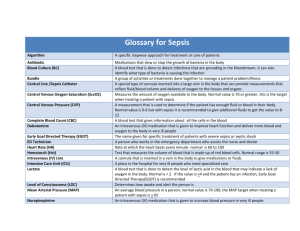
Letter of support - National Quality Forum
advertisement

TO: Consensus Standards Approval Committee FR: Helen Burstin, MD, MPH SU: Letter of Appeal on 0500 Severe Sepsis and Septic Shock: Management Bundle DA: May 3, 2013 A letter of appeal was submitted regarding measure 0500 Severe Sepsis and Septic Shock: Management Bundle endorsed in the Infectious Disease Endorsement Maintenance project. The appeal was submitted on behalf of the following organizations (the appellants): • American College of Chest Physicians • Americans Associations of Critical Care Nurses • Greater New York Hospital Association • Midwest Critical Care Collaborative • National Association for Medical Direction of Respiratory Care • Society for Academic Emergency Medicine • Society of Hospital Medicine In accordance with the NQF CDP, this measure was evaluated and recommended by the NQF Infectious Disease Steering Committee and released for member and public comment. The committee recommendations and member voting results were reviewed by the CSAC. The CSAC recommended the measures for endorsement and the Board of Directors endorsed the set of measures on March 6, 2013. The following materials are attached for your reference: ● Appendix A: 0500 Severe Sepsis and Septic Shock: Management Bundle measure specifications ● Appendix B: Letter of Appeal ● Appendix C: Response from the measure developer (Henry Ford Hospital) ● Appendix D: CSAC memo from February 12, 2013 on the measure, including voting results. ● Appendix E: Letter of support for the measure from nine organizations ○ Society of Critical Care Medicine PAGE 2 ○ ○ ○ ○ ○ ○ ○ ○ Infectious Diseases Society of America Cooper University Hospital European Society of Intensive Care Medicine Henry Ford Health System Kaiser Permanente North Shore LIJ Health System Sutter Health Institute for Healthcare Improvement The NQF Consensus Development Process version 1.9 includes an appeal process and states that “anyone may register a request for reconsideration of an endorsed voluntary consensus standard by notifying the NQF in writing within 30 days of public notification that the voluntary consensus standard had been approved by the CSAC. For an appeal to be considered, the notification letter to the NQF must include information clearly demonstrating that the appellant has interests that are directly and materially affected by the NQF-endorsed voluntary consensus standard(s), and that the NQF decision has had (or will have) an adverse effect on those interests. Appeals will be reviewed by NQF staff and management, who may consult with the project’s technical advisors, Steering Committee, and/or other sources, as appropriate, before a recommendation is provided to the CSAC and BoD. Following consultation with the CSAC, the BoD shall act on an appeal within seven calendar days of the CSAC’s recommendation to BoD regarding the appeal. The result of this BoD action shall be promulgated in the same manner as the original decision. NQF will maintain a record of all appeals, as well as post them on the web site.” Subject of the Appeals An appeal (Appendix B) was received regarding the following measure: 0500 Severe Sepsis and Septic Shock: Management Bundle (Henry Ford Hospital) This measure will focus on patients aged 18 years and older who present with symptoms of severe sepsis or septic shock. These patients will be eligible for the 3 hour (severe sepsis) and/or 6 hour (septic shock) early management bundle. The appellants requested reconsideration of the endorsement of the measure. The appeal letter and developer’s response are attached (Appendices B and C). Please refer to the letters for a complete discussion. In summary, the appellants state “we are concerned that the specification within the Severe Sepsis and Septic Shock bundle that resuscitation must be guided by a central venous catheter would have unintended deleterious effects, even 2 PAGE 3 while recognizing that this element is included in the current Surviving Sepsis Guidelines.” The appellants note additional concerns with usability, specifically that patients are identified retrospectively by ICD-9 discharge diagnoses and the time identified as “time zero” for patients that develop sepsis while in the ED or hospital. The appellants note that there are ongoing trials in the United States and Australia that will be completed in 2013 and “should provide greater clarity regarding the need for invasive monitoring.” The appellants have made specific suggestions for revisions to the measure which would be acceptable to them. Response The developers have provided detailed responses to each of the concerns (Appendix C). Specifically, in response to the concern regarding the use of CVP monitoring the developer responded “CVC placement and central venous pressure (CVP) have been part of management of the critically ill patient for almost 50 years. It has been recommended by the American College of Critical Care Medicine and the SCCM for the management of severe sepsis and septic shock since 1999. Recent studies have shown a significant association between the time to CVC placement and a decrease in organ failure and mortality particularly following publication of the initial Surviving Sepsis Campaign (SSC) guidelines. Further evidence indicates a mortality benefit with a minimum CVP target of 8 mm Hg and shows that a higher CVP target of 12 mm Hg may further lower mortality, particularly among those with an APACHE IV-predicted moderate risk of dying. When used in an algorithmic context as recommended in the measure, there is a significant reduction in 30-day mortality.” The developer refers to the letter of support from the Society of Critical Care Medicine (Appendix E) to respond to the issue of on-going trials that may provide new information in this area and states “These trials, regardless of the outcomes, will not yield new performance metrics ready for testing in the short term.” The developer indicates that revisions to the measure as suggested would create a new measure that would need empiric testing for reliability and validity. Use of the sepsis bundle has been demonstrated to reduce mortality during the Surviving Sepsis Campaign and the bundle is aligned with the current sepsis management guidelines. As described in the evaluation and voting tables below, the issues raised by the appellants were discussed by the Steering Committee during their initial evaluation and in response to the comments submitted. The Committee considered the comments and developer’s 3 PAGE 4 responses when making their recommendation for endorsement. Overall, the issues raised in the appeal have been considered during the Consensus Development Process. Though a cornerstone of the evidence evaluation process relates to the consistency of evidence, the Committee considered the evidence carefully and concluded that the evidence of mortality reduction associated with use of the sepsis bundle currently includes CVP monitoring as indicated. As new evidence emerges from ongoing clinical trials, NQF is committed to an immediate ad hoc re-evaluation of the measure. Evaluation of Measures during the Consensus Development Process 0500 Severe sepsis and septic shock: Management bundle Status: Maintenance, Original Endorsement: Oct 24, 2008 Description: This measure will focus on patients aged 18 years and older who present with symptoms of severe sepsis or septic shock. These patients will be eligible for the 3 hour (severe sepsis) and/or 6 hour (septic shock) early management bundle. Numerator Statement: If: A. measure lactate level B. obtain blood cultures prior to antibiotics C. administer broad spectrum antibiotics D. administer 30 ml/kg crystalloid for hypotension or lactate >=4 mmol/L E. apply vasopressors (for hypotension that does not respond to initial fluid resuscitation to maintain a mean areterial pressure >= 65) F. In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate >=4 mmol/L (36 mg/dl) measure central venous pressure and central venous oxygen saturation G. remeasure lactate if initial lactate is elevated represent processes of care: Numerator statement: Patients from the denominator who received all the following: A, B, and C within 3 hours of time of presentation† AND IF septic shock is present (as either defined as hypotension* or lactate >=4 mmol/L) who also received D and E and F and G within 6 hours of time of presentation. † ”time of presentation” is defined as the time of triage in the Emergency Department or, if presenting from another care venue, from the earliest chart annotation consistent with all elements severe sepsis or septic shock ascertained through chart review. * “hypotension” is defined as systolic blood pressure (SBP) <90 mm Hg or mean arterial pressure (MAP) <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline. Denominator Statement: Number of patients presenting with severe sepsis or septic shock. Exclusions: A) Patients with advanced directives for comfort care are excluded. B) Clinical conditions that preclude total measure completion should be excluded (e.g. mortality within the first 6 hours of presentation as defined above in 2a1.1). C) Patients for whom a central line is clinically contraindicated (e.g. coagulopathy that cannot be corrected, inadequate internal jugular or subclavian central venous access due to repeated cannulations). D) Patients for whom a central line was attempted but could not be successfully inserted. E) Patient or surrogate decision maker declined or is unwilling to consent to such therapies or central line placement. Adjustment/Stratification: No risk adjustment or risk stratification None Henry Ford Hospital (HFH) encourages the results of 4 PAGE 5 0500 Severe sepsis and septic shock: Management bundle this measure to be stratified by race, ethnicity, gender, and primary language, illness severity and have included these variables as recommended data elements to be collected. Level of Analysis: Facility, Integrated Delivery System Type of Measure: Composite Data Source: Electronic Clinical Data, Electronic Clinical Data : Electronic Health Record, Paper Medical Records, Electronic Clinical Data : Registry Measure Steward: Henry Ford Hospital Other organizations: Henry Ford Hospital System(HFHS) California Pacific Medical Center/Sutter Health (CPMC) Society of Critical Care Medicine (SCCM) Infectious Diseases Society of America (IDSA) Institute for Healthcare Improvement (IHI) Surviving Sepsis Campaign (SSC) Ohio State University (OSU) STEERING COMMITTEE MEETING [08/28/2012] Importance to Measure and Report: The measure met the Importance criteria (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-19; M-1; L-0; I-0; 1b. Performance Gap: H-7; M-12; L-1; I-0 1c. Evidence: Y-11; N-5; I-4 Rationale: • There are greater than 750,000 estimated cases of severe sepsis a year in the United States. Additionally, there are an estimated 400,000 ICU admissions for sepsis, approximately 200,000 deaths a year, and at an estimated cost of $17 billion a year. • More than 50 publications have reported improved survival with use of the bundle in the past decade with the vast majority of the studies being observational. Some Committee members noted the lack of randomized controlled trials and they were informed that there are three randomized controlled trials currently ongoing in the U.S., UK and Australia. • Committee members noted that there is some controversy in the field about the need for all of the bundle elements, specifically measuring central venous pressure (CVP). However, only about 15 percent of patients end up needing a CVP line because of the care algorithm in the bundle. • Meta-analyses have shown survival benefit. National and international guidelines have been created for the management of severe sepsis and septic shock based on the data. The recommendations in the guidelines mirror the bundle in this measure. i • The developer pointed to the recent GENESIS trial published in the Journal of Intensive Care Medicine of 6000 patients in 11 hospitals throughout the U.S.; hospitals ranging from 100 to 1,000 patients found that meeting the bundle in a prospective, observational cohort resulted in mortality reduction of 14 percent. 2. Scientific Acceptability of Measure Properties: The measure met the Scientific Acceptability criteria (2a. Reliability – precise specifications, testing; 2b. Validity – testing, threats to validity) Initial review: 2a. Reliability: H-1; M-7; L-5; I-7 2b. Validity: NA Rationale: • Committee members asked how the measure clearly distinguishes patients with severe sepsis versus those with septic shock. o Developer response: The key difference is hypotension refractory to fluid administration that requires a vasopressor or a persistent lactate level greater than 4 is septic shock as specified. • After several questions regarding the specifications, NQF staff realized that an attachment containing the data collection tool submitted by the developer had not been provided to the Committee. NQF staff provided the document to the Committee after the meeting. 5 PAGE 6 0500 Severe sepsis and septic shock: Management bundle • Committee members questioned whether the inter-rater reliability study of 498 patients in one institution would apply to other institutions. The developer responded that the measure is being used in a variety of health care systems such as Kaiser, Loma Linda University, University of Kansas and Intermountain Health in Utah. NOTE: During the meeting, the Committee decided there was insufficient information included in the submission to determine whether the measure met the reliability criteria. Because the Committee had not been given all of the submitted information and the developer indicated additional data on reliability testing could be provided, the Committee agreed to revisit this measure. Additional information was provided to address the questions on reliability. After Review of all Submitted Information and Additional Information Addressing Reliability via Email: 2a. Reliability: H-5; M-11; L-1; I-0 2b. Validity: H-1; M-14; L-2; I-0 Rationale: • The term ‘broad spectrum antibiotics’ is not defined. This could potentially be problematic for a data abstractor to precisely, accurately and reproducibly identify antimicrobials that will satisfy the measure. A Committee member noted that the term ‘broad spectrum antibiotics’ was not used in the reliability testing results, instead, the term ‘timely antibiotics’ was used, which seemed to be more specific to measure o Developer response: The surviving sepsis campaign defined "broad spectrum antibiotics" as those with both Gram positive and Gram negative bacterial coverage. The rationale for antibiotic selection is further discussed in the 2004 and 2008 sepsis guidelines publications. Credit for timely antibiotics was assigned in the data set used for the analyses only if both species were covered. • • The ICD-9 diagnostic codes to identify the denominator were thought to be appropriate. The measure was tested both at the data element and measure score levels for reliability. For validity the measure was only tested at the measure score level. • In review of the validity testing, a Committee member noted that measuring central venous pressure (CVP) and central venous oxygen saturation (ScvO2) were not a part of the validity testing. • Committee members noted that the validity testing indicated that after adjusting for baseline characteristics, only administration of broad spectrum antibiotics and obtaining blood cultures before their initiation were associated with lower hospital mortality. • The question of whether the sepsis bundle as a whole should be incorporated versus specific validated elements of the bundle (e.g., antibiotic selection and timing) was discussed. Though a few members supported individual measure, the majority support the bundle. • The question of how the specifications indicate accountability was raised. A member commented that time zero is triage for time limited Emergency Department (ED) therapies. If a patient presents to the ED triage and does not qualify as severe sepsis or septic shock but develops it later, would the hospital and/or physician be held accountable? Another accountability example was if a patient presents to the ED with pneumonia without severe sepsis or septic shock, and 4 hours later the patient becomes hypotensive, would the ED physicians and/or hospital be held accountable for not providing care over a timeline that had elapsed once the patient developed symptoms? Although unit and ICU time zero is based upon when the patient is diagnosed, in the ED it is time of triage which may or may not be the time at which the patient developed symptoms. The Committee member questioned how it would be reconciled. o Developer response: The patient is somewhere on the natural trajectory of becoming septic regardless of the point of presentation. If the patient who becomes hypotensive or has a high lactate does so in the ED, the reason for presentation to the ED is severe sepsis or shock. Likewise, the patient who presents with septic 6 PAGE 7 0500 Severe sepsis and septic shock: Management bundle physiology on the floor and becomes hypotensive there after an initial admit for something else need to have time to start the clock. In both instances, we are relying on the presence of key features of severe sepsis or shock to make the attribution. Specifying triage time in the ED is not only reasonable since that is most likely what occasioned their visit to the ED, but also provides a standard time. The evidence in the literature also is consistent with picking triage time on this basis. There is less certainty with the floor patient, but again, a proper review yields the time that all the key features were first present. Thus, while there may be some admitted variability between the wards and the ED time of presentation in terms of precision, both are accurate for purposes of measurement. The data in the reliability and validity sections of the NQF submission accept this loss of precision in favor of accuracy. The evidence and data cited demonstrate a high degree of reliability at the level of a performance measure even with this known variability. Thus, we do not need to view it as a threat to reliability. According to the RAND paper, these very high scores on the signal-to-noise reliability indicator actually mean that meaningful comparisons can be drawn in performance using this metric “as is” even with some known variability. 3. Usability: H-1; M-15; L-1; I-0 (Meaningful, understandable, and useful to the intended audiences for 3a. Public Reporting/Accountability and 3b. Quality Improvement) Rationale: • This measure is currently in wide use for public reporting and quality improvement by Kaiser Permanente, Surviving Sepsis Campaign, Catholic Healthcare West, Intermountain Healthcare and Sutter Healthcare. • Highmark has been using the measure in its pay for performance program for the past two years. They initially had some data collection issues but those were resolved. • The University of Kansas is currently using the measure in their EHR with real-time notifications. 4. Feasibility: H-1; M-10; L-6; I-0 (4a. Clinical data generated during care delivery; 4b. Electronic sources; 4c.Susceptibility to inaccuracies/ unintended consequences identified 4d. Data collection strategy can be implemented) Rationale: • The measure requires chart review and manual abstraction. • The measure has elements that may not be captured completely by EHR. The amount of data that needs to be collected may be overwhelming for facilities trying to work on improving outcomes for sepsis. Some of the individual elements may be helpful for internal monitoring within the institution to evaluate improvement over time. 5. Related and Competing Measures • No related or competing measures noted. Steering Committee Recommendation for Endorsement: Y-13; N-4 Public and Member Comment (October 22, 2012-November 20, 2012) General Support for the Measure • Three NQF members submitted comments in support of the measure noting that the developer had responded to questions from the Steering Committee. One commenter stated that “[the] steering committee questioned whether the sepsis quality measure addressing a bundle should be endorsed versus specific validated elements of the bundle. The SS Campaign noted that by making the bundles standard practice, there is elimination of piecemeal or chaotically applied standards for sepsis care that exist in many clinical environments today.” One supportive comment suggested that implementation may difficult with claims data. 7 PAGE 8 0500 Severe sepsis and septic shock: Management bundle Lack of Evidence for the Central Venous Pressure (CVP) Measure Component • SAEM noted that “While we recognize that the SSC recommends central venous pressure monitoring (an unreliable and seldom followed parameter), both it and measuring central venous oxygen saturation are only supported by one single center clinical trial (as such limited evidence supports its use).” • ACEP states that “ACEP has serious concerns surrounding the lack of evidence for measuring CVP as a surrogate for intravascular volume. “ “The measure developers have now cited five additional studies in which multivariate logistic regression demonstrated no independent effect on mortality in patients who achieve CVP targets versus patients who do not. (Castellanos-Ortega 2010, Nguyen 2007, Jeon 2012, Levy 2010, Cannon 2010).” • Cleveland Clinic suggested that “There may be the unintended consequence of increasing the use of central lines in situation where they may actually not be needed and potentially causing harm by their placement (bleeding pneumothorax, pain) or causing infections. By including this single item in the composite measure may encourage the over utilization of central line placement specifically not to fail the measure rather than taking care of the patients best interests.” Committee Response: The developer indicated that when the central venous pressure (CVP) component is utilized as part of the bundle, there is a decrease in mortality. Some members of the Committee did agree that there may be limited evidence for CVP use; however, the Committee concluded that use of the bundle with CVP as specified demonstrated a reduction in mortality. Lack of Evidence for Blood Culture prior to Antibiotics Element • Cleveland Clinic stated that “The whole point is that the patients receive broad spectrum antibiotics not that they are timed prior to antibiotic administration. The theoretical concern about sensitivities should not trump actual administration of those antibiotics. If not eliminated than perhaps altering the wording to simply state; “obtaining appropriate cultures” which would allow simplicity and more flexibility in the actual abstraction process. Having to identify the time of antibiotic administration along with the time of collection of cultures adds significantly to the burden and complexity of the abstraction process. Theoretically this may seem important but does the act of obtain blood cultures or any culture prior to the administration of antibiotics actually have any effect on outcomes?” Committee Response: The Committee concluded that blood cultures remain important for adjusting antibiotic coverage in patients with severe sepsis and reduced response to treatment and that the bundle of care processes are related to patient outcomes. The majority of the Committee determined that the measure met the evidence criteria (Y-12; N-0; I-2). Reliability of Triage being Time Zero for ED Patients and the Impact of ED Length of Stay • Cleveland Clinic states that “Often time’s patient present to the ED with normal vital signs then decompensate and meet criteria of sepsis. Including the initial time of presentation as the start time may not reflect patient’s condition adequately. This ambiguity of utilizing different criteria of time of presentation based on location, calls into question the measure reliability.” • ACEP suggests that “Many ED patients will present with uncomplicated pneumonia, urinary tract infection, or cellulitis only to meet the criteria for severe sepsis/septic shock hours later. If the measure calls for early goal directed therapy within three hours of triage, but the patient does not meet criteria for severe sepsis or septic shock until four hours later, then even if all required interventions are completed within an hour, the hospital will fail on this measure as currently specified. That type of measurement does not differentiate hospitals based on the quality of care provided, but rather on the ED length of stay. If used for accountability as specified, this measure could cause the unintended consequence of penalizing large volume and safety net hospitals.” • SAEM asserted that “Time-based measures that potentially start the clock ticking prior to patients meeting the defining criteria of the syndrome in question have to be recognized as invalid. The developers responded that ED patients with infections are “somewhere on the natural trajectory of becoming septic regardless of point of presentation.” Statements such as this encourage overly aggressive treatment for patients who do not initially meet criteria for severe sepsis/septic 8 PAGE 9 0500 Severe sepsis and septic shock: Management bundle shock due to provider concern of being deemed retrospectively “non-compliant” should the patients’ condition subsequently change. The developers state “if the patient who becomes hypotensive or has a high lactate does so in the ED, the reason for the presentation to the ED is severe sepsis or shock.” While this is true in cases where criteria are met at triage, it’s absolutely not the case for those who only do so hours later. Patients present with chief complaints (which are often non-specific), not diagnoses.” Committee Response: In response to the comment, the Committee again discussed the reliability of triage being time zero for Emergency Department (ED) patients and the impact of the ED length-of-stay. Some Committee members did agree that certain elements of the measure may be related to hospital situations (beds, changing clinical status) that are out of the control of the provider but indicated that the reliability presented was sufficient. The Committee reconsidered their evaluation of reliability and determined that it meets the reliability criteria at moderate to high (H-1; M-11; L-2; I-0). Feasibility of Abstracting the Composite Measure • Cleveland Clinic noted that “This new composite is far too complex for implementation as a potential accountability measure. Furthermore, all of the data elements and time stamps required to calculate this measure are not readily available discrete fields from existing electronic sources making it a significant burden on hospitals to sort and collect this data.” Committee Response: Committee members discussed the data collection burden for the input of multiple data points and the timestamps. Some members were less concerned due to the large number of hospitals who are currently collecting the data for the measure. The Committee reconsidered their evaluation of this criterion and rated feasibility as moderate. Re-vote following Public and Member Comment Following the Public and Member Comment, the Committee decided to re-vote on whether the measure met the NQF criteria for endorsement 1. Importance to Measure and Report: The measure met the Importance criteria (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-13; M-1; L-0; I-0; 1b. Performance Gap: H-5; M-9; L-0; I-0 1c. Evidence: Y-12; N-0; I-2 2. Scientific Acceptability of Measure Properties: The measure met the Scientific Acceptability criteria (2a. Reliability – precise specifications, testing; 2b. Validity – testing, threats to validity) 2a. Reliability: H-1; M-11; L-2; I-0 2b. Validity: H-0; M-14; L-0; I-0 3. Usability: H-0; M-12; L-1; I-1 (Meaningful, understandable, and useful to the intended audiences for 3a. Public Reporting/Accountability and 3b. Quality Improvement) 4. Feasibility: H-0; M-8; L-5; I-1 (4a. Clinical data generated during care delivery; 4b. Electronic sources; 4c.Susceptibility to inaccuracies/ unintended consequences identified 4d. Data collection strategy can be implemented) Steering Committee Recommendation for Endorsement: Y-11; N-3 Consensus Standards Approval Committee (CSAC) Review (February 2013): Y-14; N-0; A-0 • Decision: Approved for continued endorsement Board of Directors (March 4, 2013) • Decision: Ratified for continued endorsement 9 PAGE 10 NQF Member Voting Member voting on measure 0500: Severe sepsis and septic shock: Management bundle ended on February 6, 2013. 0500: Severe sepsis and septic shock: Management bundle Member Council Consumer Health Plan Health Professional Provider Organizations Public/Community Health Agency Purchaser QMRI Supplier/Industry All Councils Yes 10 6 9 5 0 7 4 4 45 No Abstain 0 1 0 0 2 2 12 0 0 0 0 0 0 0 0 2 14 5 Percentage of councils approving (>50%) 86% Average council percentage approval 87% Total Votes 11 6 13 17 0 7 4 6 64 % Approval 100% 100% 82% 29% 0% 100% 100% 100% 76% Discussion 1 Cannon C, Holthaus C, Zubrow M, et al. The GENESIS Project (GENeralized Early Sepsis Intervention Strategies): A Multicenter Quality Improvement Collaborative. Journal of Intensive Care Medicine, 2012. DOI: 10.1177/0885066612453025. Available at: http://jic.sagepub.com/content/early/2012/08/17/0885066612453025. Last accessed October 2012. 10 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 NATIONAL QUALITY FORUM Measure Submission and Evaluation Worksheet 5.0 This form contains the information submitted by measure developers/stewards, organized according to NQF’s measure evaluation criteria and process. The evaluation criteria, evaluation guidance documents, and a blank online submission form are available on the submitting standards web page. NQF #: 0500 NQF Project: Infectious Disease Project (for Endorsement Maintenance Review) Original Endorsement Date: Most Recent Endorsement Date: Last Updated Date: Oct 05, 2012 BRIEF MEASURE INFORMATION De.1 Measure Title: Severe Sepsis and Septic Shock: Management Bundle Co.1 Measure Steward: Henry Ford Hospital De.2 Brief Description of Measure: This measure will focus on patients aged 18 years and older who present with symptoms of severe sepsis or septic shock. These patients will be eligible for the 3 hour (severe sepsis) and/or 6 hour (septic shock) early management bundle. 2a1.1 Numerator Statement: If: A. measure lactate level B. obtain blood cultures prior to antibiotics C. administer broad spectrum antibiotics D. administer 30 ml/kg crystalloid for hypotension or lactate >=4 mmol/L E. apply vasopressors (for hypotension that does not respond to initial fluid resuscitation to maintain a mean areterial pressure >= 65) F. In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate >=4 mmol/L (36 mg/dl) measure central venous pressure and central venous oxygen saturation G. remeasure lactate if initial lactate is elevated represent processes of care: Numerator statement: Patients from the denominator who received all the following: A, B, and C within 3 hours of time of presentation† AND IF septic shock is present (as either defined as hypotension* or lactate >=4 mmol/L) who also received D and E and F and G within 6 hours of time of presentation. † ”time of presentation” is defined as the time of triage in the Emergency Department or, if presenting from another care venue, from the earliest chart annotation consistent with all elements severe sepsis or septic shock ascertained through chart review. * “hypotension” is defined as systolic blood pressure (SBP) <90 mm Hg or mean arterial pressure (MAP) <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline. 2a1.4 Denominator Statement: Number of patients presenting with severe sepsis or septic shock. 