline thin-film solar cell”
204
Conference Proceedings, 17th European Photovoltaic Solar Energy Conference, Munich,
995–1000, (2001)
[8-3] Shanli Wang, Jian Li, Jie Chen, R. W. Collins, and A. D. Compaan, “Spectroscopic
ellipsometry and atomic force microscopy studies of RF sputtered Cd1-xMnxTe films”,
Conference Record of the Thirty-First IEEE Photovoltaic Specialists Conference -2005,
480-483, (2005).
[8-4] T. J. Coutts, K. A. Emery and S. Ward, “Modeled performance of polycrystalline
thin-film tandem solar cells”, Prog. Photovolt: Res. Appl. 10, 195-203, (2002).
[8-5] G. Hansen, J. Schmit and T. Casselman, “Energy gap versus alloy composition and
temperature in Hg1-xCdxTe”, J. Appl. Phys. 53, 7099–7101, (1982).
[8-6] V. P. Singh, J. C. McClure, G. B. Lush, W. Wang, X. Wang, G. W. Thompson, and
E. Clark, “Thin film CdTe-CdS heterojunction solar cells on lightweight metal substrates”,
Sol. Energy Mater. Sol. Cells 59, 145-161, (1999).
[8-7] Y. H. Hwang, H. K. Kim, M. S. Chung, Y. H. Um, H. Y. Park and P. K. Yoo, Jpn. J.
“Spectroscopic ellipsometry studies of Cd1-xMnxTe films grown on GaAs”, Jpn. J. Appl.
Phys. 40, 5247-5250, (2001).
[8-8] P. Lautenschlager, S. Logothetidis, L. Vina, and M. Cardona, “Ellipsometric studies
of the dielectric function of Cd1-xMnxTe alloys”, Phys. Rev. B 32, 3811-3818, (1985).
[8-9] D. E. Aspnes, Handbook on Semiconductors, Vol. 2, North-Holland, Amsterdam, P.
126, (1980).
[8-10] H. Arwin and D. E. Aspnes, “Nondestructive analysis of Hg1-xCdxTe (x = 0.00,
205
0.20, 0.29, and 1.00) by spectroscopic ellipsometry. II. Substrate, oxide, and interface
properties”, J. Vac. Sci. Technol. A 2, 1316-1323, (1984).
[8-11] F. D’Amore, M. Di Giulio, S. M. Pietralunga, A. Zappettini, L. Nasi, V. Rigato,
and M. Martinelli, “Sputtered stoichiometric TeO2 glass films: Dispersion of linear and
nonlinear optical properties”, J. Appl. Phys. 94, 1654-1661, (2003).
[8-12] D. E. Aspnes and H. Arwin, “Nondestructive analysis of Hg1-xCdxTe (x=0.00, 0.20,
0.29, and 1.00) by spectroscopic ellipsometry. I. Chemical oxidation and etching”, J. Vac.
Sci. Technol. A 2, 1309-1315, (1984).
[8-13] G. Fiorito, G. Gasparrini, and D. Passoni, “A possible method for the growth of
homogeneous
Mercury
Cadmium
Telluride
single
crystals”,
Journal
of
the
Electrochemical Society 125, 315-317, (1978).
[8-14] A.D. Compaan, R. W. Collins, V. G. Karpov, and D. Giolando, “Sputtered II-VI
alloys and structures for tandem PV: final subcontract report, 9 December 2003 - 30 July
2007”, NREL/SR-520-43954, September 2008
206
Appendix A Dielectric functions
A.1
Dielectric function of uncoated soda lime glass
nm
190.76
192.33
193.91
195.49
197.06
198.64
200.22
201.8
203.37
204.95
206.53
208.11
209.69
211.27
212.85
214.43
216.01
217.59
219.18
220.76
222.34
223.92
225.51
227.09
228.67
230.26
231.84
233.42
235.01
236.59
238.18
239.76
241.35
242.94
244.52
246.11
247.69
249.28
250.87
252.45
n
1.649
1.6458
1.6428
1.6398
1.6369
1.6341
1.6313
1.6287
1.6261
1.6236
1.6212
1.6189
1.6166
1.6144
1.6123
1.6102
1.6081
1.6062
1.6042
1.6024
1.6006
1.5988
1.5971
1.5954
1.5938
1.5922
1.5906
1.5891
1.5877
1.5862
1.5848
1.5835
1.5821
1.5808
1.5796
1.5783
1.5771
1.5759
1.5748
1.5736
k
1.65E-05
1.77E-05
1.91E-05
2.06E-05
2.24E-05
2.43E-05
2.64E-05
2.87E-05
3.12E-05
3.39E-05
3.68E-05
3.99E-05
4.32E-05
4.67E-05
5.03E-05
5.42E-05
5.82E-05
6.23E-05
6.66E-05
7.09E-05
7.53E-05
7.98E-05
8.43E-05
8.88E-05
9.33E-05
9.77E-05
0.00010202
0.00010622
0.00011026
0.00011413
0.0001178
0.00012124
0.00012444
0.00012737
0.00013002
0.00013237
0.00013442
0.00013617
0.0001376
0.00013873
254.04
255.63
257.22
258.8
260.39
261.98
263.57
265.16
266.74
268.33
269.92
271.51
273.1
274.69
276.28
277.87
279.46
281.05
282.64
284.23
285.82
287.41
289
290.59
292.18
293.77
295.36
296.95
298.54
300.13
301.72
303.31
304.9
306.49
308.08
309.68
311.27
312.86
314.45
316.04
317.63
1.5725
1.5715
1.5704
1.5694
1.5684
1.5674
1.5664
1.5655
1.5646
1.5637
1.5628
1.5619
1.5611
1.5603
1.5595
1.5587
1.5579
1.5571
1.5564
1.5556
1.5549
1.5542
1.5535
1.5529
1.5522
1.5515
1.5509
1.5503
1.5497
1.5491
1.5485
1.5479
1.5473
1.5468
1.5462
1.5457
1.5451
1.5446
1.5441
1.5436
1.5431
207
0.00013957
0.00014012
0.00014042
0.00014049
0.0001404
0.00014013
0.00013962
0.00013884
0.00013778
0.00013641
0.00013473
0.00013275
0.00013045
0.00012785
0.00012497
0.0001218
0.00011837
0.0001147
0.00011081
0.00010673
0.00010247
9.81E-05
9.36E-05
8.90E-05
8.43E-05
7.97E-05
7.50E-05
7.04E-05
6.58E-05
6.14E-05
5.70E-05
5.27E-05
4.86E-05
4.47E-05
4.10E-05
3.74E-05
3.40E-05
3.08E-05
2.78E-05
2.50E-05
2.25E-05
319.22
320.81
322.41
324
325.59
327.18
328.77
330.36
331.95
333.55
335.14
336.73
338.32
339.91
341.5
343.1
344.69
346.28
347.87
349.46
351.05
352.64
354.24
355.83
357.42
359.01
360.6
362.19
363.78
365.38
366.97
368.56
370.15
371.74
373.33
374.92
376.51
378.1
379.69
381.29
382.88
1.5426
1.5422
1.5417
1.5412
1.5408
1.5403
1.5399
1.5395
1.539
1.5386
1.5382
1.5378
1.5374
1.537
1.5366
1.5362
1.5359
1.5355
1.5351
1.5348
1.5344
1.5341
1.5337
1.5334
1.5331
1.5328
1.5324
1.5321
1.5318
1.5315
1.5312
1.5309
1.5306
1.5303
1.53
1.5297
1.5295
1.5292
1.5289
1.5286
1.5284
2.01E-05
1.79E-05
1.59E-05
1.41E-05
1.24E-05
1.10E-05
9.62E-06
8.43E-06
7.37E-06
6.43E-06
5.61E-06
4.88E-06
4.25E-06
3.70E-06
3.23E-06
2.81E-06
2.46E-06
2.16E-06
1.90E-06
1.68E-06
1.50E-06
1.34E-06
1.21E-06
1.09E-06
9.99E-07
9.20E-07
8.53E-07
7.97E-07
7.49E-07
7.08E-07
6.74E-07
6.44E-07
6.18E-07
5.96E-07
5.76E-07
5.59E-07
5.43E-07
5.28E-07
5.15E-07
5.03E-07
4.91E-07
384.47
386.06
387.65
389.24
390.83
392.42
394.01
395.6
397.19
398.78
400.37
401.96
403.55
405.14
406.73
408.32
409.91
411.5
413.09
414.68
416.27
417.86
419.45
421.04
422.63
424.22
425.81
427.4
428.99
430.57
432.16
433.75
435.34
436.93
438.52
440.11
441.7
443.28
444.87
446.46
448.05
449.64
451.22
452.81
454.4
1.5281
1.5279
1.5276
1.5274
1.5271
1.5269
1.5266
1.5264
1.5262
1.5259
1.5257
1.5255
1.5253
1.525
1.5248
1.5246
1.5244
1.5242
1.524
1.5238
1.5236
1.5234
1.5232
1.523
1.5228
1.5226
1.5224
1.5222
1.522
1.5219
1.5217
1.5215
1.5213
1.5212
1.521
1.5208
1.5206
1.5205
1.5203
1.5202
1.52
1.5198
1.5197
1.5195
1.5194
4.81E-07
4.70E-07
4.61E-07
4.51E-07
4.42E-07
4.34E-07
4.26E-07
4.18E-07
4.10E-07
4.02E-07
3.95E-07
3.88E-07
3.81E-07
3.74E-07
3.68E-07
3.61E-07
3.55E-07
3.49E-07
3.43E-07
3.37E-07
3.32E-07
3.27E-07
3.21E-07
3.16E-07
3.11E-07
3.07E-07
3.02E-07
2.98E-07
2.93E-07
2.89E-07
2.85E-07
2.81E-07
2.77E-07
2.74E-07
2.70E-07
2.67E-07
2.64E-07
2.60E-07
2.57E-07
2.54E-07
2.52E-07
2.49E-07
2.46E-07
2.44E-07
2.42E-07
455.99
457.58
459.16
460.75
462.34
463.93
465.51
467.1
468.69
470.27
471.86
473.45
475.04
476.62
478.21
479.8
481.38
482.97
484.56
486.14
487.73
489.31
490.9
492.49
494.07
495.66
497.24
498.83
500.42
502
503.59
505.17
506.76
508.34
509.93
511.51
513.1
514.68
516.27
517.85
519.44
521.02
522.61
524.19
525.78
1.5192
1.5191
1.5189
1.5188
1.5186
1.5185
1.5183
1.5182
1.5181
1.5179
1.5178
1.5176
1.5175
1.5174
1.5172
1.5171
1.517
1.5169
1.5167
1.5166
1.5165
1.5164
1.5162
1.5161
1.516
1.5159
1.5158
1.5156
1.5155
1.5154
1.5153
1.5152
1.5151
1.515
1.5149
1.5147
1.5146
1.5145
1.5144
1.5143
1.5142
1.5141
1.514
1.5139
1.5138
208
2.40E-07
2.37E-07
2.35E-07
2.34E-07
2.32E-07
2.30E-07
2.29E-07
2.27E-07
2.26E-07
2.25E-07
2.24E-07
2.22E-07
2.22E-07
2.21E-07
2.20E-07
2.19E-07
2.19E-07
2.18E-07
2.18E-07
2.18E-07
2.18E-07
2.18E-07
2.18E-07
2.18E-07
2.18E-07
2.19E-07
2.19E-07
2.20E-07
2.20E-07
2.21E-07
2.22E-07
2.23E-07
2.24E-07
2.25E-07
2.27E-07
2.28E-07
2.30E-07
2.31E-07
2.33E-07
2.35E-07
2.36E-07
2.38E-07
2.41E-07
2.43E-07
2.45E-07
527.36
528.95
530.53
532.12
533.7
535.28
536.87
538.45
540.04
541.62
543.21
544.79
546.37
547.96
549.54
551.12
552.71
554.29
555.87
557.46
559.04
560.63
562.21
563.79
565.37
566.96
568.54
570.12
571.71
573.29
574.87
576.46
578.04
579.62
581.2
582.79