2a1.8 Denominator Exclusions: A) Patients with advanced directives for comfort care are excluded. B) Clinical conditions that preclude total measure completion should be excluded (e.g. mortality within the first 6 hours of presentation as defined above in 2a1.1). C) Patients for whom a central line is clinically contraindicated (e.g. coagulopathy that cannot be corrected, inadequate internal jugular or subclavian central venous access due to repeated cannulations). See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 1 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 D) Patients for whom a central line was attempted but could not be successfully inserted. E) Patient or surrogate decision maker declined or is unwilling to consent to such therapies or central line placement. 1.1 Measure Type: Composite 2a1. 25-26 Data Source: Electronic Clinical Data, Electronic Clinical Data : Electronic Health Record, Electronic Clinical Data : Registry, Paper Medical Records 2a1.33 Level of Analysis: Facility, Integrated Delivery System 1.2-1.4 Is this measure paired with another measure? No De.3 If included in a composite, please identify the composite measure (title and NQF number if endorsed): NQF #0500: Severe Sepsis and Septic Shock: Early Management Bundle STAFF NOTES (issues or questions regarding any criteria) Comments on Conditions for Consideration: E.4 If component measures of the composite are aggregate-level measures, all must be either NQF-endorsed or submitted for consideration for NQF endorsement All component measures are NQF-endorsed measures Is the measure untested? Yes endorsement: No If untested, explain how it meets criteria for consideration for time-limited 1a. Specific national health goal/priority identified by DHHS or NPP addressed by the measure (check De.5): 5. Similar/related endorsed or submitted measures (check 5.1): Other Criteria: Staff Reviewer Name(s): 1. IMPACT, OPPORTUITY, EVIDENCE - IMPORTANCE TO MEASURE AND REPORT Extent to which the specific measure focus is important to making significant gains in health care quality (safety, timeliness, effectiveness, efficiency, equity, patient-centeredness) and improving health outcomes for a specific high impact aspect of healthcare where there is variation in or overall poor performance. Measures must be judged to be important to measure and report in order to be evaluated against the remaining criteria. (composite measure evaluation criteria) (for NQF staff use) Specific NPP goal: 1d.1 Describe the purpose/objective of the composite measure: The purpose of Henry Ford Hospital´s severe sepsis and septic shock early management bundle is to support the efficient, effective, and timely delivery of high quality sepsis care in support of the IOM´s aims for improvement. This is consistent with the HHS National Quality Strategy´s priorities directed at one of the leading causes of mortality. By providing timely patient-centered care, and making sepsis care more affordable through early intervention, reduced resource use and complication rates can result. The Severe Sepsis and Septic Shock Early Management Bundle provides a standard operating procedure for the early risk stratification and management of a patient with severe infection. Through applying this standard operating procedure a clinically and statistically significant decrease in organ failure, mortality, and the utlization of health care resources has been demonstrated for over ten years. The current measure project aimed to review and update the existing NQF #0500 Severe Sepsis and Septic Shock Management Bundle to ensure it reflects the latest guideline recommendations, address areas most in need of performance improvement, and incorporate results of worldwide data collection and quality improvement initiatives. Henry Ford Hospital consulted with leadership and representatives from critical care medicine (Society of Critical Care Medicine), infectious diseases (Infectious Diseases Society of America), and emergency physicians to review and update the Severe Sepsis and Septic Shock Early Management Bundle. 1d.2 Describe the quality construct used in developing the composite: Over the last 25 years, diseases such as stroke, acute myocardial infarction and trauma have resulted in lowered mortality rates through a continuous quality improvement (CQI) and See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 2 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 standardized protocols for early intervention. The Surviving Sepsis Campaign (SSC), the GENeralized Early Sepsis Intervention Strategies (GENESIS) Project, and other sepsis quality initiatives are multifaceted continuous quality improvement (CQI) initiatives which includes: 1)early identification of high risk patients; 2)mobilization of resources for evidence based early interventions; 3)timely initiation of life-saving processes of care 4) a reduction of health care resource consumption and 5) reduced mortality, decreased organ failure, and reduced length of stay. Compliance with the bundle saves 1 in 6 patients who would otherwise die. The current mortality of severe sepsis and septic shock over 5 times higher than any of these aforementioned diseases. Only in the last decade has there been a similar approach to severe sepsis and septic shock. 1e.1 Describe how the component measures/items are consistent with and representative of the quality construct: The sepsis quality initiative is a multifaceted continuous quality improvement (CQI) initiative which includes: 1)early identification of high risk patients; 2)performance of the quality measures appropriate cultures, antibiotics and aggressive reversal of early hemodynamic abnormalities using available best practice; 3) assessment of compliance; 4) dedicated education and feedback to health care providers 5) quantification of health care resource consumption and 6)assessment of outcomes. If the component measures are combined at the patient level, complete 1a, 1b, and 1c. If the component measures are combined at the aggregate level, skip to criterion 2, Scientific Acceptability of Measure Properties (individual measures are either NQF-endorsed or submitted individually). 1a. High Impact: H M L I (The measure directly addresses a specific national health goal/priority identified by DHHS or NPP, or some other high impact aspect of healthcare.) De.4 Subject/Topic Areas (Check all the areas that apply): Infectious Diseases, Infectious Diseases : Respiratory, Pulmonary/Critical Care, Pulmonary/Critical Care : Critical Care, Pulmonary/Critical Care : Pneumonia De.5 Cross Cutting Areas (Check all the areas that apply): Disparities, Safety, Safety : Healthcare Associated Infections 1a.1 Demonstrated High Impact Aspect of Healthcare: Affects large numbers, A leading cause of morbidity/mortality, Frequently performed procedure, High resource use, Patient/societal consequences of poor quality, Severity of illness 1a.2 If “Other,” please describe: 1a.3 Summary of Evidence of High Impact (Provide epidemiologic or resource use data): Sepsis, severe sepsis and septic shock can arise from a simple infection such as pneumonia, insect bite or urinary tract infection. Although it can affect anyone at any age, it is more common in infants, the elderly and patients with chronic health conditions such as diabetes and immunosuppressive disorders such as transplant patients. This condition is associated with a mortality rates of over 16-49%, which is over eight times higher than the rate for inpatient stays for other hospital admission.[1] Findings from the National Hospital Discharge Survey indicate that the number of hospital stays for septicemia more than doubled between 2000 and 2008, and patients with this conditions were more severely ill than patients hospitalized for other conditions.[3] Severe sepsis and septic shock is a frequent cause of re-hospitalizations, especially during the first year after the initial hospitalization.[4] From 1997 to 2008, costs related to septicemia grew at almost three times the rate of costs for overall hospital stays related to other conditions. The national bill for sepsis, pneumonia (sepsis caused by a lung infection) grew twice as fast as the overall growth in hospital charges—about a 180 percent increase from 1997 to 2005, accounting for over $54 Billion per year. When combined with pneumonia, sepsis is the 3rd largest consumer of Medicare, 4th largest consumer of Medicaid and 5th largest consumer of private insurance financial resources and total hospital days. This figure is expected to increase as over 50% of the U.S. population will be over 60 years of age within the next 20 years. Thus, sepsis is also one of the top 5 most costly diseases treated in hospitals in the U.S. Based on national discharge data reported by AHRQ (1), sepsis was the sixth most common principal reason for hospitalization in the United States in 2009, accounting for 836,000 hospital stays. There were an additional 829,500 stays with a secondary diagnosis of sepsis for a total of 1,665,400 inpatient stays and 258,000 deaths. From 1993 to 2009, sepsisrelated hospital stays increased by 153%, with an average annual increase of 6%. Medicare was the predominant payer for sepsisrelated hospital stays, covering 58.1% of patients. Sepsis cases and sepsis-related deaths are expected to continue to increase with the aging of the population. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 3 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Improvements in survival for acute myocardial infarctions or heart attacks (mortality 10%), trauma (mortality 5%), and stroke (mortality 15-20%) which are in the top 20 most expensive disease have been realized through early identification and implementation of time-sensitive therapies at the most proximal stage of disease presentation in the Emergency Department (ED). However, similar approaches to patients with severe sepsis and septic shock have been lacking. In a landmark study by Rivers et. al., it has been shown that an absolute and relative reduction in mortality from sepsis can be reduced 16 and 30%, respectively, when aggressive care is provided within 6 hours of hospital arrival. Furthermore, a recent study of a large national inpatient sample determined that patients admitted through the Emergency Department had a 17% lower likelihood of dying from sepsis than when directly admitted.[5] From 2007 to 2009, over 2,047,038 patients were admitted with a sepsis-related illness.(6) For sepsis patients presenting to the hospital, 52.4% are diagnosed in the ED, 12.8% in the ICU and 34.8% on the hospital wards. The mortality is 27.6%, 41.3% and 46.8% for each of these respective locations.(7) Over 825,300 cases of sepsis present to Emergency Departments (ED) yearly and is the most common diagnosis resulting in hospital stays.(3) It is critically important that patients are diagnosed as soon as possible as mortality can almost 20% higher if a patient is sent the general floors instead of the ICU from the ED. It has also been shown that 12.8% of in-hospital cardiac arrests admitted from home have an admitting diagnosis of pneumonia.(8) This speaks to the fact that these patients are misdiagnosed and sent to the hospital wards to later deteriorate into cardiac arrest. In contrast, other common diseases such as stroke, acute myocardial infarction and trauma have even lower mortalities because of standard operating procedures or quality measures for early diagnosis and management. Only in the last decade has there been a similar approach to severe sepsis and septic shock which occurs just as frequent and is 5 times more deadly.(9) The incidence of sepsis increased 83% over the last decade and two-thirds of the patients affected were over the age of 65 years.(10) It is projected that over 50% of the U.S. population will be over 50 years of age by 2020.(2, 12 These observations speak to the inordinate burden on Medicare and Medicaid resources both present and future that make this proposal highly relevant. Interventions to improve sepsis care would lead to significant reduction in morbidity, mortality, and health care resource consumption.(4, 13-20) 1a.4 Citations for Evidence of High Impact cited in 1a.3: 1. Elixhauser, A., Friedman, B., and Stranges, E. Septicemia in U.S. Hospitals, 2009. HCUP Statistical Brief #122. October 2011 2. Wang, H.E., et al., National estimates of severe sepsis in United States emergency departments. Crit Care Med, 2007 3. Hall, M.J., Williams, S.N., DeFrances, C.J., Golosinskiy, A. Inpatient care for septicemia or sepsis: A challenge for patients and hospitals. NCHS data brief, no 62. Hyattsville, MD: National Center for Health Statistics. 2011 4. Lee H, Doig CJ, Ghali WA, Donaldson C, Johnson D, Manns B. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med. Apr 2004;32(4):981-985. 5. Powell, ES, Khare, RK, Courtney, DM, Feinglass, J. Lower mortality in sepsis patients admitted through the ED vs direct admission. Am J Emerg Med, 2011. 30(3): p. 432-439 . 6. Reed K, McBratney S, Thompson D, Nicholas C. Healthgrades emergency medicine in american hospitals study. Health Grades. June, 2010 2011;The First Annual Report(1):1-28. 7. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine. Feb 2010;38(2):367-374. 8. Carr GE, Edelson DP, Yuen TC, et al. In-Hospital cardiac arrest among patients with coexisting pneumonia: a report from the american heart association´s get with the guidelines - resuscitation program. Am. J. Respir. Crit. Care Med. May 1, 2011 2011;183(Meeting Abstracts):A6339. 9. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. Nov 8 2001;345(19):1368-1377. 10. Burt CW, McCaig LF, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2005. Adv Data. Jun 29 2007(388):115. 11. Andrews R, Elixhauser A. The national hospital bill: growth trends and 2005 update on the most expensive conditions by payer. Healthcare Cost and Utilization Project. 2007;http://www.hcup-us.ahrq.gov/reports/statbriefs/sb42.pdf(Statistical Brief #42):1-13. 12. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. 13. Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. Feb 1 2008;177(3):279-284. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 4 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 14. http://www.hcupus.ahrq.gov/reports/factsandfigures/2008/pdfs/FF_report_2008.pdf. HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States. 2008. 15. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. Nov 2011;140(5):1223-1231. 16. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. Apr 17 2003;348(16):1546-1554. 17. Thompson D, Nicholas C, M.S., T.C., May R, MD, et al. The Second Annual HealthGrades Emergency Medicine in American Hospitals Study April 2011. HealthGrades. 2010;2:1-32. 18. Reed K, May R. Healthgrades emergency medicine in american hospitals study. Health Grades. June, 2010 2010;The First Annual Report(1):1-28. 19. Rogers FB, Osler T, Lee JC, et al. In a mature trauma system, there is no difference in outcome (survival) between Level I and Level II trauma centers. J Trauma. Jun 2011;70(6):1354-1357. 20. Bennett KM, Vaslef S, Pappas TN, Scarborough JE. The volume-outcomes relationship for United States Level I trauma centers. J Surg Res. May 1 2011;167(1):19-23. 1b. Opportunity for Improvement: H M L I (There is a demonstrated performance gap - variability or overall less than optimal performance) 1b.1 Briefly explain the benefits (improvements in quality) envisioned by use of this measure: The benefits of the improvement in quality of care delivered to patients with severe sepsis and septic shock is improved mortality, decreased organ failure and decreases in the utlization of health care resources such as hosptial length of stay, total costs of hospitalization, mechanical ventilation, hemodialysis and time spent in long term care facilities. 1b.2 Summary of Data Demonstrating Performance Gap (Variation or overall less than optimal performance across providers): [For Maintenance – Descriptive statistics for performance results for this measure - distribution of scores for measured entities by quartile/decile, mean, median, SD, min, max, etc.] MEASURE LACTATE: Measurement of lactate levels has been specifically associated with improved outcomes in sepsis, and an elevated lactate value identifies patients at higher risk for poor outcomes.(1,2) Up to 10% of inhospital cardiac arrest in the US per year is secondary to sepsis (pneumonia). These patients are often misdiagnosed and sent to the medical floors only to suffer acute hemodynamic deterioration. These outcomes could be potentially avoided with lactate measurement upon admission providing risk stratification triggering alternative dispositions. In the first quarter of a multicenter quality improvement program for sepsis care, only 61.0% of patients had lactate values measured consistent with guidelines.(3) In addition, prior studies have shown that care prompted by measurement of lactate levels in sepsis patients reduces resource utilization and cost.(4) This leads to lower likelihood of hospital-acquired conditions. This performance measure has been previously used as a core component of multicenter (5) and national quality improvement initiatives.(3) Formalizing it as a national performance measure will provide direct targets for intervention that are closely linked with improvements in mortality and cost. BLOOD CULTURES: In the first quarter of a multicenter quality improvement program for sepsis care, only 64.5% of patients had blood cultures collected.(3) Collecting blood cultures has been specifically associated with improved outcomes in sepsis, and pathogens identified by blood cultures allow for customized therapy. As a result, this is a recommendation of the current Surviving Sepsis Guidelines.(5) By obtaining blood cultures, antibiotic regimens can be customized to treat the specific infecting organism. This will result in less unneeded exposure to antibiotics, reducing complications associated with antibiotic use, including drug reactions, allergies and 5 adverse events, the development of drug-resistant organisms, and the occurrence of Clostridium difficile colitis. The performance measure for collecting blood cultures for suspected sepsis has been previously used as a core component of multicenter and national quality improvement initiatives.(3,5-6) TIMELY ANTIBIOTICS: In a multicenter observational study of antibiotics in septic shock, the median time to appropriate antibiotics was 6 h after shock.(7) In the first quarter of a multicenter quality improvement program for sepsis care, only 60.4% of patients received timely See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 5 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 antibiotics.(3) Multiple studies have demonstrated that delays in administration of appropriate antibiotics in patients with sepsis and other severe infections are associated with longer lengths of stay, higher costs, and higher mortality.(6) In septic shock, a multicenter cohort study demonstrated that every hour in delay of appropriate antibiotics was associated with a 7.6% higher mortality. In a multicenter quality improvement project, the timely administration of broad-spectrum antibiotics was associated with significantly higher risk adjusted survival.(3) Based on a preponderance of data, the current recommendations in the international guidelines for the management of severe sepsis and septic shock includes the administration of broad-spectrum antibiotic therapy within 1 h of diagnosis of septic shock and severe sepsis.(5) FLUID RESUSCITATION: A common finding in patients with septic shock, manifested by low blood pressure and/or other signs of organ hypoperfusion, such as elevated serum lactate levels, is intravascular volume depletion. The degree of the intravascular volume deficit in sepsis varies, yet nearly all patients require initial volume resuscitation and many patients require continuing fluid resuscitation over the first 24 h. Early fluid resuscitation is associated with improved outcomes for patients with ALI due to septic shock.(8) International guidelines recommend that patients with suspected hypovolemia be initially treated with at least 1,000 mL of crystalloid over 30 min to determine clinical response.(5) In the first quarter of a multicenter quality improvement program for sepsis care, only 59.8% of patients received fluid resuscitation consistent with guidelines.(3) Timely fluid resuscitation avoids an error of omission in which indicated therapy is delayed or omitted. By improving outcomes, length of stay is reduced. This leads to lower likelihood of hospitalacquired conditions. This performance measure has been previously used as a core component of multicenter and national quality improvement initiatives.(3,9) Formalizing it as a national performance measure will provide direct targets for intervention that are closely linked with improvements in mortality and cost. LACTATE CLEARANCE: Elevated lactate levels prompt the consideration of specific care practices toward hemodynamic optimization guided by either central venous oxygen saturation (10) or lactate clearance.(11) International guidelines(5) recommend that patients with sepsis and continued elevated lactate values have additional therapies until lactate levels are normalized. However, normal lactate levels can be seen in septic shock. VASOPRESSORS, CVP, and ScvO2: Performance gaps in individual bundle elements can range from 79% (CI 69-89%) for vasopressors, to 27% (CI 18-36%) for CVP measurement, and as low as 15% (CI 7-23%) for ScvO2 in some community emergency departments.(12) These numbers increase (50-75%) in larger hospital settings. CVP has been shown to have a significant association with mortality (20) and multiple studies and meta-analysis have shown a significant association with reaching an ScvO2 of 70% and improved mortality.(20-26). OVERALL BUNDLE COMPLIANCE: Multicenter efforts to promote bundles of care for severe sepsis and septic shock was associated with improved guideline compliance and lower hospital mortality.(6) Even with compliance rates of less than 30%, absolute reductions in mortality of 4-6% has been noted.(3,6) Absolute reductions in mortality of over 20% has been seen with compliance rates of 52%.3 Coba et al has shown that when all bundle elements are completed and compared to patients who do not have bundle completion, the mortality difference is 14%.(27) Thus, there is a direct association between bundle compliance and improved mortality. Without a continuous quality initiative (CQI), even these compliance rates will not improve and will decrease over time.(6) Multiple studies have shown that standardized order sets, enhanced bedside monitor display, telemedicine and comprehensive CQI feedback is feasible, modifies clinician behavior and is associated with decreased hospital mortality.(14-19) 1b.3 Citations for Data on Performance Gap: [For Maintenance – Description of the data or sample for measure results reported in 1b.2 including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included] 1. Trzeciak S, Dellinger RP, Abate NL, Cowan RM, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. CHEST.2006 Feb; 129(2):225-32. 2. Drumheller B, Goyal M, Pines J et al. Elevated point-of-care lactate at triage is predictive of admission among sepsis patients presenting to the emergency department. Ann Emerg Med 2007; 50: S21–2. 3. Levy MM, Dellinger RP, et al.; Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guidelinebased performance improvement program targeting severe sepsis. Crit Care Med. 2010 Feb;38(2):367-74 4. Otero R, Nguyen B, Huang D et al. Early Goal-Direct therapy in severe sepsis and septic shock revisted. CHEST 2006 Nov; 130(5):1579-95. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 6 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 5. Dellinger RP, Levy MM, Carlet JM, Bion J, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008 Jan;36(1):296-327. 6. Ferrer R, Artigas A, et al. Improvement in Process of Care and Outcome After a Multicenter Severe Sepsis Education Program. JAMA 2008;299(19):2294-2303 7. Kumar A, Roberts D, Wood K, Light B, et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy is the Critical Determinant of Survival in Human Septic Shock. Crit Care Med. 2006;34 (6):1589-96 8. Murphy C, Schramm G, Doherty J, et al. The Importance of Fluid Management in Acute Lung Injury Secondary to Septic Shock. CHEST . 2009 July; vol 136, no. 1;102-109 9. Suarez D, Ferrer R, Artigas A. et al. Cost-effectiveness of the Surviving Sepsis Campaign protocol for severe sepsis. Intensive Care Med. 2011 Mar;37(3):444-52 10. Rivers E, Nguyen B, Havstad S et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345: 1368–77. 11. Jones AE, Shapiro NI, Trzeciak S et al. for the Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy. JAMA 2010; 303: 739–46. 12. O´Neill R, Morales J, and Jule M. Early goal directed therapy for severe sepsis and septic shock: which components of treatment are more difficult to implement in a community-based emergency department. Journal of Emergency Medicine 2011. 1-8. 13. Nguyen HB, Corbett SW, Clark RT, Cho T, Wittlake WA. Improving the Uniformity of Care with a Sepsis Bundle in the Emergency Department. Ann Emerg Med. 2005;46(3, Supplement 1):83-83. 14. Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. Mar 2009;37(3):819-824. 15. Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. Nov 2006;34(11):2707-2713. 16. Winterbottom F, Seoane L, Sundell E, Niazi J, Nash T. Improving Sepsis Outcomes for Acutely Ill Adults Using Interdisciplinary Order Sets. Clin Nurse Spec. July/August 2011;25(4):180-185. 17. Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: A multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med. Feb 2011;39(2):252-258. 18. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. Apr 2007;35(4):1105-1112. 19. Loyola S, Wilhelm J, Fornos J. An innovative approach to meeting early goal-directed therapy using telemedicine. Crit Care Nurs Q. Jul-Sep 2011;34(3):187-199. 20. Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. Jun 23 2005;31:1066-1071. 21. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI. Multicenter study of central venous oxygen saturation (ScvO(2)) as a predictor of mortality in patients with sepsis. Ann Emerg Med. Jan 2010;55(1):40-46 e41. 22. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Impact of the surviving sepsis campaign protocols on hospital length of stay and mortality in septic shock patients: Results of a 3-year follow-up quasi-experimental study. Crit Care Med. Feb 11 2010. 23. Jeong SJ, Song YG, Kim CO, et al. Measurement of Plasma sTREM-1 in Patients with Severe Sepsis Receiving Early GoalDirected Therapy and Evaluation of Its Usefulness. Shock. Mar 5 2012. 24. Cannon CM, for the Multicenter Severe S, Septic Shock Collaborative G. The GENESIS Project (GENeralization of Early Sepsis InterventionS): A Multicenter Quality Improvement Collaborative. Acad Emerg Med. 2010;17(11):1258. 25. Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: A meta-analysis. Aust Crit Care. Feb 14 2011. 26. Grissom CK, Morris AH, Lanken PN, et al. Association of physical examination with pulmonary artery catheter parameters in acute lung injury. Crit Care Med. Oct 2009;37(10):2720-2726. 27. Coba V, Whitmill M, Mooney R, et al. Resuscitation Bundle Compliance in Severe Sepsis and Septic Shock: Improves Survival, Is Better Late than Never. J Intensive Care Med. Jan 10 2011. 1b.4 Summary of Data on Disparities by Population Group: [For Maintenance –Descriptive statistics for performance results for this measure by population group] Although it can affect anyone at any age, it is more common in infants, elderly, minorities and patients with chronic health conditions. Patients, particularly the elderly, who present with sepsis can have symptoms for over 24 before presenting to the hospital. Medicaid and Medicare beneficiaries also suffer disproportionately compared to those with private insurance or selfSee Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 7 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 payers.[1-4] It is projected that over 50% of the U.S. population will be over 50 years of age by 2020.[5] This makes it imperative to examine quality initiatives that will not only improve care but also ameliorate the increasing pressure on Medicare Trust Fund which is projected to be insolvent by 2024. Because of diminished access to health care, minorities have a disproportionate greater use of the ED and are thus affected in greater numbers and of higher illness severity upon presentation.[6] These disparities can be partially ameliorated by focused interventions directed at these high risk patient populations.[7,8,9] 1b.5 Citations for Data on Disparities Cited in 1b.4: [For Maintenance – Description of the data or sample for measure results reported in 1b.4 including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included] 1. National Healthcare Disparities Report 2011. Agency for Healthcare Research and Quality. March 2012: p. 146-147. 2. Wilper, A.P., et al., Waits to see an emergency department physician: U.S. trends and predictors, 1997-2004. Health Aff (Millwood), 2008. 27(2): p. w84-95. 3. Barnato, A.E., et al., Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med, 2008. 177(3): p. 279-84. 4. Rodriguez RM, Passanante M, Phelps MA, et al. Delayed emergency department presentation in critically ill patients. Crit Care Med. Dec 2001;29(12):2318-2321. 5. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. 6. Burt CW, McCaig LF, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2005. Adv Data. Jun 29 2007(388):1-15. 7. Wilper AP, Woolhandler S, Lasser KE, et al. Waits to see an emergency department physician: U.S. trends and predictors, 1997-2004. Health Aff (Millwood). Mar-Apr 2008;27(2):w84-95. 8. Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. Feb 1 2008;177(3):279-284. 9. El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of Septic Shock in Older Adults After Implementation of the Sepsis "Bundle". J Am Geriatr Soc. Nov 27 2007. 1c. Evidence (Measure focus is a health outcome OR meets the criteria for quantity, quality, consistency of the body of evidence.) Is the measure focus a health outcome? Yes No If not a health outcome, rate the body of evidence. Quantity: H M L I Quality: H M L I Consistency: H M L I Quantity Quality Consistency Does the measure pass subcriterion1c? M-H M-H M-H Yes L M-H M Yes IF additional research unlikely to change conclusion that benefits to patients outweigh harms M-H L M-H Yes IF potential benefits to patients clearly outweigh potential harms L-M-H L-M-H L No Health outcome – rationale supports relationship to at least one healthcare structure, process, intervention, or service Does the measure pass subcriterion1c? Yes IF rationale supports relationship 1c.1 Structure-Process-Outcome Relationship (Briefly state the measure focus, e.g., health outcome, intermediate clinical outcome, process, structure; then identify the appropriate links, e.g., structure-process-health outcome; process- health outcome; intermediate clinical outcome-health outcome): The foci of this composite are the processes of early management for patients with severe sepsis and septic shock. All bundle elements are associated with improved outcomes for severe sepsis and septic shock patients including mortality and length of stay and have been consistently observed with implementation of early best practice intervention strategies. 1c.2-3 Type of Evidence (Check all that apply): Clinical Practice Guideline, Selected individual studies (rather than entire body of evidence), Systematic review of body of evidence (other than within guideline development) See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 8 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 1c.4 Exclusions Justified A) Patients with advanced directives for comfort care are excluded. B) Clinical conditions that preclude total measure completion should be excluded (e.g. mortality within the first 6 hours of presentation as defined above in 2a1.1). C) Patients for whom a central line is clinically contraindicated (e.g. coagulopathy that cannot be corrected, inadequate internal jugular or subclavian central venous access due to repeated cannulations). D) Patients for whom a central line was attempted but could not be successfully inserted. E) Patient or surrogate decision maker declined or is unwilling to consent to such therapies or central line placement. Please note that the exclusions are highly intuitive and reasonable. Thus, imagining a world for testing purposes, where patients who did not consent for lines received them or who wished to be made comfort measures were treated aggressively is wholly unlikely. Such a study most likely could not be conducted due to appropriate IRB constraints. 1c.5 Directness of Evidence to the Specified Measure (State the central topic, population, and outcomes addressed in the body of evidence and identify any differences from the measure focus and measure target population): The measure focus is on adults 18 years and older with a diagnosis of severe sepsis and septic shock. Consistent with Surviving Sepsis Campaign guidelines, it recommends measurement of lactate, obtaining blood cultures, administering broad spectrum antibiotics, fluid resuscitation, repeat lactate measurement for lactate clearance, and measuring central venous pressure (CVP) and central venous oxygen saturation (ScvO2). The evidence cited for all components of this measure is directly related to decreases in organ failure, overall reductions in hospital mortality, length of stay, and costs of care. For more information, please see attachment entitled NQF 0500 Tables and Forest Plots under the section "Scientific Acceptability". 1c.6 Quantity of Studies in the Body of Evidence (Total number of studies, not articles): The SSC guidelines support for this measure recommendation comes from a particular emphasis on the following: 1. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. 2. Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005;9:R764-70. 3. Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 2005;127:1729-43. 4. Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based "standard operating procedure" and outcome in septic shock. Crit Care Med 2006;34:943-9. 5. Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006;34:1025-32. 6. Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 2006;129:225-32. 7. Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006;34:2707-13. 8. Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 2006;26:551-7. 9. Qu HP, Qin S, Min D, Tang YQ. [The effects of earlier resuscitation on following therapeutic response in sepsis with hypoperfusion]. Zhonghua Wai Ke Za Zhi 2006;44:1193-6. 10. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007;35:1105-12. 11. Chen ZQ, Jin YH, Chen H, Fu WJ, Yang H, Wang RT. [Early goal-directed therapy lowers the incidence, severity and mortality of multiple organ dysfunction syndrome]. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:1892-5. 12. Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 2007;132:425-32. 13. Sebat F, Musthafa AA, Johnson D, et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med 2007;35:2568-75. 14. El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis "bundle". J Am Geriatr Soc 2008;56:272-8. 15. He ZY, Gao Y, Wang XR, Hang YN. [Clinical evaluation of execution of early goal directed therapy in septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007;19:14-6. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 9 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 16. Castro R, Regueira T, Aguirre ML, et al. An evidence-based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiol 2008;74:223-31. 17. Zambon M, Ceola M, Almeida-de-Castro R, Gullo A, Vincent JL. Implementation of the Surviving Sepsis Campaign guidelines for severe sepsis and septic shock: we could go faster. J Crit Care 2008;23:455-60. 18. Zubrow MT, Sweeney TA, Fulda GJ, et al. Improving care of the sepsis patient. Jt Comm J Qual Patient Saf 2008;34:187-91. 19. Peel M. Care bundles: resuscitation of patients with severe sepsis. Nurs Stand 2008;23:41-6. 20. Focht A, Jones AE, Lowe TJ. Early goal-directed therapy: improving mortality and morbidity of sepsis in the emergency department. Jt Comm J Qual Patient Saf 2009;35:186-91. 21. Moore LJ, Jones SL, Kreiner LA, et al. Validation of a screening tool for the early identification of sepsis. J Trauma 2009;66:1539-46; discussion 46-7. 22. Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care 2009;13:R167. 23. Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. Jama 2008;299:2294-303. 24. Girardis M, Rinaldi L, Donno L, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care 2009;13:R143. 25. Wang JL, Chin CS, Chang MC, et al. Key process indicators of mortality in the implementation of protocol-driven therapy for severe sepsis. J Formos Med Assoc 2009;108:778-87. 26. Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med 2009;37:819-24. 27. Pestana D, Espinosa E, Sanguesa-Molina JR, et al. Compliance With a Sepsis Bundle and Its Effect on Intensive Care Unit Mortality in Surgical Septic Shock Patients. J Trauma 2010. 28. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010;38:1036-43. 29. Lefrant JY, Muller L, Raillard A, et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: A multicenter study. Ann Fr Anesth Reanim 2010. 30. Cardoso T, Carneiro AH, Ribeiro O, Teixeira-Pinto A, Costa-Pereira A. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit Care 2010;14:R83. 31. [The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2010;22:331-4. 32. Patel GW, Roderman N, Gehring H, Saad J, Bartek W. Assessing the Effect of the Surviving Sepsis Campaign Treatment Guidelines on Clinical Outcomes in a Community Hospital (November). Ann Pharmacother 2010. 33. Crowe CA, Mistry CD, Rzechula K, Kulstad CE. Evaluation of a modified early goal-directed therapy protocol. Am J Emerg Med 2010;28:689-93. 34. Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011;28:507-12. 35. Gerber K. Surviving sepsis: a trust-wide approach. A multi-disciplinary team approach to implementing evidence-based guidelines. Nurs Crit Care 2010;15:141-51. 36. Gurnani PK, Patel GP, Crank CW, et al. Impact of the implementation of a sepsis protocol for the management of fluidrefractory septic shock: A single-center, before-and-after study. Clin Ther 2010;32:1285-93. 37. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 2010;38:367-74. 38. Macredmond R, Hollohan K, Stenstrom R, Nebre R, Jaswal D, Dodek P. Introduction of a comprehensive management protocol for severe sepsis is associated with sustained improvements in timeliness of care and survival. Qual Saf Health Care 2010. 39. Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest 2010;138:551-8. 40. Coba V, Whitmill M, Mooney R, et al. Resuscitation Bundle Compliance in Severe Sepsis and Septic Shock: Improves Survival, Is Better Late than Never. J Intensive Care Med 2011. 41. Sivayoham N, Rhodes A, Jaiganesh T, van Zyl Smit N, Elkhodhair S, Krishnanandan S. Outcomes from implementing early goal-directed therapy for severe sepsis and septic shock : a 4-year observational cohort study. Eur J Emerg Med 2011. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 10 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 42. Westphal GA, Koenig A, Caldeira Filho M, et al. Reduced mortality after the implementation of a protocol for the early detection of severe sepsis. J Crit Care 2011;26:76-81. 43. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011;36:542-7. 44. Jones AE, Troyer JL, Kline JA. Cost-effectiveness of an emergency department-based early sepsis resuscitation protocol. Crit Care Med 2011;39:1306-12. 45. O´Neill R, Morales J, Jule M. Early Goal-directed Therapy (EGDT) for Severe Sepsis/Septic Shock: Which Components of Treatment are More Difficult to Implement in a Community-based Emergency Department? J Emerg Med 2011. 46. Casserly B, Baram M, Walsh P, Sucov A, Ward NS, Levy MM. Implementing a collaborative protocol in a sepsis intervention program: lessons learned. Lung 2011;189:11-9. 47. Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: A multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med 2011;39:252-8. 48. Suarez D, Ferrer R, Artigas A, et al. Cost-effectiveness of the Surviving Sepsis Campaign protocol for severe sepsis: a prospective nation-wide study in Spain. Intensive Care Med 2011;37:444-52. 49. Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011;15:R229. 50. Shiramizo SC, Marra AR, Durao MS, Paes AT, Edmond MB, Pavao dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS ONE 2011;6:e26790. 51. Tromp M, Tjan DH, van Zanten AR, et al. The effects of implementation of the Surviving Sepsis Campaign in the Netherlands. Neth J Med 2011;69:292-8. 52. Winterbottom F, Seoane L, Sundell E, Niazi J, Nash T. Improving Sepsis Outcomes for Acutely Ill Adults Using Interdisciplinary Order Sets. Clin Nurse Spec 2011;25:180-5. 53. Bastani A, Galens S, Rocchini A, et al. ED identification of patients with severe sepsis/septic shock decreases mortality in a community hospital. Am J Emerg Med 2011. 54. Jeon K, Shin TG, Sim MS, et al. Improvements in Compliance of Resuscitation Bundles and Achievement of End Points After an Educational Program on the Management of Severe Sepsis and Septic Shock. Shock 2012. 55. Cannon CM, for the Multicenter Severe S, Septic Shock Collaborative G. The GENESIS Project (GENeralization of Early Sepsis InterventionS): A Multicenter Quality Improvement Collaborative. Acad Emerg Med 2010;17:1258. 1c.7 Quality of Body of Evidence (Summarize the certainty or confidence in the estimates of benefits and harms to patients across studies in the body of evidence resulting from study factors. Please address: a) study design/flaws; b) directness/indirectness of the evidence to this measure (e.g., interventions, comparisons, outcomes assessed, population included in the evidence); and c) imprecision/wide confidence intervals due to few patients or events): DETERMINATION OF QUALITY OF EVIDENCE UNDERLYING METHODOLOGY: A. RCT B. Downgraded RCT or upgraded observational studies C. Well-done observational studies D. Case series or expert opinion FACTORS THAT MAY DECREASE THE STREGNTH OF EVIDENCE: 1. Poor quality of planning and implementation of available RCTs, suggesting high likelihood of bias 2. Inconsistency of results (including problems with subgroup analyses) 3. Indirectness of evidence (differing population, intervention, control, outcomes, comparison) 4. Imprecision of results 5. High likelihood of reporting bias MAIN FACTORS THAT MAY INCREASE STRENGTH OF EVIDENCE: 1. Large magnitude of effect (direct evidence, RR 2 with no plausible confounders) 2. Very large magnitude of effect with RR 5 and no threats to validity (by two levels) 3. Dose-response gradient RCT, randomized controlled trial; RR, relative risk. FACTORS DETERMINING STRONG VS WEAK RECOMMENDATION: See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 11 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 1. Quality of evidence: the lower the quality of evidence, the less likely a strong recommendation 2. Relative importance of the outcomes: if values and preferences vary widely, a strong recommendation becomes less likely 3. Baseline risks of outcomes: the higher the risk, the greater the magnitude of benefit 4. Magnitude of relative risk, including benefits, harms, and burden: larger relative risk reductions or larger increases in relative risk of harm make a strong recommendation more or less likely, respectively 5. Absolute magnitude of the effect: the larger the absolute benefits and harms, the greater or lesser likelihood, respectively, of a strong recommendation 6. Precision of the estimates of the effects: the greater the precision, the more likely a strong recommendation 7. Costs: the higher the cost of treatment, the less likely a strong recommendation 1c.8 Consistency of Results across Studies (Summarize the consistency of the magnitude and direction of the effect): Although there is no explicit statement in the Surviving Sepsis Campaign (SSC)2008 guidelines regarding the overall consistency of results across studies supporting the guideline recommendations, the development of the SSC 2008 guidelines was by a committee of 68 international experts using the modified Delphi process in developing recommendations for the best current care of patients with severe sepsis and septic shock. These individuals represented 29 Sponsoring organizations: American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, American Thoracic Society, Asia Pacific Association of Critical Care Medicine, Australian and New Zealand Intensive Care Societies, Brazilian Society of Critical Care, Canadian Critical Care Society, Chinese Society of Critical Care Medicine, Chinese Society of Critical Care Medicine - China Medical Association, Emirates Intensive Care Society, European Respiratory Society, European Society of Clinical Microbiology and Infectious Diseases, European Society of Intensive Care Medicine, European Society of Pediatric and Neonatal Intensive Care, Infectious Diseases Society of America, Indian Society of Critical Care Medicine, International Pan Arabian Critical Care Medicine Society, Japanese Association for Acute Medicine, Japanese Society of Intensive Care Medicine, Pediatric Acute Lung Injury and Sepsis Investigators, Society for Academic Emergency Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, Surgical Infection Society, World Federation of Critical Care Nurses, World Federation of Pediatric Intensive and Critical Care Societies; World Federation of Societies of Intensive and Critical Care Medicine. Participation and endorsement by the German Sepsis Society and the Latin American Sepsis Institute. Over the last decade the external validity and generalizability of the various components of the early management bundle have been established in over 50 publications containing over 20,000 patients in community and tertiary hospitals, ED and ICU settings, and medical and surgical patients. In addition, a meta-analysis found that in eight unblinded trials, one randomized and seven with historical controls, sepsis bundles were associated with a consistent (I2 = 0%, p = .87) and significant increase in survival (odds ratio, 1.91; 95% confidence interval, 1.49-2.45; p < .0001). For all studies reporting such data, there were consistent (I2 = 0%, p > or = .64) decreases in time to antibiotics, and increases in the appropriateness of antibiotics (p < or = .0002 for both).(2) Similar findings were noted in a meta-analysis by Chamberlain et al.(3) 1. Dellinger RP, Levy MM, Carlet JM, Bion J, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008 Jan;36(1):296-327. 2. Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. Feb 2010;38(2):668-678. 3. Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: A meta-analysis. Aust Crit Care. Feb 14 2011. 1c.9 Net Benefit (Provide estimates of effect for benefit/outcome; identify harms addressed and estimates of effect; and net benefit - benefit over harms): A meta-analysis found that in eight unblinded trials, one randomized and seven with historical controls, sepsis bundles were associated with a consistent (I2 = 0%, p = .87) and significant increase in survival (odds ratio, 1.91; 95% confidence interval, 1.492.45; p < .0001).(1) Similar findings were noted in a more recent and larger meta-analysis by Chamberlain.(7) In the presence of septic shock each hour delay in achieving administration of effective antibiotics is associated with a measurable 7.6% increase in mortality.(2) Although restriction of antibiotics as a strategy to reduce the development of antimicrobial resistance or to reduce cost is not an appropriate initial strategy in this patient population, once the causative pathogen has been identified, it may become apparent that none of the empirical drugs offers optimal therapy; that is, there may be another drug proven to produce superior clinical outcome that should therefore replace empirical agents. Narrowing the spectrum of antibiotic coverage and reducing the See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 12 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 duration of antibiotic therapy will reduce the likelihood that the patient will develop superinfection with pathogenic or resistant organisms, such as Candida species, Clostridium difficile, or vancomycin-resistant Enterococcus faecium. However, the desire to minimize superinfections and other complications should not take precedence over the need to give the patient an adequate course of therapy to cure the infection that caused the severe sepsis or septic shock.3 After adjustment for baseline characteristics, administration of broad-spectrum antibiotics (OR, 0.86; 95%, CI 0.79– 0.93; p .0001), obtaining blood cultures before their initiation (OR, 0.76; 95% CI, 0.70 – 0.83; p < .0001) were all associated with lower hospital mortality.(4) Blood pressure and lactate targets are predictors of outcome, detect early organ dysfunction and sudden hemodynamic compensation. Early aggressive fluid therapy is associated with improved outcomes over later aggressive fluid therapy.(5) ScvO2 is one of the most important bundle elements, predictive of outcome, and is superior to physical examination in detecting low cardiac index.(6)Patients attaining an ScvO2 of 70% have a two-fold improved mortality than patients treating without it.(7) 1. Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. Feb 2010;38(2):668-678. 2. Kumar A, Roberts D, Wood KE, et al: Duration of hypotension prior to initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596. 3. Dellinger RP, Levy MM, Carlet JM, Bion J, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008 Jan;36(1):296-327. 4. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 2010;38:367-74. 5. Murphy C, Schramm G, Doherty J, et al. The Importance of Fluid Management in Acute Lung Injury Secondary to Septic Shock. CHEST. 2009 July; vol 136, no. 1;102-109 6. Rivers EP, Katranji M, Jaehne KA, Brown S, Abou Dagher G, Cannon C, Coba V. Early interventions in severe sepsis and septic shock: a review of the evidence one decade later. Minerva Anestesiol. 2012 Jun;78(6):712-24 7. Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: A meta-analysis. Aust Crit Care. Feb 14 2011. 1c.10 Grading of Strength/Quality of the Body of Evidence. Has the body of evidence been graded? Yes 1c.11 If body of evidence graded, identify the entity that graded the evidence including balance of representation and any disclosures regarding bias: The Surviving Sepsis Campaign is comprised of a consensus committee of over 50 international experts using the modified Delphi process. These individuals represented 29 Sponsoring organizations: American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, American Thoracic Society, Asia Pacific Association of Critical Care Medicine, Australian and New Zealand Intensive Care Societies, Brazilian Society of Critical Care, Canadian Critical Care Society, Chinese Society of Critical Care Medicine, Chinese Society of Critical Care Medicine - China Medical Association, Emirates Intensive Care Society, European Respiratory Society, European Society of Clinical Microbiology and Infectious Diseases, European Society of Intensive Care Medicine, European Society of Pediatric and Neonatal Intensive Care, Infectious Diseases Society of America, Indian Society of Critical Care Medicine, International Pan Arabian Critical Care Medicine Society, Japanese Association for Acute Medicine, Japanese Society of Intensive Care Medicine, Pediatric Acute Lung Injury and Sepsis Investigators, Society for Academic Emergency Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, Surgical Infection Society, World Federation of Critical Care Nurses, World Federation of Pediatric Intensive and Critical Care Societies; World Federation of Societies of Intensive and Critical Care Medicine. Participation and endorsement by the German Sepsis Society and the Latin American Sepsis Institute. The 2008 guidelines process was funded fully by the Society of Critical Care Medicine. No industry funding was accepted or utilized. Nominal groups were assembled at key international meetings (for those committee members attending the conference). A stand-alone meeting was held for all sub-group heads, co- and vice chairs, and selected key individuals. Teleconferences and electronic-based discussion among subgroups and among the entire committee served as an integral part of the development. Methods: The Grading of Recommendations Assessment, Development and Evaluation. (GRADE) system to guide assessment of quality of evidence from high (A) to very low (D) and to determine the strength of recommendations was used. A strong recommendation (1) indicates that an intervention’s desirable effects clearly outweigh its undesirable effects (risk, burden, cost) or clearly do not. Weak recommendations (2) indicate that the tradeoff between desirable and undesirable effects is less clear. Some recommendations are ungraded (UG). The grade of strong or weak is considered of greater clinical importance than a difference in letter level of quality of evidence. In areas without complete agreement, a formal process of resolution was developed and applied. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 13 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Recommendations are in 3 groups: 1) those directly targeting severe sepsis; 2) recommendations targeting general care of the critically ill patient that are considered high priority in severe sepsis; and 3) pediatric considerations. A formal conflict of interest policy (COI) was developed at the onset of the process and enforced throughout. The entire guidelines process was conducted independent of any industry funding. 1c.12 System Used for Grading the Body of Evidence: GRADE 1c.13 If other, identify and describe the grading scale with definitions: 1c.14 Grade Assigned to the Body of Evidence: EARLY MANAGEMENT WITHIN 6 HOURS=1C, MEASURE LACTATE=1C, BLOOD CULTURES=1C, ANTIBIOTICS=1B, FLUIDS=1B, VASOPRESSORS=1D, MEASURE CVP & ScVO2=1C 1c.15 Summary of Controversy/Contradictory Evidence: 1c.16 Citations for Evidence other than Guidelines(Guidelines addressed below): 1. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. 2. Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005;9:R764-70. 3. Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 2005;127:1729-43. 4. Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based "standard operating procedure" and outcome in septic shock. Crit Care Med 2006;34:943-9. 5. Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 2006;34:1025-32. 6. Trzeciak S, Dellinger RP, Abate NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 2006;129:225-32. 7. Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006;34:2707-13. 8. Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 2006;26:551-7. 9. Qu HP, Qin S, Min D, Tang YQ. [The effects of earlier resuscitation on following therapeutic response in sepsis with hypoperfusion]. Zhonghua Wai Ke Za Zhi 2006;44:1193-6. 10. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007;35:1105-12. 11. Chen ZQ, Jin YH, Chen H, Fu WJ, Yang H, Wang RT. [Early goal-directed therapy lowers the incidence, severity and mortality of multiple organ dysfunction syndrome]. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:1892-5. 12. Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 2007;132:425-32. 13. Sebat F, Musthafa AA, Johnson D, et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med 2007;35:2568-75. 14. El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis "bundle". J Am Geriatr Soc 2008;56:272-8. 15. He ZY, Gao Y, Wang XR, Hang YN. [Clinical evaluation of execution of early goal directed therapy in septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007;19:14-6. 16. Castro R, Regueira T, Aguirre ML, et al. An evidence-based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiol 2008;74:223-31. 17. Zambon M, Ceola M, Almeida-de-Castro R, Gullo A, Vincent JL. Implementation of the Surviving Sepsis Campaign guidelines for severe sepsis and septic shock: we could go faster. J Crit Care 2008;23:455-60. 18. Zubrow MT, Sweeney TA, Fulda GJ, et al. Improving care of the sepsis patient. Jt Comm J Qual Patient Saf 2008;34:18791. 19. Peel M. Care bundles: resuscitation of patients with severe sepsis. Nurs Stand 2008;23:41-6. 20. Focht A, Jones AE, Lowe TJ. Early goal-directed therapy: improving mortality and morbidity of sepsis in the emergency See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 14 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 department. Jt Comm J Qual Patient Saf 2009;35:186-91. 21. Moore LJ, Jones SL, Kreiner LA, et al. Validation of a screening tool for the early identification of sepsis. J Trauma 2009;66:1539-46; discussion 46-7. 22. Puskarich MA, Marchick MR, Kline JA, Steuerwald MT, Jones AE. One year mortality of patients treated with an emergency department based early goal directed therapy protocol for severe sepsis and septic shock: a before and after study. Crit Care 2009;13:R167. 23. Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. Jama 2008;299:2294-303. 24. Girardis M, Rinaldi L, Donno L, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care 2009;13:R143. 25. Wang JL, Chin CS, Chang MC, et al. Key process indicators of mortality in the implementation of protocol-driven therapy for severe sepsis. J Formos Med Assoc 2009;108:778-87. 26. Thiel SW, Asghar MF, Micek ST, Reichley RM, Doherty JA, Kollef MH. Hospital-wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med 2009;37:819-24. 27. Pestana D, Espinosa E, Sanguesa-Molina JR, et al. Compliance With a Sepsis Bundle and Its Effect on Intensive Care Unit Mortality in Surgical Septic Shock Patients. J Trauma 2010. 28. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 2010;38:1036-43. 29. Lefrant JY, Muller L, Raillard A, et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: A multicenter study. Ann Fr Anesth Reanim 2010. 30. Cardoso T, Carneiro AH, Ribeiro O, Teixeira-Pinto A, Costa-Pereira A. Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit Care 2010;14:R83. 31. [The effect of early goal-directed therapy on treatment of critical patients with severe sepsis/septic shock: a multi-center, prospective, randomized, controlled study]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2010;22:331-4. 32. Patel GW, Roderman N, Gehring H, Saad J, Bartek W. Assessing the Effect of the Surviving Sepsis Campaign Treatment Guidelines on Clinical Outcomes in a Community Hospital (November). Ann Pharmacother 2010. 33. Crowe CA, Mistry CD, Rzechula K, Kulstad CE. Evaluation of a modified early goal-directed therapy protocol. Am J Emerg Med 2010;28:689-93. 34. Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011;28:507-12. 35. Gerber K. Surviving sepsis: a trust-wide approach. A multi-disciplinary team approach to implementing evidence-based guidelines. Nurs Crit Care 2010;15:141-51. 36. Gurnani PK, Patel GP, Crank CW, et al. Impact of the implementation of a sepsis protocol for the management of fluidrefractory septic shock: A single-center, before-and-after study. Clin Ther 2010;32:1285-93. 37. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 2010;38:367-74. 38. Macredmond R, Hollohan K, Stenstrom R, Nebre R, Jaswal D, Dodek P. Introduction of a comprehensive management protocol for severe sepsis is associated with sustained improvements in timeliness of care and survival. Qual Saf Health Care 2010. 39. Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest 2010;138:551-8. 40. Coba V, Whitmill M, Mooney R, et al. Resuscitation Bundle Compliance in Severe Sepsis and Septic Shock: Improves Survival, Is Better Late than Never. J Intensive Care Med 2011. 41. Sivayoham N, Rhodes A, Jaiganesh T, van Zyl Smit N, Elkhodhair S, Krishnanandan S. Outcomes from implementing early goal-directed therapy for severe sepsis and septic shock : a 4-year observational cohort study. Eur J Emerg Med 2011. 42. Westphal GA, Koenig A, Caldeira Filho M, et al. Reduced mortality after the implementation of a protocol for the early detection of severe sepsis. J Crit Care 2011;26:76-81. 43. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Ortiz F, Llorca J, Delgado-Rodriguez M. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011;36:542-7. 44. Jones AE, Troyer JL, Kline JA. Cost-effectiveness of an emergency department-based early sepsis resuscitation protocol. Crit Care Med 2011;39:1306-12. 45. O´Neill R, Morales J, Jule M. Early Goal-directed Therapy (EGDT) for Severe Sepsis/Septic Shock: Which Components of See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 15 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Treatment are More Difficult to Implement in a Community-based Emergency Department? J Emerg Med 2011. 46. Casserly B, Baram M, Walsh P, Sucov A, Ward NS, Levy MM. Implementing a collaborative protocol in a sepsis intervention program: lessons learned. Lung 2011;189:11-9. 47. Schramm GE, Kashyap R, Mullon JJ, Gajic O, Afessa B. Septic shock: A multidisciplinary response team and weekly feedback to clinicians improve the process of care and mortality. Crit Care Med 2011;39:252-8. 48. Suarez D, Ferrer R, Artigas A, et al. Cost-effectiveness of the Surviving Sepsis Campaign protocol for severe sepsis: a prospective nation-wide study in Spain. Intensive Care Med 2011;37:444-52. 49. Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011;15:R229. 50. Shiramizo SC, Marra AR, Durao MS, Paes AT, Edmond MB, Pavao dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS ONE 2011;6:e26790. 51. Tromp M, Tjan DH, van Zanten AR, et al. The effects of implementation of the Surviving Sepsis Campaign in the Netherlands. Neth J Med 2011;69:292-8. 52. Winterbottom F, Seoane L, Sundell E, Niazi J, Nash T. Improving Sepsis Outcomes for Acutely Ill Adults Using Interdisciplinary Order Sets. Clin Nurse Spec 2011;25:180-5. 53. Bastani A, Galens S, Rocchini A, et al. ED identification of patients with severe sepsis/septic shock decreases mortality in a community hospital. Am J Emerg Med 2011. 54. Jeon K, Shin TG, Sim MS, et al. Improvements in Compliance of Resuscitation Bundles and Achievement of End Points After an Educational Program on the Management of Severe Sepsis and Septic Shock. Shock 2012. 55. Cannon CM, for the Multicenter Severe S, Septic Shock Collaborative G. The GENESIS Project (GENeralization of Early Sepsis InterventionS): A Multicenter Quality Improvement Collaborative. Acad Emerg Med 2010;17:1258. 1c.17 Quote verbatim, the specific guideline recommendation (Including guideline # and/or page #): GOALS OF INITIAL RESUSCITATION (Strong Recommendation): This protocol should be initiated as soon as hypoperfusion is recognized and should not be delayed pending ICU admission. During the first 6 hrs of resuscitation, the goals of initial resuscitation of sepsis-induced hypoperfusion should include all of the following as one part of a treatment protocol: central venous pressure 8–12 mm Hg, mean arterial pressure (MAP) >=65 mm Hg, urine output >=0.5 mL·kg-1·hr-1, central venous (superior vena cava) or mixed venous oxygen saturation >=70% or >=65%, respectively (grade 1C). MEASURE LACTATE (Strong Recommendation): We recommend the protocolized resuscitation of a patient with sepsis induced shock, defined as tissue hypoperfusion (hypotension persisting after initial fluid challenge or blood lactate concentration > or =4 mmol/L). (grade 1C). APPROPRIATE BLOOD CULTURES (Strong Recommendation): We recommend obtaining appropriate cultures before antimicrobial therapy is initiated if such cultures do not cause significant delay in antibiotic administration. To optimize identification of causative organisms, we recommend at least two blood cultures be obtained before antibiotics with at least one drawn percutaneously and one drawn through each vascular access device, unless the device was recently (<48 hrs) inserted. Cultures of other sites (preferably quantitative where appropriate), such as urine, cerebrospinal fluid, wounds, respiratory secretions, or other body fluids that may be the source of infection should also be obtained before antibiotic therapy if not associated with significant delay in antibiotic administration (grade 1C). ANTIBIOTIC THERAPY (Strong Recommendations): 1. We recommend that intravenous antibiotic therapy be started as early as possible and within the first hour of recognition of septic shock (1B) and severe sepsis without septic shock (1D). Appropriate cultures should be obtained before initiating antibiotic therapy but should not prevent prompt administration of antimicrobial therapy (grade 1D). 