584.37
585.95
587.53
589.12
590.7
592.28
593.86
595.45
597.03
1.5137
1.5136
1.5135
1.5134
1.5133
1.5132
1.5131
1.513
1.5129
1.5129
1.5128
1.5127
1.5126
1.5125
1.5124
1.5123
1.5122
1.5121
1.5121
1.512
1.5119
1.5118
1.5117
1.5116
1.5116
1.5115
1.5114
1.5113
1.5112
1.5112
1.5111
1.511
1.5109
1.5108
1.5108
1.5107
1.5106
1.5105
1.5105
1.5104
1.5103
1.5103
1.5102
1.5101
1.51
2.47E-07
2.50E-07
2.53E-07
2.55E-07
2.58E-07
2.61E-07
2.64E-07
2.67E-07
2.71E-07
2.74E-07
2.77E-07
2.81E-07
2.85E-07
2.89E-07
2.92E-07
2.97E-07
3.01E-07
3.05E-07
3.09E-07
3.14E-07
3.19E-07
3.23E-07
3.28E-07
3.33E-07
3.38E-07
3.44E-07
3.49E-07
3.54E-07
3.60E-07
3.66E-07
3.72E-07
3.78E-07
3.84E-07
3.90E-07
3.97E-07
4.03E-07
4.10E-07
4.17E-07
4.23E-07
4.31E-07
4.38E-07
4.45E-07
4.52E-07
4.60E-07
4.68E-07
598.61
600.19
601.78
603.36
604.94
606.52
608.1
609.69
611.27
612.85
614.43
616.01
617.6
619.18
620.76
622.34
623.92
625.5
627.09
628.67
630.25
631.83
633.41
634.99
636.58
638.16
639.74
641.32
642.9
644.48
646.06
647.65
649.23
650.81
652.39
653.97
655.55
657.13
658.71
660.29
661.88
663.46
665.04
666.62
668.2
1.51
1.5099
1.5098
1.5098
1.5097
1.5096
1.5096
1.5095
1.5094
1.5094
1.5093
1.5092
1.5092
1.5091
1.509
1.509
1.5089
1.5088
1.5088
1.5087
1.5087
1.5086
1.5085
1.5085
1.5084
1.5084
1.5083
1.5082
1.5082
1.5081
1.5081
1.508
1.508
1.5079
1.5078
1.5078
1.5077
1.5077
1.5076
1.5076
1.5075
1.5075
1.5074
1.5074
1.5073
4.76E-07
4.84E-07
4.92E-07
5.00E-07
5.09E-07
5.17E-07
5.26E-07
5.35E-07
5.44E-07
5.53E-07
5.62E-07
5.72E-07
5.81E-07
5.91E-07
6.01E-07
6.11E-07
6.21E-07
6.32E-07
6.42E-07
6.53E-07
6.64E-07
6.74E-07
6.86E-07
6.97E-07
7.08E-07
7.20E-07
7.31E-07
7.43E-07
7.55E-07
7.67E-07
7.80E-07
7.92E-07
8.05E-07
8.17E-07
8.30E-07
8.43E-07
8.56E-07
8.70E-07
8.83E-07
8.97E-07
9.11E-07
9.25E-07
9.39E-07
9.53E-07
9.67E-07
669.78
671.36
672.94
674.52
676.1
677.68
679.26
680.85
682.43
684.01
685.59
687.17
688.75
690.33
691.91
693.49
695.07
696.65
698.23
699.81
701.39
702.97
704.55
706.13
707.71
709.29
710.87
712.45
714.03
715.61
717.19
718.77
720.35
721.93
723.51
725.09
726.67
728.25
729.83
731.41
732.99
734.57
736.15
737.73
739.31
1.5072
1.5072
1.5071
1.5071
1.507
1.507
1.5069
1.5069
1.5068
1.5068
1.5067
1.5067
1.5066
1.5066
1.5065
1.5065
1.5064
1.5064
1.5064
1.5063
1.5063
1.5062
1.5062
1.5061
1.5061
1.506
1.506
1.5059
1.5059
1.5058
1.5058
1.5058
1.5057
1.5057
1.5056
1.5056
1.5055
1.5055
1.5055
1.5054
1.5054
1.5053
1.5053
1.5052
1.5052
209
9.82E-07
9.96E-07
1.01E-06
1.03E-06
1.04E-06
1.06E-06
1.07E-06
1.09E-06
1.10E-06
1.12E-06
1.13E-06
1.15E-06
1.17E-06
1.18E-06
1.20E-06
1.22E-06
1.23E-06
1.25E-06
1.27E-06
1.28E-06
1.30E-06
1.32E-06
1.34E-06
1.35E-06
1.37E-06
1.39E-06
1.41E-06
1.43E-06
1.44E-06
1.46E-06
1.48E-06
1.50E-06
1.52E-06
1.54E-06
1.55E-06
1.57E-06
1.59E-06
1.61E-06
1.63E-06
1.65E-06
1.67E-06
1.69E-06
1.71E-06
1.73E-06
1.75E-06
740.89
742.47
744.05
745.63
747.21
748.78
750.36
751.94
753.52
755.1
756.68
758.26
759.84
761.41
762.99
764.57
766.15
767.73
769.31
770.88
772.46
774.04
775.62
777.2
778.77
780.35
781.93
783.5
785.08
786.66
788.24
789.81
791.39
792.97
794.54
796.12
797.7
799.27
800.85
802.42
804
805.58
807.15
808.73
810.3
1.5052
1.5051
1.5051
1.505
1.505
1.505
1.5049
1.5049
1.5048
1.5048
1.5048
1.5047
1.5047
1.5046
1.5046
1.5046
1.5045
1.5045
1.5045
1.5044
1.5044
1.5043
1.5043
1.5043
1.5042
1.5042
1.5042
1.5041
1.5041
1.5041
1.504
1.504
1.504
1.5039
1.5039
1.5038
1.5038
1.5038
1.5037
1.5037
1.5037
1.5036
1.5036
1.5036
1.5035
1.77E-06
1.79E-06
1.81E-06
1.83E-06
1.85E-06
1.87E-06
1.89E-06
1.91E-06
1.93E-06
1.95E-06
1.97E-06
1.99E-06
2.01E-06
2.03E-06
2.05E-06
2.07E-06
2.09E-06
2.12E-06
2.14E-06
2.16E-06
2.18E-06
2.20E-06
2.22E-06
2.24E-06
2.26E-06
2.28E-06
2.31E-06
2.33E-06
2.35E-06
2.37E-06
2.39E-06
2.41E-06
2.43E-06
2.45E-06
2.47E-06
2.50E-06
2.52E-06
2.54E-06
2.56E-06
2.58E-06
2.60E-06
2.62E-06
2.64E-06
2.66E-06
2.69E-06
811.88
813.45
815.03
816.6
818.18
819.75
821.33
822.9
824.47
826.05
827.62
829.19
830.77
832.34
833.91
835.49
837.06
838.63
840.2
841.77
843.35
844.92
846.49
848.06
849.63
851.2
852.77
854.34
855.91
857.48
859.05
860.62
862.19
863.76
865.32
866.89
868.46
870.03
871.59
873.16
874.73
876.29
877.86
879.43
880.99
1.5035
1.5035
1.5034
1.5034
1.5034
1.5033
1.5033
1.5033
1.5033
1.5032
1.5032
1.5032
1.5031
1.5031
1.5031
1.503
1.503
1.503
1.5029
1.5029
1.5029
1.5029
1.5028
1.5028
1.5028
1.5027
1.5027
1.5027
1.5026
1.5026
1.5026
1.5026
1.5025
1.5025
1.5025
1.5024
1.5024
1.5024
1.5024
1.5023
1.5023
1.5023
1.5022
1.5022
1.5022
2.71E-06
2.73E-06
2.75E-06
2.77E-06
2.79E-06
2.81E-06
2.83E-06
2.85E-06
2.87E-06
2.89E-06
2.91E-06
2.94E-06
2.96E-06
2.98E-06
3.00E-06
3.02E-06
3.04E-06
3.06E-06
3.08E-06
3.10E-06
3.12E-06
3.14E-06
3.16E-06
3.18E-06
3.20E-06
3.22E-06
3.24E-06
3.25E-06
3.27E-06
3.29E-06
3.31E-06
3.33E-06
3.35E-06
3.37E-06
3.39E-06
3.41E-06
3.43E-06
3.44E-06
3.46E-06
3.48E-06
3.50E-06
3.52E-06
3.53E-06
3.55E-06
3.57E-06
882.56
884.12
885.68
887.25
888.81
890.38
891.94
893.5
895.06
896.62
898.19
899.75
901.31
902.87
904.43
905.99
907.55
909.1
910.66
912.22
913.78
915.34
916.89
918.45
920
921.56
923.11
924.67
926.22
927.77
929.33
930.88
932.43
933.98
935.53
937.08
938.63
940.18
941.73
943.27
944.82
946.37
947.91
949.46
951
1.5022
1.5021
1.5021
1.5021
1.5021
1.502
1.502
1.502
1.502
1.5019
1.5019
1.5019
1.5019
1.5018
1.5018
1.5018
1.5017
1.5017
1.5017
1.5017
1.5016
1.5016
1.5016
1.5016
1.5015
1.5015
1.5015
1.5015
1.5015
1.5014
1.5014
1.5014
1.5014
1.5013
1.5013
1.5013
1.5013
1.5012
1.5012
1.5012
1.5012
1.5011
1.5011
1.5011
1.5011
210
3.59E-06
3.60E-06
3.62E-06
3.64E-06
3.66E-06
3.67E-06
3.69E-06
3.71E-06
3.72E-06
3.74E-06
3.76E-06
3.77E-06
3.79E-06
3.81E-06
3.82E-06
3.84E-06
3.85E-06
3.87E-06
3.88E-06
3.90E-06
3.91E-06
3.93E-06
3.94E-06
3.96E-06
3.97E-06
3.99E-06
4.00E-06
4.02E-06
4.03E-06
4.05E-06
4.06E-06
4.07E-06
4.09E-06
4.10E-06
4.11E-06
4.13E-06
4.14E-06
4.15E-06
4.17E-06
4.18E-06
4.19E-06
4.20E-06
4.22E-06
4.23E-06
4.24E-06
952.55
954.09
955.63
957.18
958.72
960.26
961.8
963.34
964.88
966.41
967.95
969.49
971.02
972.56
974.09
975.63
977.16
978.69
980.22
981.75
983.28
984.81
986.34
987.87
989.4
990.92
992.45
993.97
995.49
997.01
998.54
1000.1
1012.3
1015.8
1019.2
1022.6
1026.1
1029.5
1032.9
1036.3
1039.8
1043.2
1046.6
1050
1053.5
1.5011
1.501
1.501
1.501
1.501
1.5009
1.5009
1.5009
1.5009
1.5009
1.5008
1.5008
1.5008
1.5008
1.5007
1.5007
1.5007
1.5007
1.5007
1.5006
1.5006
1.5006
1.5006
1.5006
1.5005
1.5005
1.5005
1.5005
1.5005
1.5004
1.5004
1.5004
1.5002
1.5002
1.5001
1.5001
1.5001
1.5
1.5
1.4999
1.4999
1.4998
1.4998
1.4998
1.4997
4.25E-06
4.26E-06
4.28E-06
4.29E-06
4.30E-06
4.31E-06
4.32E-06
4.33E-06
4.34E-06
4.36E-06
4.37E-06
4.38E-06
4.39E-06
4.40E-06
4.41E-06
4.42E-06
4.43E-06
4.44E-06
4.45E-06
4.46E-06
4.47E-06
4.48E-06
4.49E-06
4.50E-06
4.51E-06
4.51E-06
4.52E-06
4.53E-06
4.54E-06
4.55E-06
4.56E-06
4.57E-06
4.63E-06
4.65E-06
4.67E-06
4.68E-06
4.70E-06
4.72E-06
4.73E-06
4.75E-06
4.76E-06
4.78E-06
4.79E-06
4.81E-06
4.82E-06
1056.9
1060.3
1063.8
1067.2
1070.6
1074
1077.5
1080.9
1084.3
1087.7
1091.2
1094.6
1098
1101.5
1104.9
1108.3
1111.7
1115.2
1118.6
1122
1125.4
1128.9
1132.3
1135.7
1139.2
1142.6
1146
1149.4
1152.9
1156.3
1159.7
1163.2
1166.6
1170
1173.4
1176.9
1180.3
1183.7
1187.2
1190.6
1194
1197.4
1200.9
1204.3
1207.7
1.4997
1.4996
1.4996
1.4996
1.4995
1.4995
1.4994
1.4994
1.4994
1.4993
1.4993
1.4993
1.4992
1.4992
1.4991
1.4991
1.4991
1.499
1.499
1.499
1.4989
1.4989
1.4989
1.4988
1.4988
1.4988
1.4987
1.4987
1.4987
1.4986
1.4986
1.4986
1.4985
1.4985
1.4985
1.4984
1.4984
1.4984
1.4983
1.4983
1.4983
1.4982
1.4982
1.4982
1.4981
4.84E-06
4.85E-06
4.86E-06
4.88E-06
4.89E-06
4.90E-06
4.91E-06
4.93E-06
4.94E-06
4.95E-06
4.97E-06
4.98E-06
4.99E-06
5.00E-06
5.02E-06
5.03E-06
5.04E-06
5.06E-06
5.07E-06
5.08E-06
5.10E-06
5.11E-06
5.12E-06
5.14E-06
5.15E-06
5.16E-06
5.17E-06