2a. We recommend that initial empirical anti-infective therapy include one or more drugs that have activity against all likely pathogens (bacterial and/or fungal) and that penetrate in adequate concentrations into the presumed source of sepsis (grade 1B). FLUID THERAPY (Strong Recommendation): 1. We recommend fluid resuscitation with either natural/artificial colloids or crystalloids. There is no evidence-based support for one type of fluid over another (grade 1B). 2. We recommend that fluid resuscitation initially target a central venous pressure of >=8 mm Hg (12 mm Hg in mechanically ventilated patients). Further fluid therapy is often required (grade 1C). See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 16 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 3a. We recommend that a fluid challenge technique be applied wherein fluid administration is continued as long as the hemodynamic improvement (e.g., arterial pressure, heart rate, urine output) continues (grade 1D). 3b. We recommend that fluid challenge in patients with suspected hypovolemia be started with >=1000 mL of crystalloids or 300– 500 mL of colloids over 30 mins. More rapid administration and greater amounts of fluid may be needed in patients with sepsisinduced tissue hypoperfusion (see Initial Resuscitation recommendations)(grade 1D). 3c. We recommend that the rate of fluid administration be reduced substantially when cardiac filling pressures (central venous pressure or pulmonary artery balloon-occluded pressure) increase without concurrent hemodynamic improvement (grade 1D). VASOPRESSORS (Strong Recommendations): 1. We recommend that mean arterial pressure (MAP) be maintained >65 mm Hg(grade 1C). 2. We recommend either norepinephrine or dopamine as the first choice vasopressor agent to correct hypotension in septic shock (administered through a central catheter as soon as one is available) (grade 1C). 5. We recommend that low-dose dopamine not be used for renal protection(grade 1A). 6. We recommend that all patients requiring vasopressors have an arterial catheter placed as soon as practical if resources are available (grade 1D). MEASURE CVP & MEASURE ScvO2: (Strong Recommendation) 1.We suggest that during the first 6 hrs of resuscitation of severe sepsis or septic shock, if ScvO2 or SCvO2 of 70% or 65%, respectively, is not achieved with fluid resuscitation to the central venous pressure target (1C) 2.If the ScvO2 is <70%, CVP and MAP goals are met; then transfusion of packed red blood cells to achieve a hematocrit of >=30% and/or administration of a dobutamine infusion (up to a maximum of 20 micrograms·kg-1·min-1) be used to achieve this goal (2C). 1c.18 Clinical Practice Guideline Citation: Dellinger RP, Levy MM, Carlet JM, Bion J, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008 Jan;36(1):296-327. 1c.19 National Guideline Clearinghouse or other URL: http://www.guideline.gov/content.aspx?id=12231&search=surviving+sepsis 1c.20 Grading of Strength of Guideline Recommendation. Has the recommendation been graded? Yes 1c.21 If guideline recommendation graded, identify the entity that graded the evidence including balance of representation and any disclosures regarding bias: APPENDIX I: 2008 Surviving Ssepsis Campaign (SSC) Guidelines Committee: R. Phillip Dellinger (Chair), Tom Ahrens(a), Naoki Aikawa(b), Derek Angus, Djillali Annane, Richard Beale, Gordon R. Bernard, Julian Bion(c), Christian Brun-Buisson, Thierry Calandra, Joseph Carcillo, Jean Carlet, Terry Clemmer, Jonathan Cohen, Edwin A. Deitch(d),Jean-Francois Dhainaut, Mitchell Fink, Satoshi Gando (b), Herwig Gerlach, Gordon Guyatt (e), Maurene Harvey, Jan Hazelzet, Hiroyuki Hirasawa,f Steven M. Hollenberg, Michael Howell, Roman Jaeschke (e), Robert Kacmarek, Didier Keh, Mitchell M. Levy (g), Jeffrey Lipman, John J. Marini, John Marshall, Claude Martin, Henry Masur, Steven Opal, Tiffany M. Osborn (h), Giuseppe Pagliarello (i), Margaret Parker, Joseph Parrillo, Graham Ramsay, Adrienne Randolph, Marco Ranieri, Robert C. Read (j), Konrad Reinhart (k), Andrew Rhodes, Emanuel Rivers (h), Gordon Rubenfeld, Jonathan Sevransky, Eliezer Silva,l Charles L. Sprung, B. Taylor Thompson, Sean R. Townsend, Jeffery Vender (m), Jean-Louis Vincent (n), Tobias Welte (o), Janice Zimmerman. a American Association of Critical-Care Nurses; b Japanese Association for Acute Medicine; c European Society of Intensive Care Medicine; d Surgical Infection Society; e Grades of Recommendation, Assessment, Development and Evaluation (GRADE) f Japanese Society of Intensive Care Medicine; g Society of Critical Care Medicine; h American College of Emergency Physicians; i Canadian Critical Care Society; j European Society of Clinical Microbiology and Infectious Diseases; k German Sepsis Society; See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 17 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 l Latin American Sepsis Institute; m American College of Chest Physicians; n International Sepsis Forum; o European Respiratory Society. APPENDIX J: Author Disclosure Information 2006–2007 Dr. Dellinger has consulted for Astra-Zeneca, Talecris, and B Braun. He has received honoraria from Eli Lilly (2),Brahms (2), INO Therapeutics (1), Pulsion (1), and bioMerieux (1). He has also received grant support from AstraZeneca and Artisan. Dr. Levy has received honoraria from Eli Lilly and Edwards Lifesciences. He has also received grant support from Philips Medical Systems, Edwards Lifesciences, Philips Medical Systems, Novartis, Biosite, and Eisai. Dr. Carlet has consulted for Forrest, Wyeth, Chiron, bioMerieux, and GlaxoSmithKline. He has also received honoraria from Eli Lilly, Becton Dickinson, Jansen, Cook, AstraZeneca, Hutchinson, Bayer, Gilead, MSD, and Targanta. Dr. Bion has not disclosed any potential conflicts of interest. Dr. Parker has consulted for Johnson & Johnson. Dr. Jaeschke has received honoraria from AstraZeneca, Boehringer, Eli Lilly, GlaxoSmithKline, and MSD. Dr. Reinhart has consulted for Eli Lilly and Edwards Lifesciences. He has also received honoraria from B Braun and royalties from Edwards Lifesciences. Dr. Angus has consulted for or received speaking fees from AstraZeneca, BrahmsDiagnostica, Eisai, Eli Lilly, Glaxo-SmithKline, OrthoBiotech, Takeda, and Wyeth-Ayerst. He has also received grant support from GlaxoSmithKline, Ortho-Biotech, and Amgen. Dr. Brun-Buisson has not disclosed any potential conflicts of interest. Dr. Beale has received honoraria from Eisai and speaking fees (paid to university) from Lilly UK, Philips, Lidco, and Chiron. Dr. Calandra has consulted for Baxter, received honoraria from Roche Diagnostics, and received grant support from Baxter and Roche Diagnostics. He also served on the advisory board for Biosite. Dr. Dhainaut has consulted for Eli Lilly and Novartis. He has also received honoraria from Eli Lilly. Dr. Gerlach has not disclosed any potential conflicts of interest. Ms. Harvey has not disclosed any potential conflicts of interest. Dr. Marini has consulted for KCI and received honoraria from Maquet. Dr. Marshall has consulted for Becton Dickinson, Takeda, Pfizer, Spectral Diagnostics, Eisai, and Leo-Pharma. He has also received honoraria from Spectral Diagnostics. Dr. Ranieri has served on the advisor board for Maquet and received support for a sponsored trial from Eli Lilly. He has also received grant support from Tyco, Draeger, and Hamilton. Dr. Ramsay has consulted for Edwards Lifesciences and Respironics. Dr. Sevransky has not disclosed any potential conflicts of interest. Dr. Thompson has consulted for Eli Lilly, Abbott, and AstraZeneca. He has also received grant support from the NIH for a study on computerized glucose control. Dr. Townsend has not disclosed any potential conflicts of interest. Dr. Vender has consulted and received honoraria from Eli Lilly. Dr. Zimmerman has not disclosed any potential conflicts of interest. Dr. Vincent has consulted for AstraZeneca, Biosite, bioMerieux, Edwards Lifesciences, Eli Lilly, Eisai, Ferring, Glaxo-SmithKline, Intercell, Merck, Novartis, NovoNordisk, Organon, Pfizer, Philips Medical Systems, Roche Diagnostics, Spectral Diagnostics, Takeda, and Wyeth-Lederle. He has also received honoraria from Eli Lilly, Edwards Lifesciences, Eisai, GlaxoSmithKline, Novartis, NovoNordisk, and Pfizer. 1c.22 System Used for Grading the Strength of Guideline Recommendation: GRADE 1c.23 If other, identify and describe the grading scale with definitions: 1c.24 Grade Assigned to the Recommendation: EARLY MANAGEMENT WITHIN 6 HOURS=1C, MEASURE LACTATE=1C, BLOOD CULTURES=1C, ANTIBIOTICS=1B, FLUIDS=1B, VASOPRESSORS=1D, MEASURE CVP & ScVO2=1C 1c.25 Rationale for Using this Guideline Over Others: It is the Henry Ford Hospital policy to use guidelines, which are evidencebased, actionable by facilities and health-care providers, and developed by a national specialty organization or government agency. In addition, the HFH also accepts as the evidence base for measures to include documented quality improvement (QI) initiatives or implementation projects that have demonstrated improvement in quality of care. There was strong agreement among a large cohort of 55 international experts regarding many recommendations for the best current care of patients with severe sepsis and septic shock. Evidence-based recommendations are the first step toward improved outcomes for this important group of critically ill patients. Based on the NQF descriptions for rating the evidence, what was the developer’s assessment of the quantity, quality, and See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 18 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 consistency of the body of evidence? 1c.26 Quantity: High 1c.27 Quality: High 1c.28 Consistency: High 1c.29 Attach evidence submission form: 1c.30 Attach appendix for supplemental materials: Was the threshold criterion, Importance to Measure and Report, met? (1a & 1b must be rated moderate or high and 1c yes) Yes No Provide rationale based on specific subcriteria: For a new measure if the Committee votes NO, then STOP. For a measure undergoing endorsement maintenance, if the Committee votes NO because of 1b. (no opportunity for improvement), it may be considered for continued endorsement and all criteria need to be evaluated. 2. RELIABILITY & VALIDITY - SCIENTIFIC ACCEPTABILITY OF MEASURE PROPERTIES In the future, NQF will require measure stewards to provide a URL link to a web page where current detailed specifications can be obtained? S.1 Do you have a web page where current detailed specifications for this measure can be obtained? No S.2 If yes, provide web page URL: 2a. Precisely Specified 2a.0.1 Components of the Composite. (List the components, i.e., domains/sub-composites, individual measures. If component measures are NQF-endorsed, include NQF measure number; if not NQF-endorsed, provide date of submission to NQF) If the composite measure cannot be specified with a numerator and denominator, please consult with NQF staff. If the component measures are combined at the aggregate level, do not include the individual measure specifications below. 2a1.1 Composite Numerator Statement (Brief, narrative description of the measure focus or what is being measured about the target population, e.g., cases from the target population with the target process, condition, event, or outcome): If: A. measure lactate level B. obtain blood cultures prior to antibiotics C. administer broad spectrum antibiotics D. administer 30 ml/kg crystalloid for hypotension or lactate >=4 mmol/L E. apply vasopressors (for hypotension that does not respond to initial fluid resuscitation to maintain a mean areterial pressure >= 65) F. In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate >=4 mmol/L (36 mg/dl) measure central venous pressure and central venous oxygen saturation G. remeasure lactate if initial lactate is elevated represent processes of care: Numerator statement: Patients from the denominator who received all the following: A, B, and C within 3 hours of time of presentation† AND IF septic shock is present (as either defined as hypotension* or lactate >=4 mmol/L) who also received D and E and F and G within 6 hours of time of presentation. † ”time of presentation” is defined as the time of triage in the Emergency Department or, if presenting from another care venue, from the earliest chart annotation consistent with all elements severe sepsis or septic shock ascertained through chart review. * “hypotension” is defined as systolic blood pressure (SBP) <90 mm Hg or mean arterial pressure (MAP) <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline. 2a1.2 Numerator Time Window (The time period in which the target process, condition, event, or outcome is eligible for inclusion): See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 19 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Bundle elements should be *completed* in the times outlined in the numerator statement, however patients are *eligible* for inclusion in the numerator if diagnosed with severe sepsis or septic shock at anytime during their hospitalization. 2a1.3 Numerator Details (All information required to identify and calculate the cases from the target population with the target process, condition, event, or outcome such as definitions, codes with descriptors, and/or specific data collection items/responses: Following the scheme outlined in 2a1.1 “A” requires a response of “yes” to the question: “Was a lactate level obtained within 3 hours of time of presentation?” “B” requires a response of “yes” to the question: “Were blood cultures obtained prior to antibiotic administration and within 3 hours of time of presentation?” “C” requires a response of “yes” to the question: “Were broad spectrum antibiotics administered within 3 hours of the time of presentation?” “Septic Shock” requires a response of “yes” to the question: “Was either hypotension (defined as SBP < 90 or MAP < 65 or decrease in SBP 30 mmHg from baseline) OR lactate >=4 mmol/L present in the first 6 hour of the time of presentation?” “D” requires a response of “yes” or “not applicable” to the question: “Were 30ml/kg of crystalloid administered for hypotension or lactate >= 4 mmol/L within 6 hours of the time of presentation?” “E” requires a response of “yes” or “not applicable” to the question: “Were vasopressors applied within 6 hours of the time of presentation for hypotension that did not respond to initial fluid resuscitation to maintain a mean arterial pressure >= 65 mmHg?” “F” requires a response of “yes” or “not applicable” to the question: “Were central venous pressure (CVP) and central venous oxygen saturation (ScVO2) measured within 6 hours of presentation in the event of hypotension despite volume resuscitation or initial lactate >= 4 mmol/L (36 mg/dl)?” “G” requires a response of “yes” or “not applicable” to the question: “Was serum lactate re-measured if initially elevated within 6 hours of presentation.” 2a1.4 Composite Denominator Statement (Brief, narrative description of the target population being measured): Number of patients presenting with severe sepsis or septic shock. 2a1.5 Target Population Category (Check all the populations for which the measure is specified and tested if any): Adult/Elderly Care, Populations at Risk, Populations at Risk : Dual eligible beneficiaries, Populations at Risk : Individuals with multiple chronic conditions, Populations at Risk : Veterans, Senior Care 2a1.6 Denominator Time Window (The time period in which cases are eligible for inclusion): Patients are eligible for inclusion in the denominator for each episode of severe sepsis or septic shock during a hospitalization from emergency room presentation though discharge. The collection period for each increment of data reporting is monthly. 2a1.7 Denominator Details (All information required to identify and calculate the target population/denominator such as definitions, codes with descriptors, and/or specific data collection items/responses): The denominator may be derived by a) prospective real-time screening of all patients presenting for care to the facility, or b) retrospective screening through chart review of all patients presenting to the medical facility, or c) both methods. In each case the clinical diagnostic criteria for severe sepsis or septic shock as outlined below are applied to the population initially identified. The clinical criteria that must be applied in either instance do not vary whether prospective or retrospective data collection is employed. SEVERE SEPSIS: Severe sepsis is defined as a suspected source of clinical infection, 2 or more manifestations of systemic infection (SIRS criteria) and the presence of sepsis-induced organ dysfunction. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 20 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 SIRS criteria include: Temperature >38.3 C or <36.0 C, Heart rate >90 beats per minute, Respiration > 20 breaths/min, White blood cell count >12,000 or <4000/mm3, or >10% bandemia. Organ dysfunction variables include: (SBP)<90 mm Hg or mean arterial pressure <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline, Creatinine > 2.0 mg/dl (176.8 mmol/L) or Urine Output < 0.5 ml/kg/hour for > 2 hours, Bilirubin > 2 mg/dl (34.2 mmol/L), Platelet count < 100,000, Coagulopathy (INR >1.5 or aPTT >60 secs), Lactate > 2 mmol/L (18.0 mg/dl). SEPTIC SHOCK: Septic shock requires the presence of severe sepsis as above AND as sepsis-induced hypoperfusion persisting despite adequate fluid resuscitation OR lactate > 4 mmol/L. Sepsis induced tissue hypoperfusion is present with (SBP)<90 mm Hg or mean arterial pressure <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline. If clinical coding documentation is used to derive the denominator in a retrospective collection effort, the codes that should be applied include: ICD9 DX: a) b) c) d) e) f) g) h) i) j) k) l) m) n) o) p) q) r) s) t) 0031: SALMONELLA SEPTICEMIA 0362: MENINGOCOCCEMIA 0380: STREPTOCOCCAL SEPTICEMIA 03810: STAPH SEPTICEMIA NOS 03811: MSSA SEPTICEMIA 03812: MRSA SEPTICEMIA 03819: STAPH SEPTICEMIA NEC 0382: PNEUMOCOCCAL SEPTICEMIA 0383: ANAEROBIC SEPTICEMIA 03840: GRAM-NEG SEPTICEMIA NOS 03841: H. INFLUENZAE SEPTICEMIA 03842: E. COLI SEPTICEMIA 03843: PSEUDOMONAS SEPTICEMIA 03844: SERRATIA SEPTICEMIA 03849: GRAM-NEG SEPTICEMIA NEC 0388: SEPTICEMIA NEC 0389: SEPTICEMIA NOS 78552: SEPTIC SHOCK 99591: SEPSIS 99592: SEVERE SEPSIS 2a1.8 Denominator Exclusions (Brief narrative description of exclusions from the target population): A) Patients with advanced directives for comfort care are excluded. B) Clinical conditions that preclude total measure completion should be excluded (e.g. mortality within the first 6 hours of presentation as defined above in 2a1.1). C) Patients for whom a central line is clinically contraindicated (e.g. coagulopathy that cannot be corrected, inadequate internal jugular or subclavian central venous access due to repeated cannulations). D) Patients for whom a central line was attempted but could not be successfully inserted. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 21 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 E) Patient or surrogate decision maker declined or is unwilling to consent to such therapies or central line placement. 2a1.9 Denominator Exclusion Details (All information required to identify and calculate exclusions from the denominator such as definitions, codes with descriptors, and/or specific data collection items/responses): The exclusion details described in 2a1.8 must be ascertained by chart review. No specific definitions are required to discover this information from standard chart annotation. 2a1.10 Stratification Details/Variables (All information required to stratify the measure results including the stratification variables, codes with descriptors, definitions, and/or specific data collection items/responses ): Henry Ford Hospital (HFH) encourages the results of this measure to be stratified by race, ethnicity, gender, and primary language, illness severity and have included these variables as recommended data elements to be collected. If the component measures are combined at the patient level and include outcomes, complete the following 2a1.11 Risk Adjustment Type (Select type. Provide specifications for risk stratification in 2a1.10 and for statistical model in 2a1.13): No risk adjustment or risk stratification 2a1.12 If "Other," please describe: 2a1.13 Statistical Risk Model and Variables (Name the statistical method - e.g., logistic regression and list all the risk factor variables. Note - risk model development should be addressed in 2b4.): None 2a1.14-16 Detailed Risk Model Available at Web page URL (or attachment). Include coefficients, equations, codes with descriptors, definitions, and/or specific data collection items/responses. Attach documents only if they are not available on a webpage and keep attached file to 5 MB or less. NQF strongly prefers you make documents available at a Web page URL. Please supply login/password if needed: 2a1.17 Type of Score: Non-weighted score/composite/scale 2a1.19 Interpretation of Score (Classifies interpretation of score according to whether better quality is associated with a higher score, a lower score, a score falling within a defined interval, or a passing score): Better quality = Higher score 2a1.20 Method of Scoring Opportunity scoring (overall percentage) 2a1.21 If "other" scoring method, describe 2a1.22 Missing Component Score (Indicate how missing component scores are handled): At Henry Ford Hospital, missing components of the measure are considered not performed. 2a1.23 Weighting: Equal 2a1.24 If differential weighting, describe: 2a1.25 Calculation Algorithm/Measure Logic(Describe the calculation of the measure score as an ordered sequence of steps including identifying the target population; exclusions; cases meeting the target process, condition, event, or outcome; aggregating data; risk adjustment; etc.): The data calculations may be performed in one of two ways. The Surviving Sepsis Campaign Database available at SurvivingSepsis.org automatically performs all calculations if data is entered into the required fields. However, hospitals are not restricted to use of the database to perform the required calculations. Two See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 22 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 paper tools described below capture the logic. The two tools, URLs provided in 2a1.26.1, (“Individual Chart Measurement Tool” [ICMT], and “Monthly Measurement Worksheet” [MMW]) govern the calculation of the elements of the “all or nothing” composite measure. The tools, in fact, exceed the information required for calculation of the composite measure extending care to variables beyond the scope of this submission (e.g. care patterns for the first 24 hours of care such as the application of steroids or glucose control; calculation of individual component measures not requested for endorsement at this time). They are provided as a clear, yet highly detailed, statement of the logic. To simplify matters, the algorithm will be described in plain language here: 1. Find the patients who meet the initial patient population (ie, the general group of patients that the performance measure is designed to address). This is accomplished as described in 2a1.7 either through prospective, retrospective or both forms of data screening. Codes and criteria are specified in 2a1.7. 2. From the patients within the initial patient population criteria, find the patients who qualify for the denominator (ie, the specific group of patients for inclusion in a specific performance measure based on defined criteria). All exclusions identified by chart review in 2a1.8 will not, by definition, qualify for the denominator. Note: in some cases the initial patient population and denominator are identical. 3. From the patients within the denominator less those excluded, find the patients who qualify for the Numerator (ie, the group of patients in the denominator for whom a process or outcome of care occurs). The individual component elements of the composite indicator (eg, lactate collected, blood cultures obtained, etc.) will be found on each instance of the ICMT (one per patient chart reviewed). Each month, all ICMT’s will be gathered and tabulated to generate the composite numerator using the MMW. In this way the MMW consolidates all information gathered in each ICMT to create the composite numerator. For more detail, the steps are identified below: a. The logic on the ICMT captures all necessary data to be abstracted from a single chart to inform the numerator. b. The “time of presentation” is captured as defined in 2a1.1 in question 3 of the ICMT. c. Collection of lactate is determined and timed in question 4 of the ICMT. d. Administration of broad spectrum antibiotics and timing are captured in question 5 of the ICMT. e. Collection of blood cultures and timing is captured in question 6 of the ICMT. f. Next, required determinations to inform the conditional elements in the composite measure are made. Specifically, since component elements “D, E, F, G” defined in 2a1.1 above are dependent on the presence of septic shock, the shock state is documented in question 7 of the ICMT. i. If the patient has shock documentation of the administration of fluids is captured in question 7c of the ICMT. ii. If the patient has shock documentation of the application of vasopressors is captured in question 7e of the ICMT. iii. If the patient has shock documentation of the assessment of CVP and timing is captured in question 8 of the ICMT. iv. If the patient has shock documention of the assessment of ScVO2 and timing is captured in question 9 of the ICMT. g. If shock is not present, credit is assigned for the dependent elements “D, E, F, G” and documented on line 16 of the ICMT. h. The tally of affirmative responses (or where credit has been assigned) to the individual component measures on a per chart basis is recorded by placing a mark in the designated boxes in line 16 of the ICMT. i. Note: questions 10-15 on the ICMT do not apply to the composite measure under submission here. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 23 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 j. Once monthly the MMW will be employed to tabulate all of the line 16 scores on the ICMT to generate the composite numerator for the month. i. While the MMW is designed to report out the component measures as individual quality indicators, this is not required for the composite measure under consideration. Thus, questions 1 to 12 on the MMW are not necessary in this instance. ii. Question 13 on the MMW generates the monthly “all or nothing” numerator by requiring that ALL boxes on line 16 of each ICMT be marked complete. iii. If a single box on line 16 of the ICMT is not completed, then the “all or nothing” criterion is not met and the individual chart is not included in the numerator. This represents a quality failure. iv. Questions 14 and 15 also do not apply to the composite measure under consideration here. 4. Although the exclusion cases are removed from the denominator population for the performance calculation, the number of patients with valid exclusions should be calculated and reported along with performance rates to track variations in care and highlight possible areas of focus for QI. 2a1.26 Calculation Algorithm/Measure Logic Diagram URL or attachment: URL http://www.survivingsepsis.org/About_the_Campaign/Documents/individualchartmeasurementtool.pdf AND http://www.survivingsepsis.org/About_the_Campaign/Documents/monthlymeasurementworksheet.pdf 2a1.27 Sampling (Survey) Methodology. If measure is based on a sample (or survey), provide instructions for obtaining the sample, conducting the survey and guidance on minimum sample size (response rate): Not applicable. The measure does not require sampling or a survey. However, the minimum sample size recommended should be no less than 50 patients per facility. 2a1.28 Data Source (Check all the sources for which the measure is specified and tested). If other, please describe: Electronic Clinical Data, Electronic Clinical Data : Electronic Health Record, Electronic Clinical Data : Registry, Paper Medical Records 2a1.29 Data Source/Data Collection Instrument (Identify the specific data source/data collection instrument, e.g. name of database, clinical registry, collection instrument, etc.): Surviving Sepsis Campaign Electronic Database: http://www.survivingsepsis.org/manual_database/Pages/default.aspx Paper Tools: http://www.survivingsepsis.org/About_the_Campaign/Documents/monthlymeasurementworksheet.pdf http://www.survivingsepsis.org/About_the_Campaign/Documents/individualchartmeasurementtool.pdf 2a1.30-32 Data Source/data Collection Instrument Reference Web Page URL or Attachment: URL http://www.survivingsepsis.org/manual_database/Pages/default.aspx 2a1.33-35 Data Dictionary/Code Table Web Page URL or Attachment: URL http://www.survivingsepsis.org/About_the_Campaign/Documents/le_field_descriptions_and_coding_information.pdf 2a1.36 Level of Analysis (Check the levels of analysis for which the measure is specified and tested): Facility, Integrated See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 24 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Delivery System 2a1.37 Care Setting (Check all the settings for which the measure is specified and tested): Hospital/Acute Care Facility 2a2. Reliability Testing. (Reliability testing was conducted with appropriate method, scope, and adequate demonstration of reliability.) 2a2.1 Data/Sample (Description of the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): The Surviving Sepsis Campaign database provides unequivocal results that compare reliability across 200 organizations using the “all or nothing” composite measure specified in 2a1. • Surviving Sepsis Campaign Database Between January 2005 and March 2008, 15,775 subjects at 252 qualifying sites (individual hospitals) were entered into the Surviving Sepsis Campaign (SSC) database. Excluding hospitals that contributed fewer than 20 subjects, the final sample consisted of 15,022 patients at 165 hospitals (median, 57; range, 20–471 subjects per hospital). Data from up to eight quarters were analyzed from each site. Hospitals contributed data for a mean duration of 15.6 months (median, 14 months). Data from 15,022 subjects at 165 sites were analyzed to determine the compliance with bundle targets and association with hospital mortality. Sites were instructed to set up screening procedures to identify patients with severe sepsis and septic shock based on previously established criteria as provided in a manual that included specific specifications consistent with those in section 2a1 of this submission. Sites were provided a sample screening tool in the Campaign manual and on the Web site. Participating sites were asked to screen for patients in the emergency department, the clinical wards, and the ICU. To be enrolled, a subject had to have a suspected site of infection, 2 systemic inflammatory response syndrome criteria, and 1 organ dysfunction criterion. Clinical and demographic characteristics and time of presentation with severe sepsis criteria were collected for analysis of time-based measures. Data were entered into the SSC database locally at individual hospitals into pre-established, structured data fields documenting performance and the time of specific actions and findings. Hospitals could not modify these fields. Hospitals were instructed to exclude only those patients with comfort measures status. These instructions were consistent with the exclusions identified in 2a1.8 of this submission and conveyed to sites in the manual that accompanied download of the database. Variation in structured data fields was not possible with the database and full completion was required to submit any data to the master database. Data stripped of private health information were submitted every 30 days to the secure master SSC server at the Society of Critical Care Medicine via file transfer protocol or as comma delimited text files attached to e-mail submitted to the Campaign’s server. Additional data was obtained after the initial data collection period described above. The Surviving Sepsis Campaign database now contains data submitted from January 2005 through July 2012. Analyses constrained by the same criteria as above now are possible with a total of 28,150 patients with severe sepsis and septic shock at 218 international sites. 2a2.2 Analytic Method (Describe method of reliability testing & rationale): The analytic methods for reliability testing of the data is described below: • Surviving Sepsis Campaign Database For purposes of reliability testing, the SSC data was analyzed as described in the RAND Corporations “The Reliability of Provider Profiling: A Tutorial” by John L. Adams (see appendix). This methodology is specifically endorsed by the NQF to analyze the reliability of performance for proposed metrics. The analysis is sometimes referred to as a signal-to-noise analysis. As a measure of reliability, the analysis is a key determinant of suitability because it permits an assessment of how well one can confidently distinguish the performance of one entity from another. The signal in this case is the proportion of the variability in measured performance that can be explained by real differences in See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 25 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 performance. The SSC biostatician, Gary Phillips, MAS (Ohio State University Center for Biostatistics), used the Rand model to generate the reliability of the “all or nothing” composite metric specified in section 2a1 above by hospital site. The strategy involves fitting a betabinomial model for each indicator. From each model two parameters are generated (alpha and beta) that define the beta-binomial distribution. From these parameters Mr. Philips then produced the between hospital variance. Next the within hospital variance is generated based on a proportion affirmative answers for each quality indicator (the binomial distribution). Analyzing the between hospital variance and the within hospital variance generates the reliability for each hospital site. 2a2.3 Testing Results (Reliability statistics, assessment of adequacy in the context of norms for the test conducted): The testing results for the reliability of the indicator are described below: • Surviving Sepsis Campaign Database The reliability of the measure using the SSC database (on a scale of 0 to 1) was examined by each site for the resuscitation bundle as described in 2a2.2 above. The mean reliability (and its associated standard deviation) for each of the deciles of reliability for the composite measure is as written in text format below. A limitation of this online submission form is that tables cannot be easily inserted, therefore please see the text Table 1 “Reliability deciles with SD from beta-binomial model by site” just below. For specific reliabilities for each of 210 hospitals/sites with the associated number of contributed charts per hospital see Table 2 “Reliability estimated from beta-binomial model by site ID" included at the bottom of this field. Table 1 “Reliability deciles with SD from beta-binomial model by site”: 1st Decile: N = 22, Mean reliability = 0.751, SD of reliability = 0.0539. 2nd Decile: N = 22, Mean reliability = 0.850, SD of reliability = 0.0182. 3rd Decile: N = 22, Mean reliability = 0.889, SD of reliability = 0.0075. 4th Decile: N = 22, Mean reliability = 0.915, SD of reliability = 0.0077. 5th Decile: N = 21, Mean reliability = 0.929, SD of reliability = 0.0025. 6th Decile: N = 22, Mean reliability = 0.939, SD of reliability = 0.0049. 7th Decile: N = 22, Mean reliability = 0.956, SD of reliability = 0.0052. 8th Decile: N = 22, Mean reliability = 0.973, SD of reliability = 0.0041. 9th Decile: N = 22, Mean reliability = 0.988, SD of reliability = 0.0040. 10th Decile: N = 21, Mean reliability = 0.999, SD of reliability = 0.0013. Total: N = 218, Mean reliability = 0.919, SD of reliability 0.0732. Note also that although for purposes of this submission only the composite measure is being considered for endorsement, the specific reliabilities of the underlying components is known and were calculated using the same methodology. Results are summarized below as percentages: N = 28150 Serum lactate..................Mean reliability = 94.96.....SD = 4.57 Culture before antibiotics.....Mean reliability = 90.19.....SD = 8.03 Timely antibiotics.............Mean reliability = 87.66.....SD = 8.82 Fluids & Vasopressors..........Mean reliability = 94.12.....SD = 5.32 CVP Assessment.................Mean reliability = 90.81.....SD = 8.15 ScVO2 Assessment...............Mean reliability = 89.84.....SD = 89.84 Overall Bundle.................Mean reliability = 91.86.....SD = 7.30 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 26 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Table 2 “Reliability estimated from beta-binomial model by site ID": ID................N................Composite measure reliability 1.................63...............0.937 2.................38...............0.930 3.................36...............0.864 4.................34...............0.954 5.................34...............0.880 6.................73...............0.881 7.................35...............1.000 8.................50...............0.922 9.................21...............1.000 10................49...............0.919 11................62...............0.985 12................66...............0.899 13................97...............0.924 14................67...............0.976 15................46...............0.974 16................22...............1.000 17................43...............0.879 18................40...............0.885 19................29...............0.887 20................63...............0.960 21................37...............0.926 22................47...............0.880 23................52...............0.961 24................37...............0.777 25................27...............0.924 26................22...............0.898 27................29...............0.887 28................27...............1.000 29................82...............0.961 30................35...............0.832 31................28...............0.934 32................50...............0.922 33................29...............1.000 34................92...............0.938 35................31...............1.000 36................34...............0.880 37................94...............1.000 38................217..............0.991 39................96...............0.947 40................80...............0.916 41................20...............1.000 42................28...............0.880 43................20...............1.000 44................40...............0.845 45................25...............0.855 46................73...............1.000 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 27 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 47................44...............0.923 48................38...............0.901 49................306..............0.969 50................28...............0.691 51................62...............0.897 52................230..............0.968 53................285..............0.953 54................214..............0.937 55................20...............0.732 56................58...............0.916 57................183..............0.932 58................126..............0.911 59................30...............0.701 60................62...............0.888 61................684..............0.983 62................40...............0.813 63................70...............0.830 64................52...............0.809 65................310..............0.973 66................20...............0.604 67................59...............0.808 68................39...............0.880 69................75...............1.000 70................27...............0.731 71................169..............0.938 72................304..............0.974 73................100..............0.928 74................204..............0.938 75................159..............0.932 76................101..............0.948 77................97...............0.924 78................60...............0.891 79................50...............0.939 80................85...............0.855 81................117..............0.928 82................110..............0.939 83................87...............0.932 84................167..............0.959 85................36...............0.769 86................22...............0.822 87................57...............0.882 88................110..............0.895 89................44...............1.000 90................34...............0.954 91................236..............0.978 92................108..............0.961 93................47...............0.932 94................93...............0.981 95................165..............0.960 96................27...............0.929 97................27...............0.872 98................39...............1.000 99................23...............0.906 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 28 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 100...............33...............1.000 101...............140..............0.956 102...............264..............0.992 103...............114..............0.979 104...............136..............0.980 105...............312..............0.994 106...............20...............0.794 107...............203..............0.968 108...............27...............0.757 109...............62...............0.947 110...............40...............1.000 111...............67...............0.826 112...............351..............0.966 113...............37...............0.846 114...............162..............0.919 115...............155..............0.955 116...............148..............0.990 117...............58...............1.000 118...............528..............0.974 119...............105..............0.928 120...............20...............0.794 121...............58...............0.916 122...............240..............0.957 123...............87...............0.947 124...............177..............0.988 125...............414..............0.997 126...............159..............0.989 127...............51...............0.979 128...............621..............0.987 129...............264..............0.980 130...............35...............1.000 131...............207..............0.972 132...............80...............0.932 133...............200..............0.992 134...............126..............0.929 135...............148..............0.933 136...............187..............0.930 137...............104..............0.898 138...............26...............0.816 139...............35...............0.956 140...............36...............0.864 141...............87...............0.932 142...............68...............0.878 143...............31...............0.899 144...............168..............0.933 145...............22...............0.822 146...............101..............0.944 147...............52...............0.863 148...............166..............0.928 149...............107..............0.932 150...............74...............0.945 151...............551..............0.982 152...............80...............0.916 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 29 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 153...............75...............0.901 154...............89...............0.986 155...............23...............0.779 156...............153..............0.928 157...............21...............0.749 158...............23...............0.701 159...............25...............0.919 160...............421..............0.981 161...............454..............0.970 162...............167..............0.994 163...............1131.............0.998 164...............106..............0.884 165...............142..............0.911 166...............251..............0.948 167...............222..............0.942 168...............70...............0.853 169...............291..............0.953 170...............152..............0.934 171...............66...............0.892 172...............61...............0.841 173...............312..............0.997 174...............119..............0.988 175...............151..............0.969 176...............124..............0.946 177...............127..............0.918 178...............112..............0.925 179...............313..............0.992 180...............321..............0.986 181...............23...............0.834 182...............142..............0.934 183...............204..............0.961 184...............75...............0.857 185...............40...............0.748 186...............157..............0.933 187...............243..............0.948 188...............383..............0.968 189...............394..............0.985 190...............506..............0.985 191...............1023.............0.991 192...............100..............0.876 193...............34...............0.914 194...............70...............0.989 195...............473..............0.972 196...............37...............0.896 197...............28...............0.662 198...............41...............0.794 199...............290..............0.973 200...............227..............0.945 201...............93...............0.900 202...............28...............0.929 203...............26...............0.924 204...............231..............0.996 205...............150..............0.954 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 30 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 206...............93...............0.959 207...............150..............0.913 208...............44...............0.851 209...............251..............0.973 210...............31...............0.755 2b. VALIDITY. Validity, Testing, including all Threats to Validity: H M L I 2b1.1 Describe how the measure specifications (measure focus, target population, and exclusions) are consistent with the evidence cited in support of the measure focus (criterion 1c) and identify any differences from the evidence: The measure is consistent with regard to the evidence cited in criterion 1c with respect to the measure focus, the target population and the exclusions. Each will be detailed below: • Measure Focus: The “all or nothing” composite measure specifications directly pertain to the measure focus (the early management of patients with severe sepsis and septic shock) inasmuch as the measure is constrained to observations in first 6 hours of care. The specifications are identical to the evidence cited in 1c. Each cited piece of literature was evaluated using a binary (affirmative or negative) response as regards the provision of specific components of care delivery in determining the composite “all or nothing” calculation. The health outcomes assessed in each study involved no intermediate clinical outcomes, but rather focused on mortality in each instance. The reliability data provided from the SSC was derived under these conditions and, in fact, makes up the largest body of the clinical evidence for the measure focus. • Target Population: The specified target population of the measure is entirely consistent across the evidence. All studies have looked at patients with severe sepsis and septic shock as defined by the the 2001 Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society consensus sepsis definition. The data provided as regards reliability here is not to the contrary in any material form. • Exclusions: Exclusions were uniform from the specifications cited in 2a1 and the evidence cited here as regards reliability. In particular, patients were excluded in the SSC analysis with advanced directives for comfort care and/or clinical conditions that precluded total measure completion. These exclusions were conveyed to hospitals in the manual that accompanied the SSC database. 2b2. Validity Testing. (Validity testing was conducted with appropriate method, scope, and adequate demonstration of validity.) 2b2.1 Data/Sample (Description of the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): Although there may often be a distinction between the data used to inform the evidence for the importance of the measure focus and the data that informs reliability and validity testing, the authors of this submission have access to the database that produced the foundational evidence for the importance of the measure and have used the same data to produce calculations related to the reliability and validity of the data. Therefore, the distinction is not applicable in this case. The data sample then is identical to the data identified in 2a2.1 and repeated here: • Surviving Sepsis Campaign Database Between January 2005 and March 2008, 15,775 subjects at 252 qualifying sites (individual hospitals) were entered into the Surviving Sepsis Campaign (SSC) database. Excluding hospitals that contributed fewer than 20 subjects, the final sample consisted See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 31 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 of 15,022 patients at 165 hospitals (median, 57; range, 20–471 subjects per hospital). Data from up to eight quarters were analyzed from each site. Hospitals contributed data for a mean duration of 15.6 months (median, 14 months). Data from 15,022 subjects at 165 sites were analyzed to determine the compliance with bundle targets and association with hospital mortality. Sites were instructed to set up screening procedures to identify patients with severe sepsis and septic shock based on previously established criteria as provided in a manual that included specific specifications consistent with those in section 2a1 of this submission. Sites were provided a sample screening tool in the Campaign manual and on the Web site. Participating sites were asked to screen for patients in the emergency department, the clinical wards, and the ICU. To be enrolled, a subject had to have a suspected site of infection, 2 systemic inflammatory response syndrome criteria, and 1 organ dysfunction criterion. Clinical and demographic characteristics and time of presentation with severe sepsis criteria were collected for analysis of time-based measures. Data were entered into the SSC database locally at individual hospitals into pre-established, structured data fields documenting performance and the time of specific actions and findings. Hospitals could not modify these fields. Hospitals were instructed to exclude only those patients with comfort measures status. These instructions were consistent with the exclusions identified in 2a1.8 of this submission and conveyed to sites in the manual that accompanied download of the database. Variation in structured data fields was not possible with the database and full completion was required to submit any data to the master database. Data stripped of private health information were submitted every 30 days to the secure master SSC server at the Society of Critical Care Medicine via file transfer protocol or as comma delimited text files attached to e-mail submitted to the Campaign’s server. Additional data was obtained after the initial data collection period described above. The Surviving Sepsis Campaign database now contains data submitted from January 2005 through July 2012. Analyses constrained by the same criteria as above now are possible with a total of 28,150 patients with severe sepsis and septic shock at 218 international sites. 2b2.2 Analytic Method (Describe method of validity testing and rationale; if face validity, describe systematic assessment): Validity testing of the performance measure presented here is based on the measure as specified and submitted for endorsement. The SSC database was used to generate this analysis. The performance scores reported were generated for the hospitals using the specifications submitted for endorsement. As noted in the Measure Testing Task Force report, validity testing (as with reliability testing) can be conducted at the level of the critical data elements or the performance measure score. The testing presented here is testing at the level of the performance measure score based on conceptual understanding of the measure and also the data that are available. • As such, a reasonable hypothesis for validity testing of the performance measure score would be that those with higher scores on the composite performance measure should have a lower score on a risk-adjusted mortality measure. To test this hypothesis, the SSC biostatician, Gary Phillips, MAS (Ohio State University Center for Biostatistics) built a random effects logistic regression where patient level observations were nested within a particular site (hospital). The regression model is adjusted for the variables shown below. The model included the following variables (Organ Failure abbreviated OF): Sepsis origin .....ED (referent) .....Ward .....ICU Geographic region .....Europe .....North America (referent) .....South America Cardiovascular OF See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 32 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 .....Lactate > 4 mmol/L .....Cardiovascular OF with Lactate > 4 mmol/L .....No hypotension (referent) .....Hypotension with MAP < 65 mm Hg .....Hypotension with MAP = 65 mm Hg Received =20 ml/kg of crystalloid or equivalent Received vasopressors Pneumonia UTI Abdominal Meningitis Catheter Device Other infection Renal organ failure Hepatic organ failure Hematologic organ failure No mechanical ventilation and no pulmonary OF (referent) No mechanical ventilation and pulmonary OF Mechanical ventilation with plateau pressure < 30 cm H2O and no pulmonary OF Mechanical ventilation with plateau pressure < 30 cm H2O and pulmonary OF Mechanical ventilation with plateau pressure = 30 cm H2O independent of pulmonary OF Hyperthermia (> 38.3° C) or (101.0° F) Hypothermia (< 36° C) or (96.8° F) Chills with rigor Tachypnea (BPM > 20) Leukopenia (WBC count < 4,000/µL) Hyperglycemia (plasma glucose > 120 mg/dL) Acutely alter mental status • Separately, there is substantial face validity of the measure. The Measure Testing TF report indicates that NQF does allow for face validity of the performance measure score if it is systematically assessed. Here, the measure submitted for evaluation does not substantially differ from the components of the composite measure systematically assessed in the SSC peer reviewed publication “Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guidelinebased performance improvement program targeting severe sepsis. Critical Care Medicine 201 0; 38:367-74.” In fact, the analysis performed for this submission is the identical data set to the SSC data set. This publication demonstrated declining mortality associated with increased compliance. The specific results are provided in 2b2.3 for review. 2b2.3 Testing Results (Statistical results, assessment of adequacy in the context of norms for the test conducted; if face validity, describe results of systematic assessment): • In the random effects logistic regression model created specific for this submission by Mr. Phillips described in 2b2.2, the odds of hospital mortality are reduced 10% (odds ratio = 0.90, 95% CI: 0.83 to 0.97, p-value = 0.008) for patients that are compliant with the measure as described in sections 2a1.1 and 2a1.4 of this submission. • The face validity of the measure specifications is bolstered by the publication “Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 201 0; 38:367-74,” which systematically assessed a composite measure not materially different from the performance measure here. This publication demonstrated declining mortality associated with increased compliance. The specific results are recited here: “Outcome measures included hospital mortality, hospital length of stay, and ICU length of stay. Ten performance measures were See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 33 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 established, based on the individual elements of the resuscitation bundle and the management bundle. The analysis set was constructed from the subjects entered into the SSC database from its launch in January 2005 through March 2008. The a priori data analysis plan limited inclusion to sites with at least 20 subjects and at least 3 months of subject enrollment. Analysis presented here was limited to the first 2 yrs of subjects at each site. Sites were characterized by: hospital size (250, 250–500, >500 beds); teaching status; ICU type (medical, medical/surgical, other); and geographic region (Europe, North America, South America). Subjects were characterized by baseline severe sepsis information: location of enrollment (emergency department, ICU, ward); site of infection (pulmonary, urinary tract, abdominal, central nervous system, skin, bone, wound, catheter, cardiac, device, other); acute organ dysfunction (cardiovascular, pulmonary, renal, hepatic, hematologic). Data were organized by quarter through 2 yrs, with the first 3 months that a site entered subjects into the database defined as the first quarter, regardless of when those months occurred from January 2005 through March 2008. The effects of predictor variables on hospital mortality we expressed using odds ratios (ORs), including 95% confidence intervals (CIs) for risk-adjusted results. Logistic regression model fit was assessed using the Hosmer Lemeshow C statistic, the chi-square dispersion, the proportion of log-likelihood accounted for by the model, and an examination of model residuals. We constructed the databases in Access and Fox-Pro (Microsoft Corp, Redmond, WA) and conducted analyses in DataDesk (Data Description, Ithaca, NY) and SAS (SAS Institute, Cary, NC). SURVIVING SEPSIS CAMPAIGN - CHANGES IN ACHIEVEMENTS OF BUNDLE TARGETS OVER TIME: Compliance rates for achieving all bundle targets over time—both the overall bundles and the individual elements within both bundles—increased over time, although both basal achievement rates and the magnitude of improvement varied considerably across targets. Compliance with the initial bundle targets increased linearly from 10.9% of subjects in the first site quarter to 31.3% by the end of 2 yrs in the campaign, achieving statistical significance by the second quarter (10.9% vs. 14.9%, p .0001). The ability to achieve the entire bundle targets started higher, at 18.4% in the first quarter, and increased to 25.5% by the end of 2 yrs, but did not achieve statistical significance until the fourth quarter (18.4% vs. 21.5%, p < .008). SURVIVING SEPSIS CAMPAIGN - CHANGES IN HOSPITAL MORTALITY: Unadjusted hospital mortality decreased from 37.0% in the first quarter in the Campaign to 30.8% by 2 yrs (p < .001). On average, unadjusted mortality decreased by 0.91% (95% CI, 0.42–1.40) for each quarter in the Campaign. The results of the multivariable model examining the effect of time in the Campaign on hospital mortality, fit well (Hosmer and Lemeshow C statistic of 18.1 with 18 df, p <.34, accounted for 36.6% of variation in the data, with a chi-square dispersion of 1.04). In both the unadjusted and adjusted models, the chance of death decreased the longer a site was in the Campaign, resulting in an adjusted absolute drop of 0.8% per quarter and 5.4% over the first 2 yrs (95% CI, 2.5–8.4). SURVIVING SEPSIS CAMPAIGN - RELATIONSHIP BETWEEN BUNDLE ELEMENTS AND IN HOSPITAL MORTALITY: After adjustment for baseline characteristics, administration of broad-spectrum antibiotics (OR, 0.86; 95%, CI 0.79– 0.93; p .0001), obtaining blood cultures before their initiation (OR, 0.76; 95% CI, 0.70– 0.83; p < .0001) were all associated with lower hospital mortality. To control for entry of less severely ill patients in the database over time as the reason for decreasing mortality, severity was assessed based on variables linked to patient mortality that were available in the database. When mortality was adjusted accordingly, while the magnitude of the effect was slightly reduced, it remained statistically significant. The results of this study demonstrate that the use of a multifaceted performance improvement initiative was successful in changing sepsis treatment behavior as demonstrated by a significant increase in compliance with sepsis performance measures. This compliance was associated with a significant reduction in hospital mortality in patients with severe sepsis and septic shock. These results are consistent with an earlier report from Ferrer et al in Spain. The findings of this study show that the improvement in achievement of bundle targets and association with improved outcome is sustained over time and is demonstrated across a wide number of countries and settings.” POTENTIAL THREATS TO VALIDITY. (All potential threats to validity were appropriately tested with adequate results.) If the component measures are combined at the patient level, complete 2b 2b3. Measure Exclusions. (Exclusions were supported by the clinical evidence in 1c or appropriately tested with results demonstrating the need to specify them.) 2b3.1 Data/Sample for analysis of exclusions (Description of the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 34 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 The validity analysis provided above provides discriminatory power at the level of the performance measure. Please note that the exclusions are highly intuitive and reasonable. Thus, imagining a world for testing purposes, where patients who did not consent for lines received them or who wished to be made comfort measures were treated aggressively is wholly unlikely. Exclusions referenced in section 2a1.8 therefore were not independently analyzed for their effect on the performance measure score given the impropriety of such testing. Such a study most likely could not be conducted due to appropriate IRB constraints. Excluded patients are not included in data collection at the level of the data collection tool during the time of chart review (either in the sepsis campaign database or the paper equivalent tools, the “ICMT”). See 2a1.25 for specific algorithm and logic. 2b3.2 Analytic Method (Describe type of analysis and rationale for examining exclusions, including exclusion related to patient preference): Not applicable, see 2b3.1. 2b3.3 Results (Provide statistical results for analysis of exclusions, e.g., frequency, variability, sensitivity analyses): Not applicable, see 2b3.1. If the component measures are combined at the patient level and include outcomes, complete 2e 2b4. Risk Adjustment Strategy. (For outcome measures, adjustment for differences in case mix (severity) across measured entities was appropriately tested with adequate results.) 2b4.1 Data/Sample (Description of the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): This process measure composite is not risk adjusted. 2b4.2 Analytic Method (Describe methods and rationale for development and testing of risk model or risk stratification including selection of factors/variables): This process measure composite is not risk adjusted. 2b4.3 Testing Results (Statistical risk model: Provide quantitative assessment of relative contribution of model risk factors; risk model performance metrics including cross-validation discrimination and calibration statistics, calibration curve and risk decile plot, and assessment of adequacy in the context of norms for risk models. Risk stratification: Provide quantitative assessment of relationship of risk factors to the outcome and differences in outcomes among the strata): Not applicable. 2b4.4 If outcome or resource use measure is not risk adjusted, provide rationale and analyses to justify lack of adjustment: As a process measure, no risk adjustment is neccessary. 