5.19E-06
5.20E-06
5.21E-06
5.22E-06
5.24E-06
5.25E-06
5.26E-06
5.27E-06
5.28E-06
5.29E-06
5.30E-06
5.31E-06
5.32E-06
5.33E-06
5.34E-06
5.35E-06
5.36E-06
5.36E-06
1211.2
1214.6
1218
1221.5
1224.9
1228.3
1231.7
1235.2
1238.6
1242
1245.5
1248.9
1252.3
1255.8
1259.2
1262.6
1266.1
1269.5
1272.9
1276.4
1279.8
1283.2
1286.7
1290.1
1293.5
1297
1300.4
1303.8
1307.3
1310.7
1314.1
1317.6
1321
1324.4
1327.9
1331.3
1334.7
1338.2
1341.6
1345
1348.5
1351.9
1355.4
1358.8
1362.2
1.4981
1.4981
1.4981
1.498
1.498
1.498
1.4979
1.4979
1.4979
1.4978
1.4978
1.4978
1.4978
1.4977
1.4977
1.4977
1.4976
1.4976
1.4976
1.4976
1.4975
1.4975
1.4975
1.4975
1.4974
1.4974
1.4974
1.4973
1.4973
1.4973
1.4973
1.4972
1.4972
1.4972
1.4972
1.4971
1.4971
1.4971
1.4971
1.497
1.497
1.497
1.497
1.4969
1.4969
211
5.37E-06
5.38E-06
5.39E-06
5.39E-06
5.40E-06
5.40E-06
5.41E-06
5.41E-06
5.41E-06
5.41E-06
5.42E-06
5.42E-06
5.42E-06
5.42E-06
5.42E-06
5.42E-06
5.42E-06
5.41E-06
5.41E-06
5.41E-06
5.40E-06
5.40E-06
5.40E-06
5.39E-06
5.38E-06
5.38E-06
5.37E-06
5.36E-06
5.35E-06
5.34E-06
5.33E-06
5.32E-06
5.31E-06
5.30E-06
5.28E-06
5.27E-06
5.26E-06
5.24E-06
5.23E-06
5.21E-06
5.19E-06
5.18E-06
5.16E-06
5.14E-06
5.12E-06
1365.7
1369.1
1372.5
1376
1379.4
1382.9
1386.3
1389.7
1393.2
1396.6
1400
1403.5
1406.9
1410.4
1413.8
1417.2
1420.7
1424.1
1427.6
1431
1434.5
1437.9
1441.3
1444.8
1448.2
1451.7
1455.1
1458.6
1462
1465.4
1468.9
1472.3
1475.8
1479.2
1482.7
1486.1
1489.6
1493
1496.5
1499.9
1503.4
1506.8
1510.2
1513.7
1517.1
1.4969
1.4969
1.4969
1.4968
1.4968
1.4968
1.4968
1.4967
1.4967
1.4967
1.4967
1.4966
1.4966
1.4966
1.4966
1.4966
1.4965
1.4965
1.4965
1.4965
1.4964
1.4964
1.4964
1.4964
1.4964
1.4963
1.4963
1.4963
1.4963
1.4963
1.4962
1.4962
1.4962
1.4962
1.4961
1.4961
1.4961
1.4961
1.4961
1.496
1.496
1.496
1.496
1.496
1.4959
5.11E-06
5.09E-06
5.07E-06
5.05E-06
5.03E-06
5.01E-06
4.98E-06
4.96E-06
4.94E-06
4.92E-06
4.90E-06
4.87E-06
4.85E-06
4.83E-06
4.81E-06
4.78E-06
4.76E-06
4.73E-06
4.71E-06
4.69E-06
4.66E-06
4.64E-06
4.62E-06
4.59E-06
4.57E-06
4.54E-06
4.52E-06
4.50E-06
4.47E-06
4.45E-06
4.43E-06
4.40E-06
4.38E-06
4.36E-06
4.33E-06
4.31E-06
4.29E-06
4.27E-06
4.25E-06
4.23E-06
4.21E-06
4.19E-06
4.17E-06
4.15E-06
4.13E-06
1520.6
1524
1527.5
1530.9
1534.4
1537.8
1541.3
1544.7
1548.2
1551.7
1555.1
1558.6
1562
1565.5
1568.9
1572.4
1575.8
1579.3
1582.7
1586.2
1589.7
1593.1
1596.6
1600
1603.5
1606.9
1610.4
1613.9
1617.3
1620.8
1624.2
1627.7
1631.2
1634.6
1638.1
1641.5
1645
1648.5
1651.9
1655.4
1658.9
1662.3
1665.8
1.4959
1.4959
1.4959
1.4959
1.4959
1.4958
1.4958
1.4958
1.4958
1.4958
1.4957
1.4957
1.4957
1.4957
1.4957
1.4956
1.4956
1.4956
1.4956
1.4956
1.4956
1.4955
1.4955
1.4955
1.4955
1.4955
1.4955
1.4954
1.4954
1.4954
1.4954
1.4954
1.4953
1.4953
1.4953
1.4953
1.4953
1.4953
1.4952
1.4952
1.4952
1.4952
1.4952
4.11E-06
4.09E-06
4.07E-06
4.05E-06
4.04E-06
4.02E-06
4.00E-06
3.99E-06
3.97E-06
3.95E-06
3.94E-06
3.92E-06
3.91E-06
3.90E-06
3.88E-06
3.87E-06
3.86E-06
3.85E-06
3.83E-06
3.82E-06
3.81E-06
3.80E-06
3.79E-06
3.78E-06
3.77E-06
3.76E-06
3.75E-06
3.74E-06
3.73E-06
3.72E-06
3.71E-06
3.70E-06
3.69E-06
3.68E-06
3.67E-06
3.66E-06
3.65E-06
3.64E-06
3.63E-06
3.62E-06
3.60E-06
3.59E-06
3.58E-06
1669.3 1.4952
212
3.56E-06
A.2
Dielectric function of SnO2
eV
ε1
ε2
4.9743
3.8634
1.2246
4.0042
4.2701 0.47692
6.5004
3.3297
1.1928
4.9429
3.8783
1.2116
3.9837
4.2587 0.45382
6.4471
3.3326
1.1992
4.9118
3.8921
1.1987
3.9635
4.2467 0.43215
6.3947
3.3362
1.2058
4.8811
3.905
1.1859
3.9434
4.2343
6.3432
3.3405
1.2127
4.8508
3.9169
1.1736
3.9235
4.2217 0.39288
6.2924
3.3456
1.2197
4.8209
3.9282
1.1618
3.9039
4.2089 0.37512
6.2425
3.3515
1.2269
4.7913
3.939
1.1509
3.8844
4.196
6.1933
3.3581
1.2341
4.7621
3.9496
1.141
3.8652
4.1832 0.34297
6.1448
3.3655
1.2414
4.7332
3.9604
1.1319
3.8461
4.1704
6.0972
3.3738
1.2487
4.7047
3.9717
1.1238
3.8272
4.1578 0.31474
6.0502
3.3828
1.256
4.6765
3.9838
1.1164
3.8085
4.1453 0.30191
6.0039
3.3927
1.2632
4.6486
3.997
1.1096
3.79
5.9584
3.4034
1.2702
4.6211
4.0117
1.1029
3.7716
4.1209 0.27847
5.9135
3.4149
1.2771
4.5939
4.0279
1.0961
3.7534
4.109
5.8692
3.4272
1.2836
4.567
4.0457
1.0887
3.7355
4.0973 0.25761
5.8257
3.4404
1.2899
4.5405
4.065
1.0802
3.7176
4.0858 0.24802
5.7827
3.4544
1.2958
4.5142
4.0858
1.0702
3.7
4.0746 0.23894
5.7404
3.4691
1.3012
4.4882
4.1077
1.0584
3.6825
4.0635 0.23033
5.6987
3.4846
1.3061
4.4626
4.1304
1.0444
3.6652
4.0527 0.22216
5.6575
3.5009
1.3105
4.4372
4.1536
1.0279
3.648
4.042
5.617
3.5178
1.3142
4.4121
4.1767
1.0089
3.631
4.0316 0.20703
5.577
3.5355
1.3173
4.3873
4.1992 0.98722
3.6142
4.0214 0.20003
5.5376
3.5537
1.3196
4.3627
4.2207 0.96303
3.5975
4.0113
5.4987
3.5725
1.3211
4.3384
4.2407 0.93647
3.5809
4.0014 0.18704
5.4604
3.5919
1.3217
4.3144
4.2589 0.90779
3.5646
3.9917 0.18103
5.4226
3.6116
1.3214
4.2907
4.2749 0.87732
3.5483
3.9822 0.17531
5.3853
3.6318
1.3201
4.2672
4.2885 0.84541
3.5322
3.9728 0.16989
5.3485
3.6522
1.3178
4.244
4.2996 0.81247
3.5163
3.9636 0.16473
5.3122
3.6728
1.3145
4.221
4.3079
0.7789
3.5005
3.9546 0.15985
5.2764
3.6935
1.31
4.1983
4.3137
0.7451
3.4848
3.9458 0.15521
5.241
3.7141
1.3045
4.1758
4.3168 0.71142
3.4693
3.9371 0.15083
5.2062
3.7347
1.2979
4.1536
4.3174 0.67821
3.4539
3.9285 0.14668
5.1717
3.7549
1.2902
4.1315
4.3158 0.64576
3.4387
3.9202 0.14275
5.1378
3.7748
1.2814
4.1098
4.312
0.6143
3.4236
3.912
5.1042
3.7941
1.2716
4.0882
4.3064 0.58403
3.4086
3.9039 0.13556
5.0711
3.8127
1.2609
4.0669
4.2992 0.55508
3.3938
3.896
5.0384
3.8306
1.2494
4.0458
4.2906 0.52755
3.3791
3.8883 0.12919
5.0062
3.8475
1.2372
4.0249
4.2808 0.50149
3.3645
3.8807
213
4.133
0.41186
0.35851
0.3284
0.28984
0.26775
0.2144
0.19337
0.13905
0.13228
0.1263
3.35
3.8733 0.12359
2.8693
3.6968 0.073523
2.5098
3.6063 0.044057
3.3357
3.8661 0.12106
2.8588
3.6939
2.5017
3.6045 0.043454
3.3215
3.859
0.11871
2.8483
3.691 0.071687
2.4937
3.6026 0.042858
3.3074
3.8521 0.11652
2.838
3.6881 0.070784
2.4858
3.6008 0.042268
3.2934
3.8454
0.1145
2.8277
3.6853 0.069891
2.4779
3.599 0.041684
3.2795
3.8389 0.11264
2.8175
3.6826 0.069008
2.4701
3.5972 0.041107
3.2658
3.8325 0.11093
2.8074
3.6799 0.068134
2.4623
3.5955 0.040536
3.2522
3.8263 0.10937
2.7973
3.6772 0.06727
2.4546
3.5937 0.039971
3.2386
3.8204 0.10795
2.7873
3.6745 0.066415
2.4469
3.592 0.039412
3.2252
3.8147 0.10664
2.7774
3.6719 0.06557
2.4393
3.5903 0.03886
3.212
3.8093 0.10534
2.7676
3.6694 0.064734
2.4317
3.5886 0.038313
3.1988
3.804
0.10406
2.7578
3.6668 0.063906
2.4242
3.5869 0.037773
3.1857
3.7989 0.10279
2.7481
3.6643 0.063088
2.4167
3.5852 0.037238
3.1727
3.7939 0.10154
2.7384
3.6618 0.062279
2.4092
3.5835 0.036709
3.1599
3.7891
2.7289
3.6594 0.061479
2.4018
3.5819 0.036186
3.1471
3.7844 0.099079
2.7194
3.6569 0.060687
2.3945
3.5802 0.035669
3.1345
3.7798 0.09787
2.7099
3.6546 0.059904
2.3872
3.5786 0.035157
3.1219
3.7754 0.096675
2.7006
3.6522 0.059129
2.3799
3.577 0.034651
3.1095
3.771 0.095493
2.6913
3.6499 0.058363
2.3727
3.5754 0.034151
3.0971
3.7667 0.094324
2.682
3.6476 0.057605
2.3655
3.5738 0.033656
3.0849
3.7626 0.093169
2.6728
3.6453 0.056856
2.3584
3.5722 0.033166
3.0727
3.7585 0.092027
2.6637
3.643
0.056114
2.3513
3.5707 0.032682
3.0607
3.7545 0.090898
2.6547
3.6408 0.055381
2.3443
3.5691 0.032203
3.0487
3.7506 0.089782
2.6457
3.6386 0.054655
2.3373
3.5676 0.03173
3.0368
3.7468 0.088678
2.6368
3.6364 0.053937
2.3303
3.5661 0.031262
3.025
3.743 0.087586
2.6279
3.6343 0.053227
2.3234
3.5646 0.030799
3.0134
3.7393 0.086507
2.6191
3.6321 0.052525
2.3165
3.5631 0.030341
3.0018
3.7357 0.08544
2.6103
0.05183
2.3097
3.5616 0.029888
2.9902
3.7321 0.084385
2.6016
3.6279 0.051143
2.3029
3.5601 0.02944
2.9788
3.7286 0.083341
2.593
3.6259 0.050463
2.2961
3.5586 0.028998
2.9675
3.7252 0.08231
2.5844
3.6238 0.049791
2.2894
3.5572 0.02856
2.9563
3.7218 0.081289
2.5759
3.6218 0.049126
2.2827
3.5557 0.028127