2b5. Identification of Meaningful Differences in Performance. (The performance measure scores were appropriately analyzed and discriminated meaningful differences in quality.) 2b5.1 Data/Sample (Describe the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): The data sample used in this calculation of meaningful differences in performance (performed specifcially for this submission)was the enlarged SSC database (28,150 patients) identified in 2a2.1 and repeated here: • Surviving Sepsis Campaign Database Between January 2005 and March 2008, 15,775 subjects at 252 qualifying sites (individual hospitals) were entered into the Surviving Sepsis Campaign (SSC) database. Excluding hospitals that contributed fewer than 20 subjects, the final sample consisted of 15,022 patients at 165 hospitals (median, 57; range, 20–471 subjects per hospital). Data from up to eight quarters were analyzed from each site. Hospitals contributed data for a mean duration of 15.6 months (median, 14 months). Data from 15,022 subjects at 165 sites were analyzed to determine the compliance with bundle targets and association with hospital mortality. Sites were instructed to set up screening procedures to identify patients with severe sepsis and septic shock based on previously established criteria as provided in a manual that included specific specifications consistent with those in section 2a1 of this See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 35 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 submission. Sites were provided a sample screening tool in the Campaign manual and on the Web site. Participating sites were asked to screen for patients in the emergency department, the clinical wards, and the ICU. To be enrolled, a subject had to have a suspected site of infection, 2 systemic inflammatory response syndrome criteria, and 1 organ dysfunction criterion. Clinical and demographic characteristics and time of presentation with severe sepsis criteria were collected for analysis of time-based measures. Data were entered into the SSC database locally at individual hospitals into pre-established, structured data fields documenting performance and the time of specific actions and findings. Hospitals could not modify these fields. Hospitals were instructed to exclude only those patients with comfort measures status. These instructions were consistent with the exclusions identified in 2a1.8 of this submission and conveyed to sites in the manual that accompanied download of the database. Variation in structured data fields was not possible with the database and full completion was required to submit any data to the master database. Data stripped of private health information were submitted every 30 days to the secure master SSC server at the Society of Critical Care Medicine via file transfer protocol or as comma delimited text files attached to e-mail submitted to the Campaign’s server. Additional data was obtained after the initial data collection period described above. The Surviving Sepsis Campaign database now contains data submitted from January 2005 through July 2012. Analyses constrained by the same criteria as above now are possible with a total of 28,150 patients with severe sepsis and septic shock at 218 international sites. 2b5.2 Analytic Method (Describe methods and rationale to identify statistically significant and practically/meaningfully differences in performance): For purposes of this analysis, the enlarged SSC database was used with 218 sites and 28,150 patients. The data set therefore covers 16 quarters of participation in the campaign, or 4 years worth of data total. Sites were permitted to join the campaign during the duration of the 4 years. [NB, although the large majority of hospital sites joined early and some dropped out over time, few sites joined late. Given the discrepancy therefore between calendar time and time of participation in the campaign, sites were aligned by “site quarter” meaning that the first quarter of participation was the same for all sites regardless of the calendar month they joined the campaign. This pattern was maintained for all sites through 16 quarters. Of critical note, in the campaign to adjust for the confounding variable that mortality may be decreasing over time the mortality rate was determined at site quarter 1 of all participants and there was no statistical correlation with a decrease in mortality over time at the outset of participation. Thus, an underlying secular trend to lower mortality did not confound the data regardless of the variability in time when joining the campaign. The adjustment method and results of this important analysis are available in “Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 201 0; 38:367-74.”] Using the above data set, for this analysis, sites were aligned by number of site quarters of participation 1st quarter, 8th quarter (midpoint) and 16th quarter (endpoint). The total number of sites in the campaign at each time point was determined. The mean, SD, minimum, maximum, p25, p50, p75 of sites with a “yes” value for the composite indicator (i.e., a value counted in the numerator) is reported for each time referent in Table 1 below. Table 2 below reports the number of sites with decreasing performance over time compared with the number of sites with increasing performance over time. Please note the sharp attrition in sites reporting by site quarter 18, accounting for some of the declining performance detected. Efforts to promote participation in data collection had substantially fallen off by this time. 2b5.3 Results (Provide measure performance results/scores, e.g., distribution by quartile, mean, median, SD, etc.; identification of statistically significant and meaningfully differences in performance): Table 1. Descriptive summary of proportion where the composite is ´yes´ by site quarters of participation: Quarters.of....Number....Mean....SD........Min.....P25.....P50.....P75.....Max.. participation..of sites 1..................218.......0.101...0.180.....0.000...0.000...0.000...0.125...1.000 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 36 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 8..................88........0.205...0.197.....0.000...0.000...0.183...0.354...0.667 16.................8.........0.158...0.102.....0.000...0.096...0.136...0.255...0.295 Please note as regards Table 1: • • • • • Site quarters are based on a quarter of participation based on when a site entered the SSC program Site quarters do not align with calendar quarters (sites may enter variably) There were 218 sites that started the program After 2 years (8 quarters) there were 88 sites still participating After 4 years (16 quarters) there were 8 sites still participating Table 2. Descriptive summary of proportion where the composite is ´yes´ by site quarters of participation: Delta.........No.....Description..Mean...SD......Min...P25....P50....P75....Max.. ..............sites.............................................................. .............................................................................. Decreased.....21.....Proportion...0.108..0.176..0.000..0.000..0.000..0.118..0.595 ........................Delta........0.247..0.246..0.021..0.059..0.200..0.334..1.000 .............................................................................. Increased.....67.....Proportion...0.235..0.196..0.000..0.000..0.213..0.385..0.667 ........................Delta........0.175..0.164..0.000..0.000..0.158..0.326..0.600 Please note as regards Table 2: • Delta is whether or not a site decreased or increased • 21 sites (24%) decreased from the 1st quarter while 67 sites (76%) increased • Description: Proportion is those that met the resuscitation bundle and delta is the size of either the increase or decrease. Finally, a description of the 8 sites with observations during site quarter 16: • All 8 improved from the 1st quarter to the 8th quarter the mean proportion was 0.153 and the mean change from 1st to 8th quarter was 0.136 • Then from the 8th to the 16th quarter 3 sites decreased a mean proportion of 0.131 and 5 sites increased with a mean proportion of 0.087 2b6. Comparability of Multiple Data Sources/Methods. (If specified for more than one data source, the various approaches result in comparable scores.) 2b6.1 Data/Sample (Describe the data or sample including number of measured entities; number of patients; dates of data; if a sample, characteristics of the entities included): Please see attachment under "Supplemental Information" below. 2b6.2 Analytic Method (Describe methods and rationale for testing comparability of scores produced by the different data sources specified in the measure): Please see attachment under "Supplemental Information" below. 2b6.3 Testing Results (Provide statistical results, e.g., correlation statistics, comparison of rankings; assessment of adequacy in the context of norms for the test conducted): Please see attachment under "Supplemental Information" below. 2c. Disparities in Care: H M L I NA (If applicable, the measure specifications allow identification of disparities.) 2c.1 If measure is stratified for disparities, provide stratified results (Scores by stratified categories/cohorts): The Henry Ford Hospital, SCCM, IDSA, an emergency physician, and quality improvement organizations encourage the results of this measure to See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 37 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 be stratified by race, ethnicity, gender, and primary language, and have included these variables as recommended data elements to be collected. 2c.2 If disparities have been reported/identified (e.g., in 1b), but measure is not specified to detect disparities, please explain: The Henry Ford Hospital, SCCM, IDSA, an emergency physician, and quality improvement organizations advocate that performance measure data should, where possible, be stratified by race, ethnicity, and primary language to assess disparities and initiate subsequent quality improvement activities addressing identified disparities, consistent with recent national efforts to standardize the collection of race and ethnicity data. A 2008 NQF report endorsed 45 practices including stratification by the aforementioned variables.(1) A 2009 IOM report “recommends collection of the existing Office of Management and Budget (OMB) race and Hispanic ethnicity categories as well as more finegrained categories of ethnicity(referred to as granular ethnicity and based on one’s ancestry) and language need (a rating of spoken English language proficiency of less than very well and one’s preferred language for health-related encounters).”(2) References: (1)National Quality Forum Issue Brief (No.10). Closing the Disparities Gap in Healthcare Quality with Performance Measurement and Public Reporting. Washington, DC: NQF, August 2008. (2)Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. March 2010. AHRQ Publication No. 10-0058-EF. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/research/iomracereport. Accessed May 25, 2010. 2i. Component Item/Measure Analysis to Justify Inclusion in Composite 2i.1. Data/Sample Please see attachment under "Supplemental Information" below. 2i.2. Analytic Method Please see attachment under "Supplemental Information" below. 2i.3. Result Please see attachment under "Supplemental Information" below. 2j. Component Item/Measure Analysis of Contribution to Variability in Composite Score 2j.1. Data/Sample Please see attachment under "Supplemental Information" below. 2j.2. Analytic Method Please see attachment under "Supplemental Information" below. 2j.3. Result Please see attachment under "Supplemental Information" below. 2k. Analysis to Support Differential Weighting of Component Score 2k.1. Data/Sample Composite score is not weighted. 2k.2. Analytic Method Composite score is not weighted. 2k.3. Result See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 38 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Composite score is not weighted. 2k.4. Describe how the method scoring/aggregation achieves the stated purpose and represent the quality construct Composite score is not weighted. 2k.5. Indicate if any alternative scoring/aggregation methods were tested and why not chosen Composite score is not weighted. 2l. Analysis of Missing Component Scores 2l.1. Data/Sample For missing component scores fail opportunity score. 2l.2. Analytic Method For missing component scores fail opportunity score. 2l.3. Result For missing component scores fail opportunity score. 2.1-2.3 Supplemental Testing Methodology Information: Attachment NQF_0500_Tables_and_Forest_Plots.pdf Steering Committee: Overall, was the criterion, Scientific Acceptability of Measure Properties, met? (Reliability and Validity must be rated moderate or high) Yes No Provide rationale based on specific subcriteria: If the Committee votes No, STOP 3. USABILITY Extent to which intended audiences (e.g., consumers, purchasers, providers, policy makers) can understand the results of the measure and are likely to find them useful for decision making. (evaluation criteria) C.1 Intended Actual/Planned Use (Check all the planned uses for which the measure is intended): Professional Certification or Recognition Program, Public Reporting, Quality Improvement (Internal to the specific organization), Quality Improvement with Benchmarking (external benchmarking to multiple organizations), Regulatory and Accreditation Programs 3.1 Current Use (Check all that apply; for any that are checked, provide the specific program information in the following questions): Regulatory and Accreditation Programs, Quality Improvement with Benchmarking (external benchmarking to multiple organizations), Quality Improvement (Internal to the specific organization) 3a. Usefulness for Public Reporting: H M L I (The measure is meaningful, understandable and useful for public reporting.) 3a.1. Use in Public Reporting - disclosure of performance results to the public at large (If used in a public reporting program, provide name of program(s), locations, Web page URL(s)). If not publicly reported in a national or community program, state the reason AND plans to achieve public reporting, potential reporting programs or commitments, and timeline, e.g., within 3 years of endorsement: [For Maintenance – If not publicly reported, describe progress made toward achieving disclosure of performance results to the public at large and expected date for public reporting; provide rationale why continued endorsement should be considered.] For examples please see: Kaiser: See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 39 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 http://www.fiercehealthfinance.com/story/kaiser-sepsis-program-saves-36-million/2010-09-01 San Francisco Coalition: http://sfgate.com/cgi-bin/article.cgi?f=/c/a/2011/04/21/MNEM1J3QM1.DTL Catholic Healthcare West: http://www.caringisourcalling.org/case-study/catholic-healthcare-west-chw Sutter Healthcare: http://www.sutterhealth.org/quality/focus/quality_sepsis.html Kaiser sepsis program saves $36 million By Ron Shinkman Created Sep 1 2010 - 8:54am Introduced in late 2008 at 17 hospitals in Northern California, Kaiser´s sepsis prevention program is based on a six-step "bundle" of diagnostic and treatment tools. They include screening all emergency room patients who undergo blood testing for levels of lactate, which can be a sepsis indicator; providing a regimen of antibiotics through a central line catheter inserted through the patient´s clavicle; rigorous testing of a patient´s blood volume and arterial pressure; and performing a second lactate test within 12 hours of the first test. Sepsis: Bay Area hospitals sharply cut death rates Victoria Colliver, Chronicle Staff Writer Thursday, April 21, 2011 The nine Bay Area hospitals started with an average sepsis mortality rate of 27.7 percent of cases in the six months leading up to the start of the study in December 2008. By December 2010, the average across the hospitals had dropped to 16.6 percent, for a 40 percent difference in mortality. 3a.2.Provide a rationale for why the measure performance results are meaningful, understandable, and useful for public reporting. If usefulness was demonstrated (e.g., focus group, cognitive testing), describe the data, method, and results: Henry Ford Hospital, Society for Critical Care Medicine, Infectious Diseases Society of America, and the Institute for Healthcare Improvement believe that the use of the severe sepsis and septic shock early management bundle in continuous quality improvement initiatives is a beneficial way to gather scientific data with which to improve facility performance. This is appropriate since the measure has been tested and found to be reliable and valid. NQF endorsement will facilitate our ongoing progress toward this quality improvement objective. 3.2 Use for other Accountability Functions (payment, certification, accreditation). If used in a public accountability program, provide name of program(s), locations, Web page URL(s): Memorial Hospital in Jacksonville and other hospitals also receive a certification decision regarding a Sepsis Certified Program from The Joint Commission in their Certification of Quality Report. 3b. Usefulness for Quality Improvement: H M L I (The measure is meaningful, understandable and useful for quality improvement.) 3b.1. Use in QI. If used in quality improvement program, provide name of program(s), locations, Web page URL(s): [For Maintenance – If not used for QI, indicate the reasons and describe progress toward using performance results for improvement]. All Henry Ford Hospital (HFH) measures are suitable for use in quality improvment initiatives and this bundle is made freely available to the public. HFH, SCCM, IDSA, IHI and others strongly encourage the use of the early management bundle in CQI intiativies and seeks to provide information on such initiative to all hospitals. As endorsed by the Instititute for Healthcare Improvement, these bundles provide an evidence-based method for improvement in care, reduction in cost and reduction in mortality. Surviving Sepsis Campaign: http://www.survivingsepsis.org/Pages/default.aspx See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 40 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Intermountain Healthcare: http://www.ksl.com/?nid=148&sid=11060074 Rhode Island Critical Care Collaborative: http://www.pbn.com/Project-reduces-sepsis-deaths,46445?print=1 Catholic Health West: http://www.idse.net/ViewArticle.aspx?d=News&d_id=217&i=January+2011&i_id=692&a_id=16436 Kaiser Permanente: http://www.idse.net/ViewArticle.aspx?d=News&d_id=217&i=January+2011&i_id=692&a_id=16436 Institute for Healthcare Improvement: http://www.ihi.org/knowledge/Pages/ImprovementStories/SepsisCareEntersNewEra.aspx Greater New York Hospital Association STOP sepsis initiative: http://www.uhfnyc.org/assets/886 Ohio Patient Safety Institute/Mount Carmel Health System: http://www.ohiopatientsafety.org/library/Success%20Story%20PDFs/Sepsis%20Collaborative.pdf 3b.2. Provide rationale for why the measure performance results are meaningful, understandable, and useful for quality improvement. If usefulness was demonstrated (e.g., QI initiative), describe the data, method and results: Henry Ford Hospital, Society for Critical Care Medicine, Infectious Diseases Society of America, and the Insititue for Healthcare Improvement believe that the use of the severe sepsis and septic shock early management bundle in continuous quality improvement initiatives is a beneficial way to gather scientific data with which to improve facility performance. This is appropriate since the measure has been tested and found to be reliable and valid. NQF endorsement will facilitate our ongoing progress toward this quality improvement objective. 3d. Decomposition of Composite 3d.1 Describe the information that is available from decomposing the composite into its components See Supplemental Information attached under "Scientific Acceptability" entitled: NQF 0500 Forest plots and tables. See also section 2a2.3 "Reliability Testing Results" for a summary of the reliability of the component measures making up the composite. Finally, the component results as regards compliance and mortality have been individually described in "Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 201 0;38:367-74." Some component level details on compliance related to usability from that publication´s Table 3 are reproduced below for consideration: .............................Initial....Final......p value.....Remaining..p value. .............................Quarter....Quarter....Compared....Quarters...Compared .............................%Achieved..%Achieved..w/.Initial..%Acheived Measure lactate.........61.0.......78.7.......<=.0001.....72.5.......<=.0001. Blood cultures .....before antibiotics...64.5.......78.3.......<=.0001.....76.3.......<=.0001. Broad-spectrum abx......60.4.......67.9..........0002.....67.0.......<=.0001. Fluids and vasopressors.59.8.......77.0.......<=.0001.....71.1.......<=.0001. CVP >8 mm Hg............26.3.......38.0.......<=.0001.....33.9.......<=.0001. ScvO2 >70%..............13.3.......24.3.......<=.0001.....21.7.......<=.0001. All..........................10.9.......21.5.......<=.0001.....21.1.......<=.0001. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 41 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 3e. Achieved Stated Purpose 3e.1 Describe how the scores from testing or use reported in 2f demonstrate that the composite achieves the stated purpose Overall, to what extent was the criterion, Usability, met? H Provide rationale based on specific subcriteria: M L I 4. FEASIBILITY Extent to which the required data are readily available, retrievable without undue burden, and can be implemented for performance measurement. (evaluation criteria) 4a. Data Generated as a Byproduct of Care Processes: H M L I 4a.1-2 How are the data elements needed to compute measure scores generated? (Check all that apply). Data used in the measure are: generated by and used by healthcare personnel during the provision of care, e.g., blood pressure, lab value, medical condition, Coded by someone other than person obtaining original information (e.g., DRG, ICD-9 codes on claims), Abstracted from a record by someone other than person obtaining original information (e.g., chart abstraction for quality measure or registry) 4b. Electronic Sources: H M L I 4b.1 Are the data elements needed for the measure as specified available electronically (Elements that are needed to compute measure scores are in defined, computer-readable fields): Some data elements are in electronic sources 4b.2 If ALL data elements are not from electronic sources, specify a credible, near-term path to electronic capture, OR provide a rationale for using other than electronic sources: 4c. Susceptibility to Inaccuracies, Errors, or Unintended Consequences: H M L I 4c.1 Identify susceptibility to inaccuracies, errors, or unintended consequences of the measurement identified during testing and/or operational use and strategies to prevent, minimize, or detect. If audited, provide results: We are not aware of any unintended consequences related to this measurement. 4d. Data Collection Strategy/Implementation: H M L I A.2 Please check if either of the following apply (regarding proprietary measures): Proprietary measure 4d.1 Describe what you have learned/modified as a result of testing and/or operational use of the measure regarding data collection, availability of data, missing data, timing and frequency of data collection, sampling, patient confidentiality, time and cost of data collection, other feasibility/implementation issues (e.g., fees for use of proprietary measures): This measure was found to be reliable and feasible for implementation. Overall, to what extent was the criterion, Feasibility, met? H Provide rationale based on specific subcriteria: M L I OVERALL SUITABILITY FOR ENDORSEMENT Does the measure meet all the NQF criteria for endorsement? Yes Rationale: No If the Committee votes No, STOP. If the Committee votes Yes, the final recommendation is contingent on comparison to related and competing measures. See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 42 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 5. COMPARISON TO RELATED AND COMPETING MEASURES If a measure meets the above criteria and there are endorsed or new related measures (either the same measure focus or the same target population) or competing measures (both the same measure focus and the same target population), the measures are compared to address harmonization and/or selection of the best measure before a final recommendation is made. 5.1 If there are related measures (either same measure focus or target population) or competing measures (both the same measure focus and same target population), list the NQF # and title of all related and/or competing measures: 5a. Harmonization 5a.1 If this measure has EITHER the same measure focus OR the same target population as NQF-endorsed measure(s): Are the measure specifications completely harmonized? 5a.2 If the measure specifications are not completely harmonized, identify the differences, rationale, and impact on interpretability and data collection burden: 5b. Competing Measure(s) 5b.1 If this measure has both the same measure focus and the same target population as NQF-endorsed measure(s): Describe why this measure is superior to competing measures (e.g., a more valid or efficient way to measure quality); OR provide a rationale for the additive value of endorsing an additional measure. (Provide analyses when possible): CONTACT INFORMATION Co.1 Measure Steward (Intellectual Property Owner): Henry Ford Hospital, 2799 W. Grand Boulevard, 270 Clara Ford Pavillion, Detroit, Michigan, 48202 Co.2 Point of Contact: Emanuel, Rivers, MD, MPH, erivers1@hfhs.org, 313-916-1801Co.3 Measure Developer if different from Measure Steward: Henry Ford Hospital, 2799 W. Grand Boulevard, 270 Clara Ford Pavillion, Detroit, Michigan, 48202 Co.4 Point of Contact: Emanuel, Rivers, MD, MPH, erivers1@hfhs.org, 313-916-1801Co.5 Submitter: Emanuel, Rivers, MD, MPH, erivers1@hfhs.org, 313-916-1801-, Henry Ford Hospital Co.6 Additional organizations that sponsored/participated in measure development: Henry Ford Hospital System(HFHS) California Pacific Medical Center/Sutter Health (CPMC) Society of Critical Care Medicine (SCCM) Infectious Diseases Society of America (IDSA) Institute for Healthcare Improvement (IHI) Surviving Sepsis Campaign (SSC) Ohio State University (OSU) Co.7 Public Contact: Emanuel, Rivers, MD, MPH, erivers1@hfhs.org, 313-916-1801-, Henry Ford Hospital ADDITIONAL INFORMATION Workgroup/Expert Panel involved in measure development Ad.1 Provide a list of sponsoring organizations and workgroup/panel members’ names and organizations. Describe the members’ role in measure development. 1. Emmanuel Rivers, MD, MPH, FACEP, Emergency Medicine and Surgical Critical Care, Henry Ford Hospital, Institute of See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 43 NQF #0500 Severe Sepsis and Septic Shock: Management Bundle, Last Updated Date: Oct 05, 2012 Medicine Fellow: measure developer, measure steward, review of current evidence, validty, reliability, usability, feasibilty, and update of measure 2. William Conway, MD, Chief Medical Officer, Henry Ford Hospital, measure steward, review of current evidence, validty, reliability, usability, feasibilty, and update of measure 3. Mitchell M Levy, MD, FCCM, Rhode Island Hospital, Society of Critical Care Medicine (SCCM) for the Surviving Sepsis Campaign (SSC): review of current evidence, validty, reliability, usability, feasibilty, and update of measure 4. R. Phillip Dellinger, MD, FCCM, Cooper University Medical Center, Society of Critcal Care Medicine (SCCM) for the Surviving Sepsis Campaign (SSC): reivew of current evidence and update of measure 5. Sean R. Townsend, MD, Institute for Healthcare Improvement (IHI), California Pacific Medical Center, San Fransisco: review of current evidence, validty, reliability, usability, feasibilty, and update of measure 6. Lawrence Martinelli, MD, Coventry Health Systems, Chair Quality Improvement Task Force, Infectious Diseases Society of America (IDSA): review of current evidence, validty, reliability, usability, feasibilty, and update of measure 7. Gary Phillips, MAS, Biostatician, Ohio State University Center for Biostatitics, for the Surviving Sepsis Campaign (SSC): statistical support for evaluation of current evidence, validity, and reliability. Ad.2 If adapted, provide title of original measure, NQF # if endorsed, and measure steward. Briefly describe the reasons for adapting the original measure and any work with the original measure steward: Measure Developer/Steward Updates and Ongoing Maintenance Ad.3 Year the measure was first released: 2008 Ad.4 Month and Year of most recent revision: 06, 2012 Ad.5 What is your frequency for review/update of this measure? Annually for minor changes, every three years detailed review of evidence and test results. Ad.6 When is the next scheduled review/update for this measure? 06, 2013 Ad.7 Copyright statement: Performance measures and related data specifications developed by the Henry Ford Hospital in collaboration with representatives from emergency medicine, critical care medicine (SCCM), and infectious diseases (IDSA). Ad.8 Disclaimers: These performance measures are not clinical guidelines and do not establish a standard of medical care, and have not been tested for all potential applications. Neither the Henry Ford Hospital nor its affiliates or ageents shall be responsible for any use of the measures. Ad.9 Additional Information/Comments: Date of Submission (MM/DD/YY): 06/07/2012 See Guidance for Definitions of Rating Scale: H=High; M=Moderate; L=Low; I=Insufficient; NA=Not Applicable Created on: 10/08/2012 at 09:56 AM 44 EXHIBIT B SAMPLE DATA COLLECTION FORM, IF APPLICABLE Evaluation for Severe Sepsis Screening Tool Instructions: Use this optional tool to screen patients for severe sepsis in the emergency department, on the wards, or in the ICU. 1. Is the patient’s history suggestive of a new infection? Pneumonia, empyema Urinary tract infection Acute abdominal infection Meningitis Skin/soft tissue infection Bone/joint infection Wound infection Bloodstream catheter infection Endocarditis Implantable device infection Other ____________ ___ Yes ___No 2. Are any two of following signs & symptoms of infection both present and new to the patient? Note: laboratory values may have been obtained for inpatients but may not be available for outpatients. Hyperthermia > 38.3 °C (101.0 °F) Hypothermia < 36 °C (96.8°F) Tachycardia > 90 bpm Tachypnea > 20 bpm Acutely altered mental status Leukocytosis (WBC count >12,000 μL–1) Leukopenia (WBC count < 4000 μL–1) Hyperglycemia (plasma glucose >120 mg/dL) in the absence of diabetes ___ Yes ___No If the answer is yes to both either question 1 and 2, suspicion of infection is present: 9 Obtain: lactic acid, blood cultures, CBC with differential, basic chemistry labs, bilirubin. 9 At the physician’s discretion obtain: UA, chest x-ray, amylase, lipase, ABG, CRP, CT scan. 3. Are any of the following organ dysfunction criteria present at a site remote from the site of the infection that are not considered to be chronic conditions? Note: the remote site stipulation is waived in the case of bilateral pulmonary infiltrates. SBP < 90 mmHg or MAP < 65 mmHg SBP decrease > 40 mm Hg from baseline Bilateral pulmonary infiltrates with a new (or increased) oxygen requirement to maintain SpO2 > 90% Bilateral pulmonary infiltrates with PaO2/FiO2 ratio < 300 Creatinine > 2.0 mg/dl (176.8 mmol/L) or Urine Output < 0.5 ml/kg/hour for > 2 hours Bilirubin > 2 mg/dl (34.2 mmol/L) Platelet count < 100,000 Coagulopathy (INR >1.5 or aPTT >60 secs) Lactate > 2 mmol/L (18.0 mg/dl) ___ Yes ___No If suspicion of infection is present AND organ dysfunction is present, the patient meets the criteria for SEVERE SEPSIS and should be entered into the severe sepsis protocol. Adapted from the ©2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement For questions or concerns please contact the critical care fellow on call. Place patient label here. Cooper University Hospital Signature____________________________________________ Date:____/____/___ Time of recognition:____:____(24 hr clock) Individual Chart Measurement Tool: Instructions: Attach this tool to each chart of a patient with severe sepsis, septic shock at the time of data abstraction. This tool can be used for concurrent, prospective, or retrospective data collection. However, individual hospitals are strongly encouraged to choose a single approach and maintain that collection over time. Once all Individual Chart Measurement Tools are gathered for a single month, complete the Monthly Measurement Worksheet to report results. ***Important: mark the date format you will be following: ____ (dd/mm/yy) ____ (mm/dd/yy) 1. Document whether the patient met criteria for severe sepsis or septic shock. Check only one answer. Because strict definitions apply it may be helpful to consult the Sepsis Definitions Tool or the Evaluation for Severe Sepsis Screening Tool to ensure accuracy. _____ No, does not meet criteria for either severe sepsis or septic shock. Stop data collection. _____ Yes, met criteria for severe sepsis. Continue data collection. _____ Yes, met criteria for septic shock. Continue data collection. 2. Record the patient identifier number __________ 3. Question 3 establishes a uniform “time of presentation” for each patient depending upon their individual admission characteristics. The time of presentation will be the basis for answering subsequent questions and making calculations. Only one statement below (3a, 3b, or 3c) will apply to a single patient. Note: A protocol, protocol form and protocol order set are recommended to facilitate the treatment process and the accurate recording of timelines. 3a. For patients admitted to the ICU from the ED meeting criteria for severe sepsis or septic shock, record the time of triage in the emergency department as the time of presentation. _____ Not applicable. Proceed to 3b. _____ Applicable, record time of presentation below and proceed to question 4. 3b. For patients transferred to the ICU from units other than the ED: • • Preferred: if the resuscitation and management of severe sepsis was annotated as beginning on the transferring unit, record the time and date of that annotation as the time of presentation. Default: if the resuscitation and management of severe sepsis was not in annotated as beginning on the transferring unit, record the ICU admission date and time as the time of presentation. Note: it is critical to establish whether there was reasonable and straightforward annotation of the time of initiation of efforts to manage severe sepsis on the ward prior to ICU transfer. Otherwise, no credit can be assigned for key interventions performed prior to the default time of presentation, the time of ICU admission. Annotation may include a practitioner’s note, a practitioner’s timed and dated orders, a nurse’s timed and dated records documenting discussion of severe sepsis with a practitioner, timed records initiating referral to the ICU for severe sepsis. Individual Chart Measurement Tool, page 1 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement _____ Not applicable. Proceed to question 3c. _____ Applicable; the annotated time and date for the resuscitation and management of sepsis on the transferring unit is recorded below as the time of presentation. Proceed to question 4. _____ Applicable; the ICU admission date and time is recorded below as the time of presentation. Proceed to question 4. 3c. For patients admitted to the ICU with a diagnosis other than sepsis and who subsequently develop severe sepsis or septic shock on the same ICU stay, record the annotated time and date of the beginning of the resuscitation and management of severe sepsis as the time of presentation. _____ Not applicable. Stop data collection, time of presentation cannot be accurately determined. If data is being collected concurrently or prospectively, the patient may remain on the sepsis protocol without further data collection. _____ Applicable, record time of presentation below and proceed to question 4. ***Time of Presentation: __ __ /__ __/ __ __ (date format as above) __ __ : __ __ (24 hour clock).*** 4. Document whether serum lactate was obtained: _____ No. Proceed to question 5. _____ Yes. Place a mark in Box 1 on line 16 of this document. Proceed to question 4a. 4a. Record the value serum lactate value if obtained: ______ mmol/L or ____ mg/dl 4b. Record date and time of serum lactate collection: __ __ /__ __/ __ __ (date format as above) __ __:__ __ (24 hour clock). 5. Document whether the patient received a broad-spectrum antibiotic: ____ No. Proceed to question 7. ____ Yes. Proceed to question 5a. 5a. Name of Antibiotic(s): __________________ 5b. Date and time of first broad-spectrum antibiotic administration: __ __ /__ __/ __ __ (date format as above) __ __ : __ __ (24 hour clock). 5c. Calculate the difference between line 3, time of presentation above, and line 5b in hours and minutes: Difference: __ __ hours __ __ minutes 5d. Multiply the HOURS ONLY on line 5c above x 60 ____ 5e. Time in minutes to broad spectrum antibiotic administration for this patient: add the total of line 5d above to the number of MINUTES ONLY listed on line 5c above: _______ 5f. If item 3a above is marked applicable, was the number of minutes on line 5e above < 180 minutes: ____ No. Proceed to question 6. ____ Yes. Place a mark in Box 3 on line 16 of this document. Proceed to question 6. 5g. If item 3b or 3c is marked applicable, was the number of minutes on line 5e above < 60 minutes: ____ No. Proceed to question 6. ____ Yes. Place a mark in Box 3 on line 16 of this document. Proceed to question 6. Individual Chart Measurement Tool, page 2 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement 6. Document date and time of blood culture collection. ____ If not collected, enter “No” on 6a and proceed to question 7. __ __ /__ __/ __ __ (date format as above) __ __:__ __ (24 hour clock). Proceed to question 6a. 6a. Document whether the time and date listed on 6 above is earlier than the time and date listed on line 5b above: _____ No. Proceed to question 7. _____Yes. Place a mark in Box 2 on line 16 of this document. Proceed to question 7. 7. Answer the following questions regarding resuscitation of severe sepsis or septic shock: 7a. Document whether the patient was hypotensive and/or if serum lactate was > 4 mmol/L (36 mg/dl) on line 4a of this document: ____ No. Place a mark in Box 4, 5, 6, 7 on line 16 of this document. Place a mark in Box A on line 17 of this document. Proceed to question 11. ____ Yes. Proceed to question 7b. 7b. Document the basis for the diagnosis of hypotension, if present: ____ SBP < 90 mm Hg ____ MAP < 65 mm Hg Note: MAP = (2 x diastolic pressure + systolic pressure) / 3 ____ SBP decrease of > 40 mm Hg from known baseline 7c. Document whether initially the patient received > 20 ml/kg of crystalloid or > an equivalent amount of colloid in response to hypotension or lactate > 4 mmol/L (36 mg/dl): Cyrstalloid/Colloid Equivalency Chart:1 Normal Saline 20 ml/kg Lactated Ringer’s Solution 20 ml/kg Albumin 4-5% Albumin 20-25% Albumin 0.24 grams/kg 5.2 ml/kg 1.1 ml/kg Hetastarch 3% Hetastarch 6% Hetastarch 10% Hetastarch 0.29 grams/kg 9.7 ml/kg 4.8 ml/kg 2.9 ml/kg Pentastarch 10% Pentastarch 0.30 grams/kg 3 ml/kg 10% Dextran-40 0.30 grams/kg (3ml/kg) 3% Dextran-60, 6% Dextran-70 3% Dextran-60 6% Dextran-70 0.19 grams/kg 6.3 ml/kg 3.1 ml/kg Gelatins (succinylated & crosslinked 2.5, 3.0, 4.0%; urea-linked 3.5%) 0.23 grams/kg 1 Adapted from: Evidence-based Colloid Use in the Critically Ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med. 2004. Vol 170:1247-1259. For percentage solutions, listed ml/kg are calculated from the g/kg data. ____ No. Record “No” on lines 7f, 8b, 9b and 10 below. Proceed to question 11. Individual Chart Measurement Tool, page 3 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement ____ Yes. Place a mark in Box 4 on line 16 of this document. Proceed to question 7d. 7d. Document whether MAP remained > 65 in response to the initial fluid resuscitation described in 7c: i. ____ No. Proceed to question 7e. ii. ____ Yes, if lactate was < 4 mmol/L (36 mg/dl) on line 4a of this document place a mark in Box 5, Box 6 and Box 7 on line 16 of this document. Proceed to question 10. iii. ____ Yes, if lactate was > 4 mmol/L (36 mg/dl) on line 4a of this document, proceed to question 8. 7e. Document whether the patient received vasopressors: ____ No. Record “No” on lines 7f, 8b, 9b and 10 below. Proceed to question 11. ____ Yes. Place a mark in Box 5 on line 16 of this document. Proceed to question 7f. 7f. Document whether the MAP remained > 65 mm Hg without the use of vasopressors: Note: If no evidence for removal of vasopressors can be found, mark item 7f “no” and proceed to question 8. i. ____ No. Proceed to question 8. ii. ____ Yes, if lactate was < 4 mmol/L (36 mg/dl) on line 4a of this document place a mark in Box 6 and Box 7 on line 16 of this document. Proceed to question 10. iii. ____ Yes, if lactate was > 4 mmol/L (36 mg/dl) on line 4a of this document, proceed to question 8. 8. Document date and time CVP first > 8 mm Hg within 24 hours: ____ CVP not obtained or never > 8 mm Hg within 24 hours. Record line 8b as “No” and proceed to question 9. Date: __ __ /__ __/ __ __ (date format as above) Time: __ __ : __ __ (24 hour clock). Proceed to question 8a. 8a. Calculate the difference between line 3, time of presentation, and line 8 above in hours and minutes: Difference: __ __ : __ __ (hours:minutes). 8b. Document whether line 8a is < 6 hours. ____ No. Proceed to question 9. ____ Yes. Place a mark in Box 6 on line 16 of this document. Proceed to question 9. 9. Document date and time ScvO2 first > 70% (or SvO2 > 65%) within 24 hours: ____ScvO2 not obtained or never > 70% (or SvO2 > 65%) within 24 hours. Record line 9b as “No” and proceed to question 10. Date: __ __ /__ __/ __ __ (date format as above) Time: __ __ : __ __ (24 hour clock). Proceed to question 9a. 9a. Calculate the difference between line 3, time of presentation, and line 9 above in hours and minutes: Difference: __ __ : __ __ (hours:minutes). 9b. Document whether line 9a is < 6 hours. ____ No. Proceed to question 10. Individual Chart Measurement Tool, page 4 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement ____ Yes. Place a mark in Box 7 on line 16 of this document. Proceed to question 10. 10. Answer the following questions regarding low-dose steroids administration: 10a. Document whether line 7d or line 7f above has been answered affirmatively: ____ Yes. The bundle element is not applicable because the patient’s MAP was > 65 and did not have persistent arterial hypotension. Place a mark in Box A on line 17 and proceed to question 11. ____ No. Proceed to question 10b. 10b. Document whether there is a standardized ICU policy regarding low-dose steroid administration for septic shock: ____ No. Proceed to question 11. ____ Yes. Proceed to question 10c. 10c. Indicate whether there is documentation that the patient did not merit low-dose steroids based upon the standardized protocol: ____ No documentation is present. Proceed to question 10d. ____ Yes there is documentation present. Place a mark in Box A on line 17 below. Proceed to question 11. 10d. Document whether low-dose steroids were administered: Note: low-dose steroids refer to a daily dose of 200–300 mg of hydrocortisone or equivalent. Steroid Equivalency Chart:2 Steroid: Equivalent TOTAL DAILY dose: Hydrocortisone 200 – 300 mg Dexamethasone 8 – 12 mg Prednisone 50 – 75 mg Prednisolone 50 – 75 mg Methylprednisolone 40 – 60 mg Cortisone 250 – 375 mg Triamcinolone 40 – 60 mg Betamethasone 6 – 10 mg 2 Adapted from: Knoben JE, Anderson PO. Handbook of Clinical Drug Data, 6th ed. Drug Intelligence Pub, Inc. 1988. ____ No. Proceed to question 11. ____ Yes. Record date and time below. Proceed to question 10e. __ __ /__ __/ __ __(date format as above) __ __ : __ __ (24 hour clock) 10e. Time of presentation: from line 3 above: __ __ /__ __/ __ __(date format as above) __ __ : __ __ (24 hour clock) Individual Chart Measurement Tool, page 5 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement 10f. Document whether the time and date on 10d is < 24 hours from the time of presentation listed on item 10e. ____ No. Proceed to question 11. ____ Yes. Place a mark in Box A on line 17 and proceed to question 11. 11. Answer the following questions regarding Drotrecogin alfa (activated) administration: 11a. Document whether there is a standardized ICU policy regarding Drotrecogin alfa (activated) administration: ____ No. Proceed to question 12. ____ Yes. Proceed to question 11b. 11b. Indicate whether there is documentation that the patient did not merit Drotrecogin alfa (activated) administration based upon the standardized protocol: ____ No documentation is present. Proceed to question 11c. ____ Yes there is documentation present. Place a mark in Box B on line 17 below. Proceed to question 12. 11c. Document whether Drotrecogin alfa (activated) was administered: ____ No. Proceed to question 12. ____ Yes. Record date and time below. Proceed to question 11d. __ __ /__ __/ __ __(date format as above) __ __ : __ __ (24 hour clock) 11d. Time of presentation: from line 3 above: __ __ /__ __/ __ __(date format as above) __ __ : __ __ (24 hour clock) 11e. Document whether the time and date on 11c is < 24 hours from the time of presentation listed on item 11d. ____ No. Proceed to question 12. ____ Yes. Place a mark in Box B on line 17 and proceed to question 12. 12. Document the median glucose* value within 24 hours of the time of presentation: Median glucose: _____ mg/dl or ____ mmol/L If and only if median glucose is < 150 mg/dl (8.3 mmol/L) place a mark in Box C on line 17 of this document. Proceed to question 12a. 12a. Document the lower limit of normal for serum glucose at your institution: ____ 12b. Document the total number of measurements that fell below the lower limit of normal within 24 hours from the time of presentation for this patient: ____ * Refer to the optional Median Glucose Tool, if necessary. 13. Document the median inspiratory plateau pressure (IPP)* achieved within 24 hours of time of presentation: ____ Not applicable because the patient was not mechanically ventilated. Place a mark in Box D on line 17 of this document. Proceed to question 14. Median IPP: _____ If and only if < 30 cm H20, place a mark in Box D on line 17 of this document. Individual Chart Measurement Tool, page 6 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement * Refer to the optional Median IPP Calculation Tool, if necessary. 14. Date and time of hospital discharge: __ __ /__ __/ __ __(date format as above) __ __ : __ __ (24 hour clock) 15. Status at hospital discharge: ____ Alive ___ Deceased 16. Boxes 1 through 7: Box 1 Box 2 Box 3 Box 4 Box C Box D Box 5 Box 6 Box 7 17. Boxes A through D: Box A Box B Individual Chart Measurement Tool, page 7 of 7. © 2005 Surviving Sepsis Campaign and the Institute for Healthcare Improvement Description of the proportion of the sites that met the resuscitation bundle (1) October 5, 2012 Table 1: Descriptive summary of proportion where the resuscitation bundle is yes by site quarters of participation Quarters of Number mean sd min p25 p50 p75 max participation of sites 1 218 0.101 0.180 0.000 0.000 0.000 0.125 1.000 8 88 0.205 0.197 0.000 0.000 0.183 0.354 0.667 16 8 0.158 0.102 0.000 0.096 0.136 0.255 0.295 • • • • • Site quarters are based on a quarter of participation based on when a site entered the SSC program Site quarters do not align with calendar quarters There were 218 sites that started the program After 2 years (8 quarters) there were 88 sites still participating After 4 years (16 quarters) there were 8 sites still participating Table 2: Descriptive summary of proportion where the resuscitation bundle is yes by site quarters of participation Number Description mean sd min p25 p50 p75 max Delta of sites Proportion 0.108 0.176 0.000 0.000 0.000 0.118 0.595 Decreased 21 Delta 0.247 0.246 0.021 0.059 0.200 0.334 1.000 Increased • • • 67 Proportion Delta 0.235 0.175 0.196 0.164 0.000 0.000 0.000 0.000 0.213 0.158 0.385 0.326 0.667 0.600 Delta is whether or not a site decreased or increased 21 sites (24%) decreased from the 1st quarter while 67 sites (76%) increased Description: Proportion is those that met the resuscitation bundle and delta is the size of either the increase or decrease Description of the 8 sites with observations during site quarter 16 • All 8 improved from the 1st quarter to the 8th quarter the mean proportion was 0.153 and the mean change from 1st to 8th quarter was 0.136 • Then from the 8th to the 16th quarter 3 sites decreased a mean proportion of 0.131 and 5 sites increased with a mean proportion of 0.087 Page 1 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 NQF Component Item /Measure Analysis to Justify Inclusion in Composite (2i.1-3, 2j.1-3) Source: Castellanos-Ortega4 Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality: Source: Nguyen1 Page 1 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 Source: Jeon2 Source: Jeon3 Page 2 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 Surviving Sepsis Targets: Source: Levy5 Meta-analysis of Eight Trials: Source: Barochia6 Page 3 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 Meta-analysis of Eight Trials (continued): Source: Barochia6 Bundle Elements and Survival: Source: Chamberlain7 Page 4 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 Central Venous Oxygen Saturation and Survival: Source: Chamberlain7 Bundle Interventions: Therapeutic Interventions Variable in Data Base Odds Ratio 95% Confidence Interval p-value 6 hour resuscitation bundle Serum lactate measured Lactate - 0 hours 2.18 (1.89-2.52) <0.001 Blood cultures before antibiotics Blood culture in < 3 hours 1.01 (0.84-1.21) 0.92 Early treatment with antibiotics Antibiotics in < 3 hours 1.00 (0.85-1.18) 0.96 Intravenous fluids delivered Fluid Challenge in < 3 hours 1.26 (0.96-1.66) 0.09 Mean arterial pressure ≥ 65 mm Hg achieved MAP ≥ 65 mmHg - 6 hours 0.59 (0.50-0.70) <0.001 Central venous pressure ≥ 8 mm Hg CVP ≥ 8 - 6 hours 1.17 (0.88-1.57) 0.28 0.75 (0.56-0.99) 0.047 Central venous oxygen saturation ≥ 70% achieved ScvO2 ≥ 70% - 6 hours Source: Cannon8 Page 5 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 Early vs. Late Therapy Source: Jones10 Early vs. Late Therapy Source: Ferrer9 Page 6 of 7 NQF Infectious Disease Project Measure #0500: Severe Sepsis and Septic Shock Early Management Bundle 07.02.2012 References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. Apr 2007;35(4):1105-1112. Jeon K, Shin TG, Sim MS, et al. Improvements in Compliance of Resuscitation Bundles and Achievement of End Points After an Educational Program on the Management of Severe Sepsis and Septic Shock. Shock. Jan 31 2012. Jeong SJ, Song YG, Kim CO, et al. Measurement of Plasma sTREM-1 in Patients with Severe Sepsis Receiving Early Goal-Directed Therapy and Evaluation of Its Usefulness. Shock. Mar 5 2012. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Impact of the surviving sepsis campaign protocols on hospital length of stay and mortality in septic shock patients: Results of a 3-year follow-up quasi-experimental study. Crit Care Med. Feb 11 2010. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine. Feb 2010;38(2):367-374. Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. Feb 2010;38(2):668-678. Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: A meta-analysis. Aust Crit Care. Feb 14 2011. Cannon CM, for the Multicenter Severe S, Septic Shock Collaborative G. The GENESIS Project (GENeralization of Early Sepsis InterventionS): A Multicenter Quality Improvement Collaborative. Acad Emerg Med. 2010;17(11):1258. Ferrer R, Artigas A. Effectiveness of treatments for severe sepsis: data from the bundle implementation programs. Minerva Anestesiol. Mar 2011;77(3):360-365. Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, Kline JA; Emergency Medicine Shock Research Network investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008 Oct;36(10):2734-9. Page 7 of 7 April 4, 2013 NQF Board of Directors National Quality Forum 1030 15th Street, NW Suite 800 Washington, D.C. 20005 To the Steering Committee: The American College of Chest Physicians (ACCP), American Association of Critical-­‐Care Nurses (AACN), Greater New York Hospital Association (GNYHA), Midwest Critical Care Collaborative (MWCCC), National Association for Medical Direction of Respiratory Care (NAMDRC), Society for Academic Emergency Medicine (SAEM), and Society of Hospital Medicine (SHM) would like to appeal the National Quality Forum’s (NQF) recent decision to endorse the maintenance of Severe Sepsis and Septic Shock: Management Bundle. The organizations listed represent pulmonary, critical care, hospital medicine, and emergency medicine clinicians from across the United States. The ACCP would specifically like to apologize for not commenting earlier on this composite measure; however we believe that the Severe Sepsis and Septic Shock measure, as specified, creates concern about scientific acceptability, usability and feasibility. We strongly support the development and implementation of performance measures targeting severe sepsis and septic shock. In fact, as part of an NQF request to identify gaps in performance measures in critical care, the ACCP worked with its partner professional societies to propose specific performance measures in the management of severe sepsis and septic shock (see enclosure). Furthermore, we suggested performance measures very similar to the first four components of the proposed bundle (measurement of serum lactate, blood cultures, administration of broad spectrum antibiotics and administration of intravenous crystalloids for volume resuscitation). Indeed, we continue to support these target elements for performance improvement. Moreover, we would further suggest emphasizing the rapid administration of appropriate antibiotics as suggested by the recent Surviving Sepsis Guidelines1, ideally within 1 hour of recognition of septic shock (1B recommendation) or severe sepsis (1C recommendation)2-­‐4. Including time to appropriate antibiotics would be a reasonable addition to the proposed measures. On the other hand, we are concerned that the specification within the Severe Sepsis and Septic Shock bundle that resuscitation must be guided by a central venous catheter would have unintended deleterious effects, even while recognizing that this element is included in the current Surviving Sepsis Guidelines. Central venous catheter guided fluid management has not been shown to be superior (or even equivalent) to resuscitation guided by other measures, including other invasive or non-­‐invasive assessments of volume status and perfusion. Despite the fact that the measure developer responded that “when the central venous pressure component is utilized as part of the bundle, there is a decrease in mortality”, identical CVP targets were used in both arms of that clinical trial5. Additionally, the current measure does not target a specific value for CVP or central venous oxygen saturation– only that it be measured. We suggest that the available data does not support the scientific acceptability criteria for the measure to be specified as it is. In the analysis of the Surviving Sepsis Campaign efforts, neither the CVP nor the CVO2 target was independently associated with improved survival6. Along similar lines, the Greater New York Hospital Association (GNYHA)/United Hospital Fund (UHF) adopted recommendations along the same lines that the ACCP is proposing. The GNYHA/UHF was not prescriptive in the resuscitation strategy employed. This 55 hospital collaborative found that the use of a noninvasive strategy (without central venous catheterization) became more common over time even while mortality continued to improve. This experience provides a basis for the New York state regulations regarding sepsis care as introduced by Governor Cuomo. We point to other illustrations of continued equipoise surrounding this issue based upon the conduct of the ARISE study (Clinical Trials #NCT00975793) in Australia and the PROCESS study (Clinical Trials #NCT00510835) in the United States. These multi-­‐center studies are scheduled to complete in 2013 and should provide greater clarity regarding the need for invasive monitoring. Therefore, we respectfully suggest that central venous catheter guided fluid resuscitation not be specified, or that it be replaced with wording such as, “the resuscitation is objectively monitored using a method such as lactate clearance, ScvO2 monitoring or CVP monitoring”. Beyond the mandated use of central venous catheter guidance for volume resuscitation, the ACCP has additional concerns regarding the usability of the measure as written. For example, the specifications allow for identification of patients retrospectively via ICD-­‐9 codes included in the discharge record. The measure developer suggests in his responses to the submitted comments (comment 6a of the response to the ACEP) that if ICD-­‐9 codes were used retrospectively, data abstractors “would determine if severe sepsis was present in the ED and if so, time zero would be defined as triage time.” It is not clear, however, that these measure only apply to severe sepsis and septic shock patients presenting in the Emergency Department as specified. If the measure includes patients who develop severe sepsis or septic shock as an inpatient subsequent to admission, as well as patients transferred to a facility already septic, use of ED triage time as “time zero” will likely result in inaccurate assessments of performance based upon the facility’s rates of nosocomial sepsis and hospital-­‐to-­‐hospital referral patterns. Furthermore, there is no mitigation for patients presenting with severe sepsis and septic shock at one Emergency Department and transferred to another. The receiving Emergency Department will be held accountable for care provided elsewhere currently. Beyond this issue of usability, retrospective identification of discharges with sepsis may create an unreasonable burden of data collection. As the Steering Committee is aware, ICD-­‐9 codes may be included in the discharge record even when the diagnosis is ultimately not present. The specified ICD-­‐9 codes include uncomplicated sepsis, not a population targeted for intervention by the measure. The measure developer describes approximately 750,000 admissions for severe sepsis and septic shock. A recent estimate from the CDC suggests over 1.6 million admissions involving sepsis per year and the measure developer described 2 millions ED visits per year for sepsis. This means that the data will have to be abstracted from more than twice as many charts as they are ultimately targets for intervention. We reiterate our desire to have well-­‐specified performance measures for use as tools to improve care for severe sepsis and septic shock patients. We suggest the following modifications to create more scientifically acceptable, usable and feasible set of measures. We also feel these changes will be more easily implemented allowing benefit for a greater number of patients. Such modifications would in no way prohibit facilities from exceeding or supplementing these practices. 1. The Severe Sepsis and Septic Shock: Management Bundle measures should be limited to patients presenting to the reporting Emergency Department. Those transferred from other hospitals and those developing sepsis after admission should be excluded. This mirrors the performance measures developed around community-­‐acquired pneumonia. 2. For patients with severe sepsis, the bundle elements should be simplified to include: a. Blood cultures before antibiotics (with an exception for culturing resulting in delays in antibiotics administration, as consistent with current guidelines1) b. Measurement of serum lactate level c. Administration of broad spectrum antibiotics d. We would also define “time zero” as the time when blood is first drawn in the ED for patients with severe sepsis. Since severe sepsis is most often defined by laboratory abnormalities, this would tie the time-­‐based metrics to the timing of an assessment for such organ failures. 3. For patients with septic shock, we suggest the severe sepsis bundle plus the following: a. Administer 30 ml/kg crystalloid intravenous fluids b. Median time to antibiotics c. We suggest “time zero” as the time when blood was first drawn or when hypotension occurred, whichever comes earlier. 4. We suggest that CVP and central venous oxygen saturation and re-­‐measuring lactate either: a. Be removed from the current bundle or b. Be provided as examples, with other invasive and noninvasive modalities (e.g., bioimpedence, serial ultrasonographic assessments, response to fluid challenge, etc.) qualifying as acceptable alternative approaches when part of a protocol to guide resuscitation. We look forward to your response and hope to be able to endorse and promote revised measures. We again apologize for the late comments. Sincerely, Darcy Marciniuk, MD, FCCP President, American College of Chest Physicians Kathryn Roberts, MSN, RN, CNS, CCRN, CCNS President, American Association of Critical-­‐Care Nurses Kenneth Raske President, Greater New York Hospital Association Steven Simpson, MD Chair, Midwest Critical Care Collaborative Dennis Doherty, MD President, National Association for Medical Direction of Respiratory Care Cherri D. Hobgood, MD President, Society for Academic Emergency Medicine Shaun Frost, MD, SFHM, FACP President, Society of Hospital Medicine Reference List (1) Dellinger RP, Levy MM, Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med 2013; 41(2):580-­‐637. (2) Daniels R, Nutbeam T, McNamara G et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011; 28(6):507-­‐512. (3) Larosa JA, Ahmad N, Feinberg M et al. The use of an early alert system to improve compliance with sepsis bundles and to assess impact on mortality. Crit Care Res Pract 2012; 2012:980369. (4) Sebat F, Musthafa AA, Johnson D et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med 2007; 35(11):2568-­‐2575. (5) Rivers E, Nguyen B, Havstad S et al. Early goal-­‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345(19):1368-­‐1377. (6) Levy MM, Dellinger RP, Townsend SR et al. The Surviving Sepsis Campaign: results of an international guideline-­‐based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38(2):367-­‐374. April 22, 2013 NQF Board of Directors National Quality Forum 1030 15th Street NW Suite 800 Washington, DC 20005 Dear Directors: We are the developers of Infectious Diseases Measure #0500, Severe Sepsis and Septic Shock: Early Management Bundle, which was originally submitted in 2008 and endorsed by the National Quality Forum (NQF) on March 6, 2013 after repeated and rigorous examination. We appreciate the opportunity to reply to the issues brought forth on April 4, 2013 during the 30-day appeal period after this endorsement. Our letter is intended as a supplement to the letter provided by Dr. Carol Thompson, President of the Society of Critical Care Medicine (SCCM) and numerous health care organizations. The appellants have indicated they believe that measure #0500 “as specified, creates concern about scientific acceptability, usability and feasibility.” We will respond to these concerns in turn. Lastly, we will turn to the appellants’ suggestions to improve the measure. Concerns about scientific acceptability: 1. The appellants indicate that they would like to emphasize earl ier administration of antibiotics beyond the specifications of #0500. Specifically, to recognize the 1-hour standard recommended in the 2012 Surviving Sepsis Campaign (SSC) Guidelines, they suggest “including time to appropriate antibiotics would be a reasonable addition to the proposed measures.” We believe that this measure could be developed in due course and that the SSC data base which currently has nearly 40,000 patients and is growing, may be able to contribute performance data regarding this metric. However, #0500 is a composite measure and the testing done for reliability, validity, feasibility, and usability in the submission is not applicable to the appellants ’ proposed metric. It would be appropriate for the appellants to propose this metric at the next call for measures with supportive evidence. Creating a new measure, however, is not a reason to reject a well-tested and developed metric. 