2.9451
3.7185 0.08028
2.5675
3.6198 0.048468
2.2761
3.5543 0.027699
2.934
3.7153 0.079283
2.559
3.6178 0.047817
2.2695
3.5529 0.027276
2.923
3.7121 0.078296
2.5507
3.6159 0.047173
2.263
3.5515 0.026857
2.9121
3.7089 0.07732
2.5424
3.6139 0.046536
2.2564
3.5501 0.026444
2.9013
3.7058 0.076355
2.5342
3.612 0.045906
2.2499
3.5487 0.026035
2.8905
3.7027 0.075401
2.526
3.6101 0.045283
2.2435
3.5473 0.025631
2.8799
3.6997 0.074457
2.5178
3.6082 0.044667
2.2371
3.5459 0.025231
0.1003
3.63
214
0.0726
2.2307
3.5445 0.024836
2.0078
3.4975 0.012588
1.8255
3.4595 0.0053216
2.2244
3.5432 0.024445
2.0027
3.4964 0.012346
1.8213
3.4586 0.0051883
2.2181
3.5418 0.024059
1.9976
3.4954 0.012107
1.817
3.4578 0.0050575
2.2118
3.5405 0.023677
1.9925
3.4943 0.011872
1.8128
3.4569 0.0049289
2.2056
3.5392
1.9874
3.4933 0.01164
1.8087
3.456 0.0048027
2.1994
3.5379 0.022927
1.9824
3.4922 0.01141
1.8045
3.4551 0.0046789
2.1932
3.5365 0.022558
1.9774
3.4912 0.011184
1.8004
3.4543 0.0045574
2.1871
3.5352 0.022194
1.9724
3.4901 0.010961
1.7962
3.4534 0.0044382
2.181
3.534 0.021834
1.9675
3.4891 0.010741
1.7921
3.4526 0.0043213
2.175
3.5327 0.021478
1.9625
3.4881 0.010524
1.7881
3.4517 0.0042067
2.1689
3.5314 0.021126
1.9577
3.4871 0.010311
1.784
3.4508 0.0040943
2.163
3.5301 0.020779
1.9528
3.486
1.7799
3.45 0.0039842
2.157
3.5289 0.020435
1.9479
3.485 0.0098917
1.7759
3.4492 0.0038763
2.1511
3.5276 0.020096
1.9431
3.484 0.0096867
1.7719
3.4483 0.0037706
2.1452
3.5264 0.01976
1.9383
3.483 0.0094847
1.7679
3.4475 0.0036671
2.1393
3.5251 0.019429
1.9335
3.482 0.0092855
1.7639
3.4467 0.0035658
2.1335
3.5239 0.019101
1.9288
3.481 0.0090893
1.76
3.4458 0.0034667
2.1277
3.5227 0.018778
1.924
3.4801 0.0088959
1.756
3.445 0.0033697
2.1219
3.5215 0.018458
1.9193
3.4791 0.0087053
1.7521
3.4442 0.0032749
2.1162
3.5202 0.018142
1.9146
3.4781 0.0085175
1.7482
3.4434 0.0031821
2.1105
3.519
1.91
3.4771 0.0083325
1.7443
3.4426 0.0030915
2.1048
3.5179 0.017522
1.9053
3.4762 0.0081503
1.7405
3.4417 0.003003
2.0992
3.5167 0.017218
1.9007
3.4752 0.0079709
1.7366
3.4409 0.0029166
2.0936
3.5155 0.016917
1.8961
3.4742 0.0077941
1.7328
3.4401 0.0028322
2.088
3.5143 0.01662
1.8915
3.4733 0.0076201
1.729
3.4393 0.0027499
2.0825
3.5132 0.016327
1.887
3.4723 0.0074488
1.7252
3.4385 0.0026696
2.077
3.512 0.016037
1.8825
3.4714 0.0072801
1.7214
3.4377 0.0025913
2.0715
3.5108 0.015751
1.878
3.4705 0.0071141
1.7176
3.437 0.0025151
2.066
3.5097 0.015468
1.8735
3.4695 0.0069507
1.7139
3.4362 0.0024408
2.0606
3.5086 0.015189
1.869
3.4686 0.00679
1.7101
3.4354 0.0023685
2.0552
3.5074 0.014914
1.8646
3.4677 0.0066318
1.7064
3.4346 0.0022982
2.0498
3.5063 0.014642
1.8601
3.4667 0.0064762
1.7027
3.4338 0.0022299
2.0444
3.5052 0.014373
1.8557
3.4658 0.0063231
1.699
3.4331 0.0021635
2.0391
3.5041 0.014108
1.8514
3.4649 0.0061726
1.6954
3.4323 0.002099
2.0338
3.503 0.013846
1.847
3.464 0.0060246
1.6917
3.4315 0.0020365
2.0286
3.5019 0.013588
1.8427
3.4631 0.0058791
1.6881
3.4308 0.0019759
2.0233
3.5008 0.013333
1.8383
3.4622 0.005736
1.6844
3.43 0.0019171
2.0181
3.4997 0.013081
1.834
3.4613 0.0055955
1.6808
3.4292 0.0018602
2.0129
3.4986 0.012833
1.8298
3.4604 0.0054573
1.6772
3.4285 0.0018052
0.0233
0.01783
215
0.0101
1.6737
3.4277 0.0017521
1.5453
3.4009 0.0009975
1.4356
3.3785 0.0012441
1.6701
3.427 0.0017008
1.5423
3.4003 0.0010034
1.433
3.378 0.0012509
1.6666
3.4262 0.0016514
1.5393
3.3997 0.0010093
1.4304
3.3774 0.0012577
1.663
3.4255 0.0016037
1.5363
3.3991 0.0010153
1.4278
3.3769 0.0012646
1.6595
3.4248 0.0015579
1.5333
3.3985 0.0010212
1.4252
3.3764 0.0012714
1.656
3.424 0.0015139
1.5303
3.3978 0.0010272
1.4227
3.3759 0.0012783
1.6525
3.4233 0.0014716
1.5273
3.3972 0.0010332
1.4201
3.3753 0.0012852
1.6491
3.4226 0.0014311
1.5244
3.3966 0.0010392
1.4176
3.3748 0.0012922
1.6456
3.4218 0.0013924
1.5214
3.396 0.0010453
1.4151
3.3743 0.0012991
1.6422
3.4211 0.0013555
1.5185
3.3954 0.0010513
1.4125
3.3738 0.0013061
1.6387
3.4204 0.0013202
1.5156
3.3948 0.0010574
1.41
3.3732 0.0013131
1.6353
3.4197 0.0012867
1.5127
3.3943 0.0010636
1.4075
3.3727 0.0013201
1.6319
3.419 0.001255
1.5098
3.3937 0.0010697
1.405
3.3722 0.0013271
1.6285
3.4183 0.0012249
1.5069
3.3931 0.0010759
1.4025
3.3717 0.0013342
1.6252
3.4176 0.0011965
1.504
3.3925 0.001082
1.4
3.3712 0.0013413
1.6218
3.4169 0.0011698
1.5011
3.3919 0.0010882
1.3976
3.3706 0.0013484
1.6185
3.4162 0.0011448
1.4983
3.3913 0.0010945
1.3951
3.3701 0.0013556
1.6152
3.4155 0.0011215
1.4954
3.3907 0.0011007
1.3927
3.3696 0.0013627
1.6118
3.4148 0.0010998
1.4926
3.3902 0.001107
1.3902
3.3691 0.0013699
1.6085
3.4141 0.0010798
1.4898
3.3896 0.0011133
1.3878
3.3686 0.0013771
1.6053
3.4134 0.0010613
1.487
3.389 0.0011196
1.3854
3.3681 0.0013844
1.602
3.4127 0.0010445
1.4842
3.3885 0.001126
1.383
3.3676 0.0013916
1.5987
3.412 0.0010294
1.4814
3.3879 0.0011323
1.3806
3.3671 0.0013989
1.5955
3.4113 0.0010158
1.4786
3.3873 0.0011387
1.3782
3.3666 0.0014062
1.5922
3.4107 0.0010038
1.4758
3.3868 0.0011451
1.3758
3.3661 0.0014135
1.589
3.41 0.0009934
1.4731
3.3862 0.0011516
1.3734
3.3656 0.0014209
1.5858
3.4093 0.0009846
1.4703
3.3856 0.001158
1.371
3.3651 0.0014283
1.5826
3.4086 0.0009773
1.4676
3.3851 0.0011645
1.3687
3.3646 0.0014357
1.5795
3.408 0.0009716
1.4649
3.3845 0.001171
1.3663
3.3641 0.0014431
1.5763
3.4073 0.0009674
1.4622
3.384 0.0011776
1.364
3.3636 0.0014505
1.5731
3.4067 0.0009648
1.4595
3.3834 0.0011841
1.3616
3.3631 0.001458
1.57
3.406 0.0009636
1.4568
3.3829 0.0011907
1.3593
3.3626 0.0014655
1.5669
3.4054 0.000964
1.4541
3.3823 0.0011973
1.357
3.3621 0.001473
1.5637
3.4047 0.0009659
1.4514
3.3818 0.0012039
1.3547
3.3616 0.0014805
1.5606
3.4041 0.0009693
1.4487
3.3812 0.0012106
1.3524
3.3611 0.0014881
1.5576
3.4034 0.0009742
1.4461
3.3807 0.0012172
1.3501
3.3606 0.0014957
1.5545
3.4028 0.00098
1.4435
3.3801 0.0012239
1.3478
3.3601 0.0015033
1.5514
3.4022 0.0009858
1.4408
3.3796 0.0012306
1.3455
3.3596 0.0015109
1.5484
3.4015 0.0009917
1.4382
3.3791 0.0012374
1.3433
3.3591 0.0015186
216
1.341
3.3586 0.0015263
1.2591
3.3404 0.0018438
1.1188
3.305 0.0026278
1.3388
3.3582 0.001534
1.2572
3.3399 0.0018524
1.1154
3.3041 0.0026522
1.3365
3.3577 0.0015417
1.2552
3.3395 0.001861
1.1119
3.3031 0.0026768
1.3343
3.3572 0.0015495
1.2533
3.339 0.0018697
1.1085
3.3021 0.0027016
1.3321
3.3567 0.0015572
1.2514
3.3386 0.0018783
1.1052
3.3012 0.0027265
1.3299
3.3562 0.001565
1.2494
3.3381 0.001887
1.1018
3.3002 0.0027515
1.3277
3.3557 0.0015729
1.2475
3.3377 0.0018957
1.0984
3.2993 0.0027767
1.3255
3.3553 0.0015807
1.2456
3.3372 0.0019044
1.0951
3.2983 0.0028021
1.3233
3.3548 0.0015886
1.2437
3.3368 0.0019132
1.0918
3.2974 0.0028276
1.3211
3.3543 0.0015965
1.2418
3.3363 0.0019219
1.0885
3.2964 0.0028533
1.3189
3.3538 0.0016044
1.2399
3.3359 0.0019307
1.0853
3.2954 0.0028791
1.3167
3.3534 0.0016123
1.2249
3.3323 0.0020028
1.082
3.2945 0.0029051
1.3146
3.3529 0.0016203
1.2207
3.3313 0.0020232
1.0788
3.2935 0.0029312
1.3124
3.3524 0.0016282
1.2166
3.3303 0.0020437
1.0756
3.2926 0.0029575
1.3103
3.3519 0.0016363
1.2126
3.3293 0.0020644
1.0724
3.2916 0.002984
1.3081
3.3515 0.0016443
1.2085
3.3283 0.0020852
1.0692
3.2907 0.0030106
1.306
3.351 0.0016523
1.2045
3.3274 0.0021062
1.0661
3.2897 0.0030374
1.3039
3.3505 0.0016604
1.2005
3.3264 0.0021273
1.0629
3.2888 0.0030643
1.3018
3.35 0.0016685
1.1965
3.3254 0.0021485
1.0598
3.2878 0.0030914
1.2997
3.3496 0.0016766
1.1926
3.3244 0.0021699
1.0567
3.2868 0.0031186
1.2976
3.3491 0.0016848
1.1887
3.3234 0.0021914
1.0536
3.2859 0.003146
1.2955
3.3486 0.0016929
1.1848
3.3225 0.0022131
1.0506
3.2849 0.0031736
1.2934
3.3482 0.0017011
1.1809
3.3215 0.0022349
1.0475
3.284 0.0032013
1.2913
3.3477 0.0017093
1.1771
3.3205 0.0022568
1.0445
3.283 0.0032292
1.2893
3.3472 0.0017176
1.1732
3.3195 0.0022789
1.0415
3.2821 0.0032573
1.2872
3.3468 0.0017258
1.1695
3.3185 0.0023011
1.0385
3.2811 0.0032855
1.2851
3.3463 0.0017341
1.1657
3.3176 0.0023235
1.0355
3.2802 0.0033139
1.2831
3.3459 0.0017424
1.1619
3.3166 0.002346
1.0326
3.2792 0.0033424
1.2811
3.3454 0.0017507
1.1582
3.3156 0.0023687
1.0296
3.2782 0.0033711
1.279
3.3449 0.0017591
1.1545
3.3147 0.0023915
1.0267
3.2773
1.277
3.3445 0.0017675
1.1509
3.3137 0.0024145
1.0238
3.2763 0.003429
1.275
3.344 0.0017759
1.1472
3.3127 0.0024376
1.0209
3.2754 0.0034582
1.273
3.3436 0.0017843
1.1436
3.3118 0.0024608
1.018
3.2744 0.0034876
1.271
3.3431 0.0017927
1.14
3.3108 0.0024842
1.0152
3.2734 0.0035171
1.269
3.3426 0.0018012
1.1364
3.3098 0.0025078
1.0123
3.2725 0.0035468
1.267
3.3422 0.0018097
1.1328
3.3089 0.0025315
1.0095
3.2715 0.0035767
1.265
3.3417 0.0018182
1.1293
3.3079 0.0025553
1.0067
3.2706 0.0036067
1.263
3.3413 0.0018267
1.1258
3.3069 0.0025793
1.0039
3.2696 0.0036369
1.2611
3.3408 0.0018352
1.1223
3.306 0.0026035
1.0011
3.2687 0.0036673
217
0.0034
0.99836 3.2677 0.0036979
0.90118 3.2296 0.005027
0.82106 3.1899 0.0066458
0.99561 3.2667 0.0037286
0.89893 3.2286 0.0050647
0.81919 3.1888 0.0066914
0.99287 3.2658 0.0037595
0.8967
3.2276 0.0051027
0.81732 3.1878 0.0067372
0.99015 3.2648 0.0037905
0.89447 3.2266 0.0051408
0.81547 3.1867 0.0067832
0.98744 3.2638 0.0038218
0.89226 3.2256 0.0051791
0.81362 3.1857 0.0068294
0.98475 3.2629 0.0038532
0.89006 3.2246 0.0052177
0.81179 3.1846 0.0068759
0.98208 3.2619 0.0038847
0.88787 3.2236 0.0052564
0.80996 3.1836 0.0069225
0.97941 3.2609 0.0039165
0.88569 3.2226 0.0052953
0.80814 3.1825 0.0069694
0.97677
3.26 0.0039484
0.88351 3.2216 0.0053344
0.80632 3.1815 0.0070165
0.97413
3.259 0.0039805
0.88135 3.2206 0.0053737
0.80452 3.1804 0.0070638
0.97151
3.258 0.0040128
0.8792
3.2196 0.0054132
0.80272 3.1793 0.0071114
0.96891 3.2571 0.0040452
0.87707 3.2186 0.0054529
0.80093 3.1783 0.0071591
0.96632 3.2561 0.0040778
0.87493 3.2176 0.0054928
0.79915 3.1772 0.0072071
0.96374 3.2551 0.0041106
0.87282 3.2166 0.0055328
0.79737 3.1762 0.0072553
0.96117 3.2542 0.0041436
0.87071 3.2156 0.0055731
0.79561 3.1751 0.0073037
0.95862 3.2532 0.0041768
0.86861 3.2146 0.0056136
0.79385
3.174 0.0073523
0.95608 3.2522 0.0042101
0.86652 3.2136 0.0056543
0.7921
3.1729 0.0074011
0.95356 3.2513 0.0042436
0.86444 3.2126 0.0056951
0.79035 3.1719 0.0074502
0.95105 3.2503 0.0042773
0.86237 3.2115 0.0057362
0.78862 3.1708 0.0074995
0.94855 3.2493 0.0043112
0.86031 3.2105 0.0057775
0.78689 3.1697 0.007549
0.94607 3.2483 0.0043452
0.85826 3.2095 0.0058189
0.78517 3.1686 0.0075987
0.94359 3.2474 0.0043794
0.85622 3.2085 0.0058606
0.78345 3.1676 0.0076486
0.94113 3.2464 0.0044138
0.85419 3.2075 0.0059025
0.78174 3.1665 0.0076988
0.93869 3.2454 0.0044484
0.85217 3.2064 0.0059446
0.78004 3.1654 0.0077492
0.93625 3.2444 0.0044832
0.85016 3.2054 0.0059868
0.77835 3.1643 0.0077998
0.93383 3.2435 0.0045182
0.84815 3.2044 0.0060293
0.77667 3.1632 0.0078507
0.93142 3.2425 0.0045533
0.84616 3.2034 0.006072
0.77499 3.1621 0.0079018
0.92902 3.2415 0.0045886
0.84417 3.2023 0.0061149
0.77332 3.1611 0.0079531
0.92664 3.2405 0.0046241
0.8422
0.77165
3.16 0.0080046
0.92427 3.2395 0.0046598
0.84023 3.2003 0.0062013
0.77
3.1589 0.0080563
0.9219
3.2385 0.0046957
0.83828 3.1992 0.0062448
0.76835 3.1578 0.0081083
0.91956 3.2376 0.0047317
0.83633 3.1982 0.0062885
0.7667
0.91722 3.2366 0.004768
0.83439 3.1972 0.0063325
0.76507 3.1556 0.008213
0.91489 3.2356 0.0048044
0.83246 3.1961 0.0063766
0.76344 3.1545 0.0082656
0.91258 3.2346 0.004841
0.83054 3.1951 0.0064209
0.76181 3.1534 0.0083185
0.91028 3.2336 0.0048778
0.82862 3.1941 0.0064655
0.7602
0.90798 3.2326 0.0049149
0.82672
3.193 0.0065102
0.75859 3.1512 0.008425
0.9057
0.82482
3.192 0.0065552
0.75698 3.1501 0.0084786
3.2316 0.004952
0.90344 3.2306 0.0049894
3.2013 0.006158
0.82294 3.1909 0.0066004
218
0.75539
3.1567 0.0081605
3.1523 0.0083717
3.149 0.0085325
0.7538
3.1478 0.0085865
0.75221 3.1467 0.0086408
0.75064 3.1456 0.0086954
0.74907 3.1445 0.0087501
0.7475
3.1434 0.0088051
0.74594 3.1423 0.0088604
0.74439 3.1411 0.0089158
0.74285
3.14 0.0089715
219
A.3
Dielectric function of SiO2
eV
ε1
ε2
5.0062
2.3328
0
4.0458
2.2726
0
3.3938
2.2409
0
6.5004
2.4689
0
4.9743
2.3305
0
4.0249
2.2715
0
3.3791
2.2402
0
6.4471
2.4629
0
4.9429
2.3283
0
4.0042
2.2704
0
3.3645
2.2396
0
6.3947
2.4571
0
4.9118
2.3261
0
3.9837
2.2693
0
3.35
2.239
0
6.3432
2.4515
0
4.8811
2.3239
0
3.9635
2.2683
0
3.3357
2.2383
0
6.2924
2.4461
0
4.8508
2.3218
0
3.9434
2.2672
0
3.3215
2.2377
0
6.2425
2.4408
0
4.8209
2.3198
0
3.9235
2.2662
0
3.3074
2.2371
0
6.1933
2.4357
0
4.7913
2.3178
0
3.9039
2.2652
0
3.2934
2.2365
0
6.1448
2.4307
0
4.7621
2.3158
0
3.8844
2.2642
0
3.2795
2.2359
0
6.0972
2.4259
0
4.7332
2.3139
0
3.8652
2.2632
0
3.2658
2.2354
0
6.0502
2.4213
0
4.7047
2.312
0
3.8461
2.2622
0
3.2522
2.2348
0
6.0039
2.4167
0
4.6765
2.3101
0
3.8272
2.2613
0
3.2386
2.2342
0
5.9584
2.4123
0
4.6486
2.3083
0
3.8085
2.2603
0
3.2252
2.2337
0
5.9135
2.408
0
4.6211
2.3065
0
3.79
2.2594
0
3.212
2.2331
0
5.8692
2.4039
0
4.5939
2.3048
0
3.7716
2.2585
0
3.1988
2.2326
0
5.8257
2.3998
0
4.567
2.3031
0
3.7534
2.2576
0
3.1857
2.232
0
5.7827
2.3959
0
4.5405
2.3014
0
3.7355
2.2567
0
3.1727
2.2315
0
5.7404
2.3921
0
4.5142
2.2998
0
3.7176
2.2558
0
3.1599
2.231
0
5.6987
2.3883
0
4.4882
2.2982
0
3.7
2.255
0
3.1471
2.2304
0
5.6575
2.3847
0
4.4626
2.2966
0
3.6825
2.2541
0
3.1345
2.2299
0
5.617
2.3812
0
4.4372
2.295
0
3.6652
2.2533
0
3.1219
2.2294
0
5.577
2.3777
0
4.4121
2.2935
0
3.648
2.2525
0
3.1095
2.2289
0
5.5376
2.3744
0
4.3873
2.292
0
3.631
2.2517
0
3.0971
2.2284
0
5.4987
2.3711
0
4.3627
2.2906
0
3.6142
2.2509
0
3.0849
2.2279
0
5.4604
2.3679
0
4.3384
2.2891
0
3.5975
2.2501
0
3.0727
2.2274
0
5.4226
2.3648
0
4.3144
2.2877
0
3.5809
2.2493
0
3.0607
2.227
0
5.3853
2.3618
0
4.2907
2.2863
0
3.5646
2.2486
0
3.0487
2.2265
0
5.3485
2.3588
0
4.2672
2.285
0
3.5483
2.2478
0
3.0368
2.226
0
5.3122
2.3559
0
4.244
2.2837
0
3.5322
2.2471
0
3.025
2.2256
0
5.2764
2.3531
0
4.221
2.2823
0
3.5163
2.2463
0
3.0134
2.2251
0
5.241
2.3504
0
4.1983
2.2811
0
3.5005
2.2456
0
3.0018
2.2247
0
5.2062
2.3477
0
4.1758
2.2798
0
3.4848
2.2449
0
2.9902
2.2242
0
5.1717
2.345
0
4.1536
2.2785
0
3.4693
2.2442
0
2.9788
2.2238
0
5.1378
2.3425
0
4.1315
2.2773
0
3.4539
2.2435
0
2.9675
2.2233
0
5.1042
2.34
0
4.1098
2.2761
0
3.4387
2.2429
0
2.9563
2.2229
0
5.0711
2.3375
0
4.0882
2.2749
0
3.4236
2.2422
0
2.9451
2.2225
0
5.0384
2.3351
0
4.0669
2.2738
0
3.4086
2.2415
0
2.934
2.2221
0
220
2.923
2.2216
0
2.5507
2.2082
0
2.263
2.1987
0
2.0338
2.1914
0
2.9121
2.2212
0
2.5424
2.2079
0
2.2564
2.1985
0
2.0286
2.1913
0
2.9013
2.2208
0
2.5342
2.2077
0
2.2499
2.1983
0
2.0233
2.1911
0
2.8905
2.2204
0
2.526
2.2074
0
2.2435
2.1981
0
2.0181
2.1909
0
2.8799
2.22
0
2.5178
2.2071
0
2.2371
2.1979
0
2.0129
2.1908
0
2.8693
2.2196
0
2.5098
2.2068
0
2.2307
2.1977
0
2.0078
2.1906
0
2.8588
2.2192
0
2.5017
2.2066
0
2.2244
2.1975
0
2.0027
2.1905
0
2.8483
2.2188
0
2.4937
2.2063
0
2.2181
2.1973
0
1.9976
2.1903
0
2.838
2.2184
0
2.4858
2.206
0
2.2118
2.1971
0
1.9925
2.1901
0
2.8277
2.2181
0
2.4779
2.2058
0
2.2056
2.1969
0
1.9874
2.19
0
2.8175
2.2177
0
2.4701
2.2055
0
2.1994
2.1967
0
1.9824
2.1898
0
2.8074
2.2173
0
2.4623
2.2052
0
2.1932
2.1965
0
1.9774
2.1897
0
2.7973
2.2169
0
2.4546
2.205
0
2.1871
2.1963
0
1.9724
2.1895
0
2.7873
2.2166
0
2.4469
2.2047
0
2.181
2.1961
0
1.9675
2.1893
0
2.7774
2.2162
0
2.4393
2.2045
0
2.175
2.1959
0
1.9625
2.1892
0
2.7676
2.2159
0
2.4317
2.2042
0
2.1689
2.1957
0
1.9577
2.189
0
2.7578
2.2155
0
2.4242
2.204
0
2.163
2.1955
0
1.9528
2.1889
0
2.7481
2.2152
0
2.4167
2.2037
0
2.157
2.1953
0
1.9479
2.1887
0
2.7384
2.2148
0
2.4092
2.2035
0
2.1511
2.1951
0
1.9431
2.1886
0
2.7289
2.2145
0
2.4018
2.2032
0
2.1452
2.1949
0
1.9383
2.1884
0
2.7194
2.2141
0
2.3945
2.203
0
2.1393
2.1948
0
1.9335
2.1883
0
2.7099
2.2138
0
2.3872
2.2027