2. The appellants state they “are concerned that the specification within the Severe Sepsis and Septic Shock bundle that resuscitation must be guided by a central venous catheter (CVC) would have unintended deleterious effects, even while recognizing that this element is included in the current Surviving Sepsis Guidelines.” 1 CVC placement and central venous pressure (CVP) have been part of management of the critically ill patient for almost 50 years.(1, 2) It has been recommended by the American College of Critical Care Medicine and the SCCM for the management of severe sepsis and septic shock since 1999.(3) Recent studies have shown a significant association between the time to CVC placement and a decrease in organ failure (4) and mortality particularly following publication of the initial SSC guidelines.(4, 5) Further evidence indicates a mortality benefit with a minimum CVP target of 8 mm Hg and shows that a higher CVP target of 12 mm Hg may further lower mortality, particularly among those with an APACHE IV-predicted moderate risk of dying.(6) When used in an algorithmic context as recommended in the measure, there is a significant reduction in 30-day mortality.(7) Together, these findings reflect the balance of evidence that early CVP measurement is indicative of fluid responsiveness (8, 9) which is further enhanced by shock index.(10) We respectfully submit that the appellants fail to show any deleterious effects of CVC on patient outcomes. 3. The appellants state “Central venous catheter guided fluid management has not been shown to be superior (or even equivalent) to resuscitation guided by other measures, including other invasive or non-invasive assessments of volume status and perfusion.” We believe it would be inappropriate at th is stage of measure completion to engage in another extended scientific debate over the same issues that were rigorously examined and approved by the NQF Infectious Disease Steering Committee after 5 years of examination. First, we wish to point out that #0500 does not restrict any provider from using any method of volume assessment in addition to ascertaining the central venous pressure (CVP). In fact, we encourage alternate strategies in addition to CVP assessment. Second, the vast majority of cases of sepsis -induced hypoperfusion will necessitate CVC placement to safely deliver vasopressors and other intravenous therapies simultaneously. Thus, obtaining CVP from the existing line is no added burden or adverse risk. Third, the appellants provide no evidence of the effectiveness of any other particular invasive or non-invasive assessments of volume status. In contrast, the measure submission scientific evidence includes more than 50 citations favoring the use of a CVC in severe sepsis and shock (please refer to “Evidence [measure evaluation criterion 1C]”). Finally, we note that by simply stating that CVP is not superior to other modalities, the appellants are not demonstrating that it is inferior to other modalities.(11) The suggestion provided by the appellants of using lactate clearance as a substitute for central venous oxygen saturation (ScvO2) does not negate the use of CVC. Furthermore, lactate clearance may have no clinical utility in 25-50% of septic shock patients because lactate is not elevated.(12-16) Given these considerations, the essential question regarding use of CVP together with ScvO2 in #0500 is whether the tandem strategy provides an effective resuscitation that can decrease progression of organ failure and death. This tandem has undergone almost 50 years of scientific scrutiny.(1) Thus, measure #0500 and those familiar with the 2 science do not endorse the use of CVP as an isolated variable. The measure is a composite of essential elements of the cardiovascular system. Thus, the interpretation of CVP is inextricably tied to cardiac preload, after-load, contractility and vessel compliance. All of these variables ultimately affect systemic oxygen delivery and consumption. As any component of cardiovascular physiology, CVP is part of a closed system, not to be interpreted in isolation and represents a pressure-volume relationship. This measure represents an algorithmic approach to be used by clinicians in providing beneficial treatments for particular patients. 4. The appellants note, “Despite the fact that the measure developer responded that ‘when the central venous pressure component is utilized as part of the bundle, there is a decrease in mortality,’ identical CVP targets were used in both arms of [Rivers’] clinical trial.” The inclusion of CVP in both arms of the Early Goal-Directed Therapy in Severe Sepsis and Septic Shock (EGDT) study was to protect the control group (give the same care) based on contemporary management and institutional review board recommendations.(17) The control group in the EGDT study using CVP alone was noted to have a significantly improved mortality rate (5%) over routine care (without CVP) in patients examined before the study was initiated. When CVP is used in an algorithmic fashion as intended, it becomes advantageous over CVP alone. Thus, the salutary impact on morbidity and mortality as discussed above in item #3 results from its use as a part of a comprehensive protocol and not an isolated variable.(17) 5. The appellants state, “Additionally, the current measure does not target a specific value for CVP or central venous oxygen saturation—only that it be measured. We suggest that the available data does not support the scientific acceptability criteria for the measure to be specified as it is. In the analysis of the SSC efforts, neither the CVP nor the ScvO2 target was independently associated with improved survival.” No particular target for CVP and ScvO 2 is set in #0500. This permits the provider to interpret CVP in accordance with the 2012 SSC Guidelines and in the context of the patient’s particular physiology.(18) It also permits exactly the leeway that the appellants seek—freedom to interpret CVP and ScvO 2 as just additional variables to be considered alongside whatever other resuscitation modalities and endpoints they may choose. The SSC has provided guidance as to optimization of these parameters in the guidelines, which many of the appellants have also officially endorsed.(18) Thus, if there were in actuality some confusion about targets, the appellants are surely aware of the proper ranges by both training and academic interest. While the appellants properly cite the finding in the SSC observational study, they omit the overwhelming results of more than 50 previously cited investigations that conclude resuscitation guided by optimization of CVP and ScvO2 reduces mortality (see “Evidence 3 [measure evaluation criterion 1C].” As just one example, a meta-analysis of 8 trials comparing resuscitation including CVP and ScvO2 optimization to routine care showed that “sepsis care bundles were associated with consistent and significant increases in survival across eight studies.”(19, 20) Finally, we wish to note that the appellants’ letter implicitly recognizes the value of CVPand ScvO2-guided resuscitation as they plainly endorse it in their appeal. In particular, the appellants agree to accept CVC-guided fluid management provided it is one choice among others. They propose a measure element with the phrasing that: “the resuscitation is objectively monitored using a method such as lactate clearance, ScvO2 monitoring or CVP monitoring.” Certainly, the appellants would not credibly offer up a strategy in which they have no confidence. 6. The appellants state, “We point to other illustrations of continued equipoise surrounding this issue based upon the conduct of the ARISE study (Clinical Trials #NCT00975793) in Australia and the PROCESS study (Clinical Trials #NCT00510835) in the United States. These multi-center studies are scheduled to complete in 2013 and should provide greater clarity regarding the need for invasive monitoring.” These trials, regardless of the outcomes, will not yield new performance metrics ready for testing in the short term. Please refer to the comprehensive discussion in Dr. Thompson’s letter from SCCM describing long time lags until readiness for measure development. The study of early intervention strategies such as EGDT began in 1997. After its publication in 2001, EGDT and its components has withstood the rigors of reproducibility, generalizability and validity for more than a decade. To withhold lifesaving therapies pending other trials is not in the best interest of the patients we serve. Although randomized controlled trials are commonly considered a research standard, their use in evaluating therapies among the critically ill have been called into question for multiple reasons.(21) Many investigators have shown that estimates of treatment effects in large case controlled observational studies are qualitatively similar to those obtained in randomized, controlled trials.(22, 23) Thus, the results of more than 50 observational studies--including a 5467 patient multi-center implementation study such as GENESIS-(24) and the SSC database comprising nearly 40,000 patients (25) provide important and equally acceptable contributions to the outcome science of severe sepsis and septic shock. Concerns about usability or use: 1. The appellants state, “the Greater New York Hospital Association (GNYHA)/United Hospital Fund (UHF) adopted recommendations along the same lines that the ACCP is proposing. The GNYHA/UHF was not prescriptive in the resuscitation strategy employed. This 55 hospital collaborative found that the use of a noninvasive strategy (without central venous catheterization) became more common over time even while mortality continued to improve. This experience provides a basis 4 for the New York state regulations regarding sepsis care as introduced by Governor Cuomo." This remark appears to be a comment regarding usability of #0500, suggesting that a “prescriptive” resuscitation is unwieldy. In addition, the suggestion is that mortality may decline without specifying resuscitation strategies . While this group has collaborated to do excellent work and should be commended for bringing together a respected consortium of care providers, these data are not yet published. NQF sets a high bar for measure developers apropos scientific acceptability, but the minimum standard would be publication in the professional literature. The purpose of peer review is to ensure the methodology and integrity of data in answering key questions. The key issues that sepsis quality improvement data must answer in peer review are: 1) were the cited gains the result of identifying less ill patients as screening improved during the course of the collaborative; and 2) was there an underlying secular trend toward declining mortality in the hospitals due to other interventions? If either were the case, the results would be irretrievably confounded or there may be no actual gains. Therefore, it is entirely speculative to indicate that this group’s results are robust enough to substantiate the issues that the appellants assert. In contrast, the data that have informed #0500 have demonstrated robustness in the face of such scrutiny. More careful examination of these data on the GNYHA website suggests that it is deeply flawed. The GNYHA website features a summary of data through June 2012 that shows 8,556 patients with severe sepsis were reported to the collaborative. Those patients were treated under 1 of 3 possible strategies “non-invasive, invasive or no response”: Further review of this non-invasive technique reveals in the same slide presentation that 3,236 patients or 38% received “empiric fluid loading,” which is synonymous with a best estimate of needs and assessment of urine output according to documents on their website. For 47% of patients (3,990 total) the providers have not answered basic questions about the means of fluid assessment , instead providing “no response.” 5 Finally, none of the GNYHA patients appear to have been receiving the appellants’ referenced strategy of “bioimpedence” and only 2% of patients received the other referenced strategy, ultrasound. What these data lay plain, if any signal can be attributed to the data at all, is that when providers are given the “choices” that the appellants demand: 1) most of the time they have not answered the question about what resuscitation strategy they are using; 2) 38% of the time they give empiric fluid; and 3) optional sophisticated technologies are not used. Thus, if the GNYHA data are intended to show how less “prescriptive” measurement strategies lead to better results by being more usable and less cumbersome, the apparent results do not demonstrate that conclusion. As a point of clarification regarding the announcement by Governor Cuomo: " the new sepsis rules New York is adopting standards are similar to those recommended last year for the entire country by the National Quality Forum, a consortium of health care experts that provides guidance to hospitals and the Medicare system on best practices." (New York Times, 1/7/2013) These New York state regulations as published include the following: "In December 2012, the National Quality Forum included a proposed measure of adherence to treatment bundles for patients treated for sepsis. This measure, which is currently under consideration, would focus on patients 18 years of age and older who present with symptoms of severe sepsis or septic shock who are eligible for the 3 hour (severe sepsis) and/or 6 hour (septic shock) early management bundle. The regulations proposed by the Department to measure adherence with established sepsis protocols will seek to be in alignment with the NQF measure when adopted." 2. The appellants state, “It is not clear, however, that these measure [sic] only apply to severe sepsis and septic shock patients presenting in the ED as specified. If the measure includes patients who develop severe sepsis or septic shock as an inpatient subsequent to admission, as well as patients transferred to a facility already septic, use of ED triage time as “time zero” will likely result in inaccurate assessments 6 of performance based upon the facility’s rates of nosocomial sepsis and hospital -tohospital referral patterns. Furthermore, there is no mitigation for patients presenting with severe sepsis and septic shock at one ED and transferred to another. The receiving ED will be held accountable for care provided elsewhere currently .” The appellants make two points here—one regarding inpatient sepsis and the other about transfers between hospitals. Regarding the development of inpatient sepsis, the appellants have misread the measure specifications. The measure clearly indicates “‘time of presentation’ is defined as the time of triage in the ED or, if presenting from another care venue, from the earliest chart annotation consistent with all elements of severe sepsis or septic shock ascertained through chart review” in the measure specifications (measure evaluation criterion 2a1). Thus, the time of presentation for inpatient sepsis is determined by chart abstraction. The use of triage time is restricted to patients presenting from the ED. In the previously submitted material to the NQF, the 2 different start times for the ED and the inpatient medical/surgical floors were found to be statistically reliable and valid measures of performance. Regarding transfers between facilities, the appellants have made a correct point. The SSC data always excluded transfers between facilities. The measure specifications (measure evaluation criterion 2a1) should be updated to reflect exclusion of inter hospital transfers. The higher mortality seen in transfers should remain a focus of examination for the public good and quality improvement. Concerns about feasibility: 1. The appellants state, “Beyond this issue of usability, retrospective identification of discharges with sepsis may create an unreasonable burden of data collection…The specified ICD-9 codes include uncomplicated sepsis, not a population targeted for intervention by the measure…This means that the data will have to be abstracted from more than twice as many charts as they are ultimately targets for intervention.” We respectfully submit that the appellants have overstated their position. Nothing in #0500 differs from the current patient identification and data abstraction processes that all hospitals across the United States presently use to abstract data for the Center for Medicare and Medicaid Services’ (CMS) Inpatient Quality Reporting Program (IQR). Please see http://www.cms.gov/Medicare/Quality Initiatives-Patient-AssessmentInstruments/HospitalQualityInits/HospitalRHQDAPU.html for details on this program. For example, under the IQR, patients with heart failure-related diagnoses are 7 harvested from a hospital’s monthly discharges for CMS’ HF-1 measure. A sample of those patients is selected for chart abstraction at each hospital. Given that some charts harvested from ICD-9 codes will not meet measure specificati ons, they are discarded until CMS’ pre-determined number of charts is achieved. The result represents the set of charts to be abstracted to determine compliance rates with that measure. The appellants’ concern that #0500 would be applied differently is unfounded. The use of sampling and random identification from ICD -9 discharges resolves their concerns. Suggestions for a new measure: 1. The appellants suggest that Severe Sepsis and Septic Shock: Management Bundle measures should be limited to patients presenting to the reporting Emergency Department (ED). Those transferred from other hospitals and those developing sepsis after admission should be excluded. This mirrors the performance measures developed around community---acquired pneumonia. Eliminating patients developing sepsis after hospital admission ignores a very important area for reducing morbidity and mortality. Up to 36.4% of all severe sepsis and septic shock patients are diagnosed on the general floors. These patients experience a mortality of over 46% which is the highest mortality of all patients treated in the hospital including the intensive care unit (ICU).(26, 27) It has been shown that over 50% of severe sepsis and septic shock patients are admitted through the ED.(16, 25, 26) Many of these patients are inadequately risk stratified or diagnosed and are sent to the general floors. These patient decompensate to either die or become ICU admissions. Ironically pneumonia is an important source of these frequently catastrophic and deadly outcomes. In a recent study examining cardiac arrest occurring within 72 hours of hospitalization in over 500 hospitals, 12.1% (4,453 patients) carried an admitting diagnosis of pneumonia. Only 52.3% of these patients were receiving cardiac monitoring prior to cardiac arrest. In patients with preexis ting pneumonia, cardiac arrest may occur in the absence of preceding shock or respiratory failure. (28) With this information, we can obviously improve upon the pneumonia measure. Significantly improved outcomes can still be realized e ven if these patients with a delayed diagnosis receive the #0500 measure.(29, 30) 2. The appellants conclude with a thorough re-design of #0500 and its specifications. The appellants likely intend to provide helpful suggestions, however they misunderstand the NQF Measure Evaluation Criteria. These criteria state that the redesigned measure as described by the appellants would have to meet multiple evaluation criteria and therefore would require substantial measures testing. For instance, one of the fundamental problems of using lactate clearance as a sole non8 invasive resuscitation target is that a normal lactate (< 2mM/L) can be present in up to 50% of septic shock patients who are prone to multi-system organ failure and mortalities approaching 50%. (12-16) Thus, after that testing is complete, the new candidate measure could enter the Consensus Measures Development Process. Therefore, major alterations to #0500 as described by the appellants cannot be entertained at this stage. In conclusion, we appreciate the appellants’ desire to see well -specified measures developed for severe sepsis and septic shock. However, measure #0500 has not only withstood multiple examinations by the NQF over 5 years; it has contributed to the largest reduction in mortality for severe sepsis and septic shock in a generation in the United States.(31) We kindly request that that the NQF Board continue to endorse #0500. Respectfully, Emanuel P. Rivers, MD, MPH Vice Chairman and Research Director, Department of Emergency Medicine Attending Staff, Emergency Medicine and Surgical Critical Care, Henry Ford Hospital Clinical Professor, Wayne State University, Detroit, Michigan Institute of Medicine, The National Academies Respectfully, Sean R. Townsend, MD Vice President of Quality & Safety California Pacific Medical Center Clinical Assistant Professor, University of California San Francisco San Francisco, California 9 References 1. Wilson JN, Mayrhofer OMD. Rational approach to management of clincal shock, utilizing light-reflection oximetry and central venous pressure monitoring. Survey of Anesthesiology 1966;10(3):211. 2. Cohn JN. Central venous pressure as a guide to volume expansion. Ann Intern Med 1967;66(6):1283-1287. 3. Practice parameters for hemodynamic support of sepsis in adult patients in sepsis. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med 1999;27(3):639-660. 4. Kashiouris M, Kashyap R, Jaffer-Sathick I, et al. Association between delays in central venous catherter utilization and organ dysfunction in patients with severe sepsis and septic shock. Critical Care Medicine 2011;39(12)(Supplement):142. 5. Walkey AJ, Wiener RS, Lindenauer PK. Utilization Patterns and Outcomes Associated With Central Venous Catheter in Septic Shock: A Population-Based Study. Crit Care Med 2013. 6. Hayatdavoudi S, Crowell R, Rincon T, et al. Does aggressive fluid resucitation using cvp monitoring decrease mortality below the Apache IV predicted outcomes? Critical Care Medicine 2012;40(12):1-328. 7. Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med 2005;31:1066-1071. 8. Magder S. Central venous pressure: A useful but not so simple measurement. Crit Care Med 2006;34(8):2224-2227. 9. Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J Intensive Care Med 2007;22(1):44-51. 10. Lanspa MJ, Brown SM, Hirshberg EL, et al. Central venous pressure and shock index predict lack of hemodynamic response to volume expansion in septic shock: A prospective, observational study. Journal of critical care 2012;27(6):609-615. 11. Greene WL, Concato J, Feinstein AR. Claims of equivalence in medical research: are they supported by the evidence? Ann Intern Med 2000;132(9):715-722. 12. Levraut J, Ciebiera JP, Chave S, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than overproduction. Am J Respir Crit Care Med 1998;157(4 Pt 1):1021-1026. 13. Na S, Kuan WS, Mahadevan M, et al. Implementation of early goal-directed therapy and the surviving sepsis campaign resuscitation bundle in Asia. Int J Qual Health Care 2012;24(5):452-462. 10 14. Wacharasint P, Nakada TA, Boyd JH, et al. Normal-Range Blood Lactate Concentration in Septic Shock is Prognostic and Predictive. Shock 2012. 15. Dugas AF, Mackenhauer J, Salciccioli JD, et al. Prevalence and characteristics of nonlactate and lactate expressors in septic shock. J Crit Care 2012;27(4):344-350. 16. Cannon CM, Holthaus CV, Zubrow MT, et al. The GENESIS Project (GENeralized Early Sepsis Intervention Strategies): A Multicenter Quality Improvement Collaborative. J Intensive Care Med August, 2012. 17. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345(19):1368-1377. 18. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Critical Care Medicine 2013;41(2):580-637. 19. Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 2010;38(2):668-678. 20. Chamberlain DJ, Willis EM, Bersten AB. The severe sepsis bundles as processes of care: A meta-analysis. Aust Crit Care 2011. 21. Vincent JL. We should abandon randomized controlled trials in the intensive care unit. Crit Care Med 2010;38(10 Suppl):S534-538. 22. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. Am J Ophthalmol 2000;130(5):688. 23. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342(25):1887-1892. 24. Cannon C, Holthaus C, Rivers E, et al. Improving outcome in severe sepsis and septic shock: results of a prospective multicenter collaborative. The Journal of Emergency Medicine 2009;37(2):217–236. 25. Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. The Lancet Infectious Diseases 2012. 26. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical Care Medicine 2010;38(2):367-374. 27. Lundberg JS, Perl TM, Wiblin T, et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med 1998;26(6):1020-1024. 28. Carr GE, Yuen TC, McConville JF, et al. Early Cardiac Arrest in Patients Hospitalized 11 With Pneumonia: A Report From the American Heart Association's Get With the GuidelinesResuscitation Program. Chest 2012;141(6):1528-1536. 29. Coba V, Whitmill M, Mooney R, et al. Resuscitation Bundle Compliance in Severe Sepsis and Septic Shock: Improves Survival, Is Better Late than Never. J Intensive Care Med 2011. 30. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock 2011;36(6):542-547. 31. Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest 2011;140(5):1223-1231. 12 TO: Consensus Standards Approval Committee (CSAC) FR: Reva Winkler, Senior Director Alexis Morgan, Senior Project Manager RE: An Amendment to the National Voluntary Consensus Standards: Infectious Disease Endorsement Maintenance 2012, Addendum Report Member Voting Results DA: February 12, 2013 The CSAC will consider the Steering Committee’s recommendations for two remaining measures within the Infectious Disease Endorsement Maintenance Project during its February 12th conference call. The complete voting draft addendum report and detailed measure information are available on the project webpage. Member voting on the two recommended measures ended on February 6, 2013. NQF MEMBER VOTING RESULTS All of recommended measures were approved with 76% approval or higher. Representatives of 64 member organizations voted; no votes were received from the Public/Community Health Agency Council. Results for each measure are provided below. (Links are provided to the full measure summary evaluation tables.) Measure 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia Measure Council Yes Consumer 10 Health Plan 6 Health Professional 10 Provider Organizations 9 Public/Community Health Agency 0 Purchaser 7 QMRI 2 Supplier/Industry 6 All Councils 50 Percentage of councils approving (>50%) Average council percentage approval *equation: Yes/ (Total - Abstain) No 0 0 0 4 0 0 0 0 4 Abstain 1 0 3 4 0 0 2 0 10 Total Votes 11 6 13 17 0 7 4 6 64 % Approval* 100% 100% 100% 69% 0% 100% 100% 100% 93% 100% 96% Voting Comments: • No voting comments were received. 1 Measure 0500 Severe sepsis and septic shock: Management bundle Measure Council Consumer Health Plan Health Professional Provider Organizations Public/Community Health Agency Purchaser QMRI Supplier/Industry All Councils Percentage of councils approving (>50%) Average council percentage approval *equation: Yes/ (Total - Abstain) Yes No 10 6 9 5 0 7 4 4 45 0 0 2 12 0 0 0 0 14 Abstain 1 0 2 0 0 0 0 2 5 Total Votes 11 6 13 17 0 7 4 6 64 % Approval* 100% 100% 82% 29% 0% 100% 100% 100% 76% 86% 87% Voting Comments: • The Society of Critical Care Medicine and the Infectious Diseases Society of America: The letter of support is attached to this memo. (Attachment A) • GNYHA: the letter addressed to Ms. Ann Monroe (Attachment B) and a response from the developer to GNYHA (Attachment C) are attached to this memo. • American Hospital Association: While we think this is an important topic for measurement, and that this may turn out to be the right measurement, we do not see evidence that this measure has undergone a rigorous review to ensure its validity and reliability for comparative reporting or for use in pay for performance programs. It has only been used for internal quality improvement projects and collaboratives. • AmeriHealth Mercy Family of Companies agrees with concerns raised in prior public comments that measurement of Central Venous Pressure does not meet evidence based medicine criteria. The argument that it is part of a protocol that has improved mortality does not mean that the measurement of CVP contributed to that improvement. Potentially the results could have been better without CVP if the catheters contributed to further infection. So would suggest that on future review the following be removed: 6) In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate >=4 mmol/L (36 mg/dl): Measure central venous pressure (CVP) Measure central venous oxygen saturation (ScvO2) • GNYHA voting comment (see page 3) • Columbia University comment (see page 4) 2 GNYHA voting comment: Since 2010, the GNYHA/UHF STOP Sepsis Collaborative has supported 57 hospitals in enhancing clinical systems to identify and treat patients with severe sepsis and septic shock. The Collaboratives diverse participants and inclusive approach across hospitals with varying resources and staffing produced important results which were not sufficiently considered in the development of measure 0500. Many hospital emergency departments (EDs) lack the capacity to meet the aggressive therapeutic interventions associated with Early Goal Directed Therapy (EGDT) to which the NQF measure criteria map. Hospitals cite limited ED resources pointing to central venous pressure (CVP) measurement and lack of staff to insert and monitor central venous catheters as the reason. Our Collaborative offered hospitals a choice of adhering to an invasive (EGDT) or a non-invasive protocol. The latter allows hospitals to use ultrasound assessment of responsiveness to intravascular volume administration as an alternative to CVP monitoring and to assess response to treatment using serial measurements of serum lactate measurement instead of mixed venous oxygen saturation (Sv02). Results including 10,000 severe sepsis and septic shock cases show the non-invasive approach being used in >80% of cases and achieving equivalent reductions in mortality as patients monitored with CVP. NQFs measure should encourage and allow all hospitals to optimally care for patients with sepsis rather than the small number of hospitals who are able to consistently deliver EGDT. Prior to the Collaborative, most hospitals had no protocol-based approach to identification and treatment due to barriers adhering to EGDT. But when given training and evidence to support a noninvasive approach, hospitals quickly implemented these protocols in their EDs. A systematic application of the critical, best-validated components of treatment prompt, sufficient fluid resuscitation, timely, appropriate antibiotics and source control are all included in the non-invasive protocol and individually associated with reduced mortality. Further, evidence supports alternatives to invasive CVP and Sv02 monitoring. This measure fails to consider evidence that alternative markers to CVP targets exist and are equally effective. Our Collaborative focuses on adapting and translating evidence-based therapies to make them feasible in busy EDs. Jones et al.(2010) successfully tested the hypothesis of non-inferiority between lactate clearance and Scv02 as goals of early sepsis resuscitation. This study's results, which assigned septic patients to one of two protocols, did not demonstrate significantly different in-hospital mortality. If CVP properly assessed the adequacy of fluid resuscitation, then cost and harm associated with central line placement may be balanced by this benefit. However, Marik et al. 2008 meta-analysis demonstrated CVP to be a