0
2.1335
2.1946
0
1.9288
2.1881
0
2.7006
2.2135
0
2.3799
2.2025
0
2.1277
2.1944
0
1.924
2.188
0
2.6913
2.2131
0
2.3727
2.2023
0
2.1219
2.1942
0
1.9193
2.1878
0
2.682
2.2128
0
2.3655
2.202
0
2.1162
2.194
0
1.9146
2.1877
0
2.6728
2.2125
0
2.3584
2.2018
0
2.1105
2.1938
0
1.91
2.1875
0
2.6637
2.2122
0
2.3513
2.2016
0
2.1048
2.1937
0
1.9053
2.1874
0
2.6547
2.2118
0
2.3443
2.2013
0
2.0992
2.1935
0
1.9007
2.1872
0
2.6457
2.2115
0
2.3373
2.2011
0
2.0936
2.1933
0
1.8961
2.1871
0
2.6368
2.2112
0
2.3303
2.2009
0
2.088
2.1931
0
1.8915
2.187
0
2.6279
2.2109
0
2.3234
2.2007
0
2.0825
2.193
0
1.887
2.1868
0
2.6191
2.2106
0
2.3165
2.2004
0
2.077
2.1928
0
1.8825
2.1867
0
2.6103
2.2103
0
2.3097
2.2002
0
2.0715
2.1926
0
1.878
2.1865
0
2.6016
2.21
0
2.3029
2.2
0
2.066
2.1924
0
1.8735
2.1864
0
2.593
2.2097
0
2.2961
2.1998
0
2.0606
2.1923
0
1.869
2.1862
0
2.5844
2.2094
0
2.2894
2.1996
0
2.0552
2.1921
0
1.8646
2.1861
0
2.5759
2.2091
0
2.2827
2.1993
0
2.0498
2.1919
0
1.8601
2.186
0
2.5675
2.2088
0
2.2761
2.1991
0
2.0444
2.1918
0
1.8557
2.1858
0
2.559
2.2085
0
2.2695
2.1989
0
2.0391
2.1916
0
1.8514
2.1857
0
221
1.847
2.1855
0
1.6917
2.1805
0
1.5606
2.1761
0
1.4487
2.1719
0
1.8427
2.1854
0
1.6881
2.1804
0
1.5576
2.1759
0
1.4461
2.1718
0
1.8383
2.1853
0
1.6844
2.1803
0
1.5545
2.1758
0
1.4435
2.1717
0
1.834
2.1851
0
1.6808
2.1802
0
1.5514
2.1757
0
1.4408
2.1716
0
1.8298
2.185
0
1.6772
2.18
0
1.5484
2.1756
0
1.4382
2.1715
0
1.8255
2.1849
0
1.6737
2.1799
0
1.5453
2.1755
0
1.4356
2.1714
0
1.8213
2.1847
0
1.6701
2.1798
0
1.5423
2.1754
0
1.433
2.1713
0
1.817
2.1846
0
1.6666
2.1797
0
1.5393
2.1753
0
1.4304
2.1712
0
1.8128
2.1845
0
1.663
2.1796
0
1.5363
2.1752
0
1.4278
2.1711
0
1.8087
2.1843
0
1.6595
2.1794
0
1.5333
2.1751
0
1.4252
2.171
0
1.8045
2.1842
0
1.656
2.1793
0
1.5303
2.175
0
1.4227
2.1709
0
1.8004
2.1841
0
1.6525
2.1792
0
1.5273
2.1749
0
1.4201
2.1708
0
1.7962
2.1839
0
1.6491
2.1791
0
1.5244
2.1748
0
1.4176
2.1707
0
1.7921
2.1838
0
1.6456
2.179
0
1.5214
2.1747
0
1.4151
2.1706
0
1.7881
2.1837
0
1.6422
2.1789
0
1.5185
2.1745
0
1.4125
2.1705
0
1.784
2.1835
0
1.6387
2.1787
0
1.5156
2.1744
0
1.41
2.1704
0
1.7799
2.1834
0
1.6353
2.1786
0
1.5127
2.1743
0
1.4075
2.1703
0
1.7759
2.1833
0
1.6319
2.1785
0
1.5098
2.1742
0
1.405
2.1702
0
1.7719
2.1831
0
1.6285
2.1784
0
1.5069
2.1741
0
1.4025
2.1701
0
1.7679
2.183
0
1.6252
2.1783
0
1.504
2.174
0
1.4
2.17
0
1.7639
2.1829
0
1.6218
2.1782
0
1.5011
2.1739
0
1.3976
2.1699
0
1.76
2.1828
0
1.6185
2.1781
0
1.4983
2.1738
0
1.3951
2.1698
0
1.756
2.1826
0
1.6152
2.1779
0
1.4954
2.1737
0
1.3927
2.1697
0
1.7521
2.1825
0
1.6118
2.1778
0
1.4926
2.1736
0
1.3902
2.1696
0
1.7482
2.1824
0
1.6085
2.1777
0
1.4898
2.1735
0
1.3878
2.1695
0
1.7443
2.1822
0
1.6053
2.1776
0
1.487
2.1734
0
1.3854
2.1694
0
1.7405
2.1821
0
1.602
2.1775
0
1.4842
2.1733
0
1.383
2.1693
0
1.7366
2.182
0
1.5987
2.1774
0
1.4814
2.1732
0
1.3806
2.1693
0
1.7328
2.1819
0
1.5955
2.1773
0
1.4786
2.1731
0
1.3782
2.1692
0
1.729
2.1817
0
1.5922
2.1772
0
1.4758
2.173
0
1.3758
2.1691
0
1.7252
2.1816
0
1.589
2.177
0
1.4731
2.1729
0
1.3734
2.169
0
1.7214
2.1815
0
1.5858
2.1769
0
1.4703
2.1728
0
1.371
2.1689
0
1.7176
2.1814
0
1.5826
2.1768
0
1.4676
2.1727
0
1.3687
2.1688
0
1.7139
2.1812
0
1.5795
2.1767
0
1.4649
2.1726
0
1.3663
2.1687
0
1.7101
2.1811
0
1.5763
2.1766
0
1.4622
2.1725
0
1.364
2.1686
0
1.7064
2.181
0
1.5731
2.1765
0
1.4595
2.1724
0
1.3616
2.1685
0
1.7027
2.1809
0
1.57
2.1764
0
1.4568
2.1723
0
1.3593
2.1684
0
1.699
2.1808
0
1.5669
2.1763
0
1.4541
2.1722
0
1.357
2.1683
0
1.6954
2.1806
0
1.5637
2.1762
0
1.4514
2.1721
0
1.3547
2.1682
0
222
1.3524
2.1681
0
1.269
2.1644
0
1.1364
2.1575
0
1.0123
2.1493
0
1.3501
2.168
0
1.267
2.1643
0
1.1328
2.1573
0
1.0095
2.1491
0
1.3478
2.1679
0
1.265
2.1642
0
1.1293
2.1571
0
1.0067
2.1488
0
1.3455
2.1678
0
1.263
2.1641
0
1.1258
2.1569
0
1.0039
2.1486
0
1.3433
2.1677
0
1.2611
2.164
0
1.1223
2.1566
0
1.0011
2.1484
0
1.341
2.1676
0
1.2591
2.1639
0
1.1188
2.1564
0
0.99836
2.1482
0
1.3388
2.1675
0
1.2572
2.1638
0
1.1154
2.1562
0
0.99561
2.148
0
1.3365
2.1674
0
1.2552
2.1637
0
1.1119
2.156
0
0.99287
2.1478
0
1.3343
2.1673
0
1.2533
2.1636
0
1.1085
2.1558
0
0.99015
2.1476
0
1.3321
2.1672
0
1.2514
2.1635
0
1.1052
2.1556
0
0.98744
2.1473
0
1.3299
2.1671
0
1.2494
2.1634
0
1.1018
2.1554
0
0.98475
2.1471
0
1.3277
2.167
0
1.2475
2.1634
0
1.0984
2.1552
0
0.98208
2.1469
0
1.3255
2.1669
0
1.2456
2.1633
0
1.0951
2.155
0
0.97941
2.1467
0
1.3233
2.1668
0
1.2437
2.1632
0
1.0918
2.1548
0
0.97677
2.1465
0
1.3211
2.1667
0
1.2418
2.1631
0
1.0885
2.1546
0
0.97413
2.1463
0
1.3189
2.1666
0
1.2399
2.163
0
1.0853
2.1543
0
0.97151
2.146
0
1.3167
2.1665
0
1.2249
2.1622
0
1.082
2.1541
0
0.96891
2.1458
0
1.3146
2.1664
0
1.2207
2.162
0
1.0788
2.1539
0
0.96632
2.1456
0
1.3124
2.1664
0
1.2166
2.1618
0
1.0756
2.1537
0
0.96374
2.1454
0
1.3103
2.1663
0
1.2126
2.1616
0
1.0724
2.1535
0
0.96117
2.1452
0
1.3081
2.1662
0
1.2085
2.1614
0
1.0692
2.1533
0
0.95862
2.1449
0
1.306
2.1661
0
1.2045
2.1612
0
1.0661
2.1531
0
0.95608
2.1447
0
1.3039
2.166
0
1.2005
2.161
0
1.0629
2.1529
0
0.95356
2.1445
0
1.3018
2.1659
0
1.1965
2.1608
0
1.0598
2.1527
0
0.95105
2.1443
0
1.2997
2.1658
0
1.1926
2.1606
0
1.0567
2.1525
0
0.94855
2.1441
0
1.2976
2.1657
0
1.1887
2.1604
0
1.0536
2.1522
0
0.94607
2.1438
0
1.2955
2.1656
0
1.1848
2.1602
0
1.0506
2.152
0
0.94359
2.1436
0
1.2934
2.1655
0
1.1809
2.16
0
1.0475
2.1518
0
0.94113
2.1434
0
1.2913
2.1654
0
1.1771
2.1598
0
1.0445
2.1516
0
0.93869
2.1432
0
1.2893
2.1653
0
1.1732
2.1595
0
1.0415
2.1514
0
0.93625
2.143
0
1.2872
2.1652
0
1.1695
2.1593
0
1.0385
2.1512
0
0.93383
2.1427
0
1.2851
2.1651
0
1.1657
2.1591
0
1.0355
2.151
0
0.93142
2.1425
0
1.2831
2.165
0
1.1619
2.1589
0
1.0326
2.1508
0
0.92902
2.1423
0
1.2811
2.1649
0
1.1582
2.1587
0
1.0296
2.1506
0
0.92664
2.1421
0
1.279
2.1648
0
1.1545
2.1585
0
1.0267
2.1503
0
0.92427
2.1418
0
1.277
2.1647
0
1.1509
2.1583
0
1.0238
2.1501
0
0.9219
2.1416
0
1.275
2.1647
0
1.1472
2.1581
0
1.0209
2.1499
0
0.91956
2.1414
0
1.273
2.1646
0
1.1436
2.1579
0
1.018
2.1497
0
0.91722
2.1412
0
1.271
2.1645
0
1.14
2.1577
0
1.0152
2.1495
0
0.91489
2.1409
0
223
0.91258
2.1407
0
0.83054
2.1316
0
0.76181
2.1219
0
0.91028
2.1405
0
0.82862
2.1314
0
0.7602
2.1217
0
0.90798
2.1403
0
0.82672
2.1311
0
0.75859
2.1214
0
0.9057
2.14
0
0.82482
2.1309
0
0.75698
2.1211
0
0.90344
2.1398
0
0.82294
2.1307
0
0.75539
2.1209
0
0.90118
2.1396
0
0.82106
2.1304
0
0.7538
2.1206
0
0.89893
2.1394
0
0.81919
2.1302
0
0.75221
2.1204
0
0.8967
2.1391
0
0.81732
2.1299
0
0.75064
2.1201
0
0.89447
2.1389
0
0.81547
2.1297
0
0.74907
2.1198
0
0.89226
2.1387
0
0.81362
2.1294
0
0.7475
2.1196
0
0.89006
2.1384
0
0.81179
2.1292
0
0.74594
2.1193
0
0.88787
2.1382
0
0.80996
2.129
0
0.74439
2.1191
0
0.88569
2.138
0
0.80814
2.1287
0
0.74285
2.1188
0
0.88351
2.1377
0
0.80632
2.1285
0
0.88135
2.1375
0
0.80452
2.1282
0
0.8792
2.1373
0
0.80272
2.128
0
0.87707
2.1371
0
0.80093
2.1277
0
0.87493
2.1368
0
0.79915
2.1275
0
0.87282
2.1366
0
0.79737
2.1272
0
0.87071
2.1364
0
0.79561
2.127
0
0.86861
2.1361
0
0.79385
2.1267
0
0.86652
2.1359
0
0.7921
2.1265
0
0.86444
2.1357
0
0.79035
2.1262
0
0.86237
2.1354
0
0.78862
2.126
0
0.86031
2.1352
0
0.78689
2.1257
0
0.85826
2.135
0
0.78517
2.1255
0
0.85622
2.1347
0
0.78345
2.1252
0
0.85419
2.1345
0
0.78174
2.125
0
0.85217
2.1342
0
0.78004
2.1247
0
0.85016
2.134
0
0.77835
2.1245
0
0.84815
2.1338
0
0.77667
2.1242
0
0.84616
2.1335
0
0.77499
2.124
0
0.84417
2.1333
0
0.77332
2.1237
0
0.8422
2.1331
0
0.77165
2.1235
0
0.84023
2.1328
0
0.77
2.1232
0
0.83828
2.1326
0
0.76835
2.1229
0
0.83633
2.1323
0
0.7667
2.1227
0
0.83439
2.1321
0
0.76507
2.1224
0
0.83246
2.1319
0
0.76344
2.1222
0
224
A.4
Dielectric function of SnO2:F
eV
ε1
ε2
5.0062 6.5153
3.2727 4.0458 4.6146 0.11577 3.3938 4.1528 0.033239
6.5004 3.9713
4.0149 4.9743 6.5892
3.2482 4.0249 4.5946 0.10925 3.3791 4.1447 0.032638
6.4471 3.8365
3.9148 4.9429 6.6857
3.1561 4.0042 4.5753 0.10334 3.3645 4.1368 0.032069
6.3947 4.0079
3.9887 4.9118 6.7541
3.0728 3.9837 4.5568 0.097956
6.3432
4.041
4.0674 4.8811 6.8624
2.9625 3.9635 4.5388 0.093011 3.3357 4.1213 0.030999
6.2924 4.1187
3.9552 4.8508 6.8828
2.8423 3.9434 4.5215 0.088501 3.3215 4.1137 0.030498
6.2425 4.1532
3.9855 4.8209 6.9286
2.8191 3.9235 4.5048 0.08436 3.3074 4.1062 0.030019
6.1933 4.1876
3.9881 4.7913 6.8787
2.713 3.9039 4.4885 0.080529 3.2934 4.0989 0.029569
6.1448 4.2819
3.9916 4.7621 6.8868
2.666 3.8844 4.4728 0.077006 3.2795 4.0917 0.029131
6.0972 4.3261
3.9426 4.7332 6.8607
2.5396 3.8652 4.4575 0.073734 3.2658 4.0845 0.028709
6.0502 4.3707
3.9117 4.7047 6.8106
2.3692 3.8461 4.4427 0.070718 3.2522 4.0775 0.028309
6.0039 4.4314
3.868 4.6765 6.7108
2.17 3.8272 4.4283 0.067919 3.2386 4.0706 0.027921
5.9584 4.5232
3.9396 4.6486 6.5733
1.9544 3.8085 4.4143 0.065308 3.2252 4.0637 0.027551
5.9135 4.5773
3.8937 4.6211 6.4086
1.7318
5.8692 4.6024
3.872 4.5939 6.2269
4.129 0.03152
3.212 4.0569 0.027199
1.5118 3.7716 4.3875 0.060622 3.1988 4.0502 0.026859
5.8257 4.6925
3.8377
5.7827
4.755
3.8735 4.5405 5.8478
5.7404
4.828
3.8354 4.5142 5.6671 0.92812 3.7176 4.3498 0.054663 3.1599 4.0307 0.025929
5.6987
4.891
3.8227 4.4882
5.6575 4.9459
4.567 6.0375
3.79 4.4007 0.062875
3.35
1.3012 3.7534 4.3747 0.058505 3.1857 4.0436 0.02654
1.1047 3.7355
5.501 0.77349
4.362 0.056515 3.1727 4.0371 0.026221
3.7 4.3378 0.052917 3.1471 4.0243 0.02564
3.7736 4.4626 5.3554 0.64218 3.6825 4.3262 0.051279 3.1345
4.018 0.025359
5.617 5.0635
3.8069 4.4372 5.2368 0.53621 3.6652 4.3147 0.049737 3.1219 4.0119
5.577 5.1365
3.8102 4.4121 5.1496
0.455
0.0251
3.648 4.3036 0.04829 3.1095 4.0057 0.024849
5.5376 5.2317
3.7544 4.3873 5.1007 0.40485
5.4987 5.2471
3.7339 4.3627 4.9976 0.36189 3.6142
5.4604 5.3437
3.7248 4.3384 4.9634 0.32464 3.5975 4.2716 0.044418 3.0727 3.9877 0.02415
5.4226 5.4337
3.7151 4.3144 4.9301 0.29291 3.5809 4.2614 0.043262 3.0607 3.9818 0.023929
5.3853 5.5346
3.6793 4.2907 4.8976 0.26542 3.5646 4.2514 0.042167 3.0487 3.9761 0.02373
5.3485
3.5867 4.2672 4.8663 0.24169 3.5483 4.2416 0.041141 3.0368 3.9703 0.02353
5.62
3.631 4.2927 0.04693 3.0971 3.9997
4.282 0.045637 3.0849 3.9936 0.024369
5.3122 5.6826
3.5601
4.244 4.8361 0.22099 3.5322 4.2319 0.040162
5.2764 5.7345
3.5325
4.221 4.8071 0.20295 3.5163 4.2225 0.03923 3.0134
5.241 5.7963
0.0246
3.025 3.9647 0.023341
3.959 0.023159
3.5341 4.1983 4.7793 0.18707 3.5005 4.2132 0.03834 3.0018 3.9534 0.022989
5.2062 5.8783
3.4751 4.1758 4.7528 0.17307 3.4848 4.2042 0.037502 2.9902
5.1717 5.9604
3.5094 4.1536 4.7272 0.16065 3.4693 4.1952 0.036701 2.9788 3.9425 0.02266
5.1378 6.0616
3.4719 4.1315 4.7029 0.14965 3.4539 4.1865 0.035942 2.9675 3.9371 0.02251
5.1042 6.1636
3.4253 4.1098 4.6794 0.13975 3.4387 4.1778 0.03522 2.9563 3.9317 0.022359
5.0711 6.2959
5.0384 6.3878
3.389 4.0882
3.948 0.022821
4.657 0.13093 3.4236 4.1693 0.03453 2.9451 3.9264 0.02222
3.3222 4.0669 4.6353 0.12296 3.4086
225
4.161 0.033871
2.934 3.9212 0.02209
2.923
3.916 0.02196 2.5507 3.7402 0.02016
2.263 3.5951 0.022001 2.0338 3.4625 0.025969
2.9121 3.9108 0.02184 2.5424 3.7362 0.02018 2.2564 3.5916 0.02208 2.0286 3.4592 0.026101
2.9013 3.9057 0.02172 2.5342 3.7322 0.02019 2.2499 3.5881 0.022159 2.0233 3.4559 0.026229
2.8905 3.9006 0.02161
2.526 3.7283 0.02021 2.2435 3.5846 0.02224 2.0181 3.4525 0.026349
2.8799 3.8956
0.0215 2.5178 3.7244 0.02023 2.2371 3.5811 0.02232 2.0129 3.4492 0.026479
2.8693 3.8906
0.0214 2.5098 3.7204 0.02025 2.2307 3.5777
2.8588 3.8856
0.0213 2.5017 3.7166 0.02027 2.2244 3.5742 0.02249 2.0027 3.4426 0.026741
2.8483 3.8808 0.02121 2.4937 3.7127
0.0224 2.0078 3.4459 0.02661
0.0203 2.2181 3.5707 0.02257 1.9976 3.4392 0.026871
2.838 3.8759 0.02113 2.4858 3.7088 0.02032 2.2118 3.5673 0.02266 1.9925 3.4359 0.027011
2.8277 3.8711 0.02104 2.4779 3.7049 0.02035 2.2056 3.5638 0.02275 1.9874 3.4327 0.027139
2.8175 3.8663 0.02097 2.4701 3.7011 0.02038 2.1994 3.5604 0.02284 1.9824 3.4293 0.02728
2.8074 3.8615 0.02089 2.4623 3.6973 0.02042 2.1932
3.557 0.022929 1.9774
3.426 0.02741
2.7973 3.8568 0.02082 2.4546 3.6935 0.02045 2.1871 3.5535 0.02302 1.9724 3.4227 0.027549
2.7873 3.8521 0.02076 2.4469 3.6897 0.02049
2.181 3.5501 0.02312 1.9675 3.4194 0.027691
2.7774 3.8475 0.02069 2.4393 3.6859 0.02052
2.175 3.5466 0.023211 1.9625 3.4161 0.027829
2.7676 3.8428 0.02063 2.4317 3.6821 0.02056 2.1689 3.5433 0.023309 1.9577 3.4128 0.027971
2.7578 3.8382 0.02058 2.4242 3.6783 0.02061
2.163 3.5398 0.023411 1.9528 3.4095 0.028111
2.7481 3.8337 0.02053 2.4167 3.6746 0.02065
2.157 3.5364
2.7384 3.8292 0.02048 2.4092 3.6709 0.02069 2.1511
3.533
0.0235 1.9479 3.4062 0.028259
0.0236 1.9431 3.4029
0.0284
2.7289 3.8247 0.02044 2.4018 3.6672 0.02074 2.1452 3.5296 0.02371 1.9383 3.3996 0.02854
2.7194 3.8202
0.0204 2.3945 3.6635 0.02079 2.1393 3.5262 0.02381 1.9335 3.3963 0.02869
2.7099 3.8158 0.02036 2.3872 3.6598 0.02084 2.1335 3.5228 0.02391 1.9288
2.7006 3.8114 0.02033 2.3799 3.6561 0.02089 2.1277 3.5195 0.02402
2.6913
1.924 3.3897 0.028989
3.807 0.02029 2.3727 3.6524 0.02094 2.1219 3.5161 0.024119 1.9193 3.3864 0.02914
2.682 3.8026 0.02027 2.3655 3.6488
0.021 2.1162 3.5127 0.02423 1.9146 3.3831 0.029289
2.6728 3.7983 0.02024 2.3584 3.6451 0.02105 2.1105 3.5093 0.02434
2.6637
3.393 0.028841
3.794 0.02022 2.3513 3.6415 0.02111 2.1048
2.6547 3.7897
1.91 3.3798 0.029441
3.506 0.024449 1.9053 3.3765 0.029589
0.0202 2.3443 3.6379 0.02117 2.0992 3.5026 0.02456 1.9007 3.3732 0.02974
2.6457 3.7855 0.02018 2.3373 3.6342 0.02123 2.0936 3.4992 0.02467 1.8961 3.3699
2.6368 3.7812 0.02017 2.3303 3.6307 0.02129
2.088 3.4959 0.02478 1.8915 3.3667 0.030059
2.6279 3.7771 0.02016 2.3234 3.6271 0.02136 2.0825 3.4925 0.024901
2.6191 3.7729 0.02015 2.3165 3.6235 0.02142
3.36 0.030371
1.878 3.3567 0.030532
2.066 3.4825 0.02524 1.8735 3.3534 0.030691
2.593 3.7605 0.02014 2.2961 3.6128 0.02163 2.0606 3.4791 0.025361
2.5844 3.7564 0.02014 2.2894 3.6092
1.887 3.3633 0.03021
2.077 3.4892 0.025011 1.8825
2.6103 3.7687 0.02014 2.3097 3.6199 0.02149 2.0715 3.4858 0.025131
2.6016 3.7646 0.02014 2.3029 3.6163 0.02156
0.0299
1.869 3.3502 0.03085
0.0217 2.0552 3.4758 0.025481 1.8646 3.3468 0.031012
2.5759 3.7523 0.02014 2.2827 3.6057 0.02177 2.0498 3.4725
0.0256 1.8601 3.3436 0.031179
2.5675 3.7482 0.02015 2.2761 3.6022 0.02185 2.0444 3.4692 0.025729 1.8557 3.3403 0.031339
2.559 3.7443 0.02015 2.2695 3.5986 0.02192 2.0391 3.4658 0.025849 1.8514 3.3369 0.031512
226
1.847 3.3337 0.03167 1.6917
3.204 0.03899 1.5606 3.0711 0.047926 1.4487 2.9333 0.058515
1.8427 3.3303 0.031842 1.6881 3.2007 0.039202 1.5576 3.0675 0.048184 1.4461 2.9296 0.05881
1.8383 3.3271 0.032009 1.6844 3.1974 0.039408 1.5545 3.0641 0.048432 1.4435 2.9259 0.059115
1.834 3.3238 0.032178 1.6808
3.194 0.039618 1.5514 3.0606 0.048679 1.4408 2.9224 0.059407
1.8298 3.3205 0.032352 1.6772 3.1906 0.039828 1.5484 3.0571 0.048943 1.4382 2.9188
0.0597
1.8255 3.3172 0.03252 1.6737 3.1872 0.040042 1.5453 3.0537 0.049189 1.4356 2.9152 0.060001
1.8213 3.3138 0.032692 1.6701 3.1839 0.04026 1.5423 3.0501 0.049451
1.433 2.9115 0.060301
1.817 3.3106 0.032868 1.6666 3.1804 0.040472 1.5393 3.0466 0.049713 1.4304 2.9079
1.8128 3.3073 0.033038
0.0606
1.663 3.1771 0.040688 1.5363 3.0431 0.049973 1.4278 2.9043 0.060898
1.8087 3.3039 0.033221 1.6595 3.1737 0.040909 1.5333 3.0396 0.050222 1.4252 2.9007 0.061195
1.8045 3.3007
0.0334
1.656 3.1704 0.041129 1.5303 3.0362 0.050491 1.4227
1.8004 3.2973 0.033571 1.6525
2.897 0.061512
3.167 0.041348 1.5273 3.0327 0.050748 1.4201 2.8934 0.061807
1.7962 3.2941 0.033748 1.6491 3.1635 0.041572 1.5244 3.0291 0.051013 1.4176 2.8897 0.062122
1.7921 3.2908 0.033928 1.6456 3.1602 0.041789 1.5214 3.0257 0.051268 1.4151 2.8862 0.062414
1.7881 3.2874 0.034112 1.6422 3.1567 0.042012 1.5185 3.0221 0.051541 1.4125 2.8825 0.062727
1.784 3.2841
0.0343 1.6387 3.1534 0.042227 1.5156 3.0186 0.051803
1.7799 3.2808 0.034478 1.6353
1.41 2.8788 0.063039
3.15 0.042458 1.5127 3.0151 0.052074 1.4075 2.8752 0.063349
1.7759 3.2775 0.034659 1.6319 3.1466 0.042688 1.5098 3.0115 0.052344
1.7719 3.2741 0.03485 1.6285 3.1432 0.042907 1.5069
1.7679 3.2708 0.035039 1.6252 3.1397 0.043141
3.008 0.052613 1.4025 2.8679 0.063977
1.504 3.0045 0.052881
1.7639 3.2675 0.035218 1.6218 3.1364 0.043368 1.5011
1.405 2.8715 0.063659
1.4 2.8642 0.064284
3.001 0.053148 1.3976 2.8605 0.064603
1.76 3.2642 0.035411 1.6185 3.1329 0.043601 1.4983 2.9974 0.053423 1.3951 2.8569 0.064917
1.756 3.2609 0.035598 1.6152 3.1295 0.043833 1.4954
2.994 0.053697 1.3927 2.8531 0.065244
1.7521 3.2575 0.035789 1.6118 3.1262 0.044057 1.4926 2.9904 0.05397 1.3902 2.8495 0.065546
1.7482 3.2542 0.035989 1.6085 3.1227 0.044297 1.4898 2.9868 0.054252 1.3878 2.8458 0.06587
1.7443 3.2509 0.036178 1.6053 3.1192 0.044533
1.7405 3.2475 0.036372
1.487 2.9832 0.054533 1.3854 2.8421 0.066193
1.602 3.1158 0.044771 1.4842 2.9797 0.054803
1.383 2.8384 0.066525
1.7366 3.2442 0.036569 1.5987 3.1124 0.044998 1.4814 2.9761 0.055082 1.3806 2.8347 0.066845
1.7328 3.2409 0.036761 1.5955
3.109 0.045241 1.4786 2.9726 0.05536 1.3782
2.831 0.067165
1.729 3.2375 0.036962 1.5922 3.1056 0.045476 1.4758 2.9691 0.055646 1.3758 2.8273 0.067493
1.7252 3.2342 0.037162
1.589 3.1021 0.045718 1.4731 2.9655 0.055932 1.3734 2.8236 0.06781
1.7214 3.2308 0.037361 1.5858 3.0987 0.045958 1.4703
2.962 0.056207
1.7176 3.2275 0.03756 1.5826 3.0953 0.046198 1.4676 2.9583
1.371
2.82 0.068136
0.0565 1.3687 2.8162 0.068474
1.7139 3.2241 0.037762 1.5795 3.0917 0.046454 1.4649 2.9547 0.056783 1.3663 2.8125 0.068797
1.7101 3.2208 0.037959 1.5763 3.0883 0.046691 1.4622 2.9511 0.057074
1.364 2.8087 0.069133
1.7064 3.2175 0.03817 1.5731 3.0849 0.046937 1.4595 2.9475 0.057364 1.3616 2.8051 0.069454
1.7027 3.2141 0.03837
1.57 3.0814 0.047181 1.4568
2.944 0.057654 1.3593 2.8014 0.069787
1.699 3.2108 0.038579 1.5669 3.0779 0.047433 1.4541 2.9404 0.057942
1.357 2.7976 0.07012
1.6954 3.2074 0.038783 1.5637 3.0745 0.047676 1.4514 2.9368 0.058229 1.3547 2.7939 0.070461
227
1.3524 2.7901 0.070791
1.269
1.3501 2.7864 0.071129
1.267 2.6381 0.085101 1.1328 2.3327 0.11737 1.0095 1.9451 0.16424
1.3478 2.7827 0.071467
1.265 2.6343 0.085477 1.1293 2.3232 0.11845 1.0067 1.9346 0.16559
1.3455
1.263 2.6305 0.085851 1.1258 2.3137 0.11952 1.0039 1.9241 0.16695
2.779 0.071803
2.642 0.084723 1.1364
2.342 0.11632 1.0123 1.9556 0.16289
1.3433 2.7751 0.072153 1.2611 2.6265 0.086244 1.1223 2.3042
0.1206 1.0011 1.9137
1.341 2.7714 0.072486 1.2591 2.6227 0.086616 1.1188 2.2947 0.12168 0.99836
1.3388 2.7676 0.072834 1.2572 2.6188 0.087015 1.1154
1.3365 2.7639 0.073165 1.2552
2.285
0.1683
1.903 0.16968
0.1228 0.99561 1.8925 0.17106
2.615 0.087385 1.1119 2.2756 0.12388 0.99287 1.8819 0.17245
1.3343 2.7601 0.07352 1.2533 2.6111 0.087782 1.1085
2.266 0.12499 0.99015 1.8713 0.17384
1.3321 2.7563 0.073864 1.2514 2.6071 0.088167 1.1052 2.2561 0.12614 0.98744 1.8607 0.17524
1.3299 2.7525 0.074216 1.2494 2.6034 0.088542 1.1018 2.2466 0.12724 0.98475
1.3277 2.7487 0.074567 1.2475 2.5995 0.088935 1.0984
1.3255
1.85 0.17665
2.237 0.12836 0.98208 1.8393 0.17806
2.745 0.074908 1.2456 2.5956 0.089327 1.0951 2.2273 0.12951 0.97941 1.8286 0.17949
1.3233 2.7412 0.075257 1.2437 2.5917 0.089717 1.0918 2.2175 0.13065 0.97677 1.8179 0.18092
1.3211 2.7374 0.075614 1.2418 2.5878 0.090106 1.0885 2.2078 0.13179 0.97413 1.8071 0.18236
1.3189 2.7337 0.07596 1.2399 2.5839 0.090494 1.0853 2.1978 0.13298 0.97151 1.7963 0.18381
1.3167
2.73 0.076305 1.2249 2.5522 0.093724
1.082 2.1882 0.13412 0.96891 1.7855 0.18526
1.3146 2.7261 0.076665 1.2207 2.5435 0.094619 1.0788 2.1783 0.13531 0.96632 1.7746 0.18673
1.3124 2.7224 0.077017 1.2166 2.5346 0.09554 1.0756 2.1684 0.13648 0.96374 1.7638
0.1882
1.3103 2.7185 0.077384 1.2126 2.5255 0.096477 1.0724 2.1585 0.13766 0.96117 1.7529 0.18968
1.3081 2.7148 0.077724 1.2085 2.5167 0.097396 1.0692 2.1487 0.13884 0.95862
1.306
1.742 0.19116
2.711 0.078089 1.2045 2.5076 0.098342 1.0661 2.1386 0.14006 0.95608
1.731 0.19266
1.3039 2.7071 0.078452 1.2005 2.4987 0.099281 1.0629 2.1288 0.14125 0.95356
1.72 0.19416
1.3018 2.7033 0.078815 1.1965 2.4897 0.10022 1.0598 2.1188 0.14247 0.95105
1.709 0.19567
1.2997 2.6995 0.079176 1.1926 2.4806 0.10119 1.0567 2.1088 0.14369 0.94855
1.698 0.19719
1.2976 2.6956 0.079535 1.1887 2.4714 0.10216 1.0536 2.0988 0.14491 0.94607 1.6869 0.19872
1.2955 2.6918 0.079904 1.1848 2.4624 0.10313 1.0506 2.0886 0.14617 0.94359 1.6758 0.20025
1.2934 2.6881 0.080261 1.1809 2.4533
0.1041 1.0475 2.0787
0.1474 0.94113 1.6647
0.2018
1.2913 2.6843 0.080626 1.1771 2.4441 0.10509 1.0445 2.0685 0.14866 0.93869 1.6536 0.20335
1.2893 2.6803 0.081008 1.1732 2.4351 0.10606 1.0415 2.0583 0.14992 0.93625 1.6424 0.20491
1.2872 2.6766 0.081361 1.1695 2.4257 0.10707 1.0385 2.0482 0.15118 0.93383 1.6312 0.20648
1.2851 2.6728 0.081723 1.1657 2.4166 0.10806 1.0355 2.0381 0.15244 0.93142
1.62 0.20805
1.2831 2.6689 0.082101 1.1619 2.4075 0.10906 1.0326 2.0276 0.15375 0.92902 1.6088 0.20963
1.2811
2.665 0.082478 1.1582 2.3982 0.11008 1.0296 2.0176
1.279 2.6613 0.082845 1.1545 2.3889
0.155 0.92664 1.5975 0.21123
0.1111 1.0267 2.0072 0.15631 0.92427 1.5862 0.21283
1.277 2.6574 0.08322 1.1509 2.3794 0.11215 1.0238 1.9969 0.15762 0.9219 1.5749 0.21444
1.275 2.6535 0.083592 1.1472 2.3702 0.11317 1.0209 1.9866 0.15892 0.91956 1.5635 0.21605
1.273 2.6497 0.083974 1.1436 2.3608 0.11422
1.271 2.6458 0.084344
1.018 1.9764 0.16023 0.91722 1.5521 0.21768
1.14 2.3514 0.11527 1.0152 1.9658 0.16158 0.91489 1.5407 0.21931
228
0.91258 1.5293 0.22095 0.83054 1.0622 0.29172 0.76181 0.55339 0.37653
0.91028 1.5178
0.2226 0.82862 1.0498 0.29371 0.7602 0.53972 0.37891
0.90798 1.5064 0.22426 0.82672 1.0372 0.29572 0.75859 0.52609 0.38129
0.9057 1.4948 0.22593 0.82482 1.0246 0.29773 0.75698 0.51249 0.38367
0.90344 1.4833
0.2276 0.82294
1.012 0.29975 0.75539 0.49874 0.38608
0.90118 1.4717 0.22929 0.82106 0.99934 0.30178 0.7538 0.48502 0.38849
0.89893 1.4601 0.23098 0.81919 0.98667 0.30382 0.75221 0.47133
0.3909
0.8967 1.4485 0.23268 0.81732 0.97405 0.30587 0.75064 0.45749 0.39336
0.89447 1.4369 0.23439 0.81547 0.9613 0.30793 0.74907 0.44369
0.89226 1.4252 0.23611 0.81362 0.94859
0.3958
0.31 0.7475 0.42991 0.39824
0.89006 1.4135 0.23783 0.81179 0.93576 0.31207 0.74594 0.41607
0.4007
0.88787 1.4017 0.23957 0.80996 0.92297 0.31416 0.74439 0.40216 0.40318
0.88569
1.39 0.24131 0.80814 0.91012 0.31627 0.74285 0.3882 0.40567
0.88351 1.3782 0.24306 0.80632 0.89732 0.31836
0.88135 1.3664 0.24482 0.80452 0.8844 0.32048
0.8792 1.3546 0.24659 0.80272 0.87151
0.3226
0.87707 1.3427 0.24837 0.80093 0.85858 0.32474
0.87493 1.3308 0.25016 0.79915 0.8456 0.32688
0.87282 1.3188 0.25195 0.79737 0.83265 0.32904
0.87071 1.3069 0.25376 0.79561 0.81958
0.3312
0.86861 1.2949 0.25557 0.79385 0.80654 0.33337
0.86652 1.2829 0.25739 0.7921 0.79346 0.33557
0.86444 1.2709 0.25922 0.79035 0.7804 0.33775
0.86237 1.2589 0.26106 0.78862 0.76723 0.33996
0.86031 1.2468 0.26291 0.78689 0.75408 0.34216
0.85826 1.2347 0.26477 0.78517 0.74087 0.34439
0.85622 1.2225 0.26664 0.78345 0.7277 0.34662
0.85419 1.2104 0.26851 0.78174 0.71448 0.34885
0.85217 1.1982
0.2704 0.78004 0.70121
0.3511
0.85016 1.1859 0.27229 0.77835 0.6879 0.35337
0.84815 1.1737 0.27419 0.77667 0.67454 0.35564
0.84616 1.1614
0.2761 0.77499 0.66118 0.35792
0.84417 1.1492 0.27802 0.77332 0.6478 0.36021
0.8422 1.1368 0.27995 0.77165 0.63443 0.36252
0.84023 1.1245 0.28189
0.83828
0.77 0.62094 0.36484
1.112 0.28384 0.76835 0.60748 0.36716
0.83633 1.0996 0.28579 0.7667 0.59403 0.36948
0.83439 1.0872 0.28776 0.76507 0.58046 0.37183
0.83246 1.0747 0.28973 0.76344 0.56691 0.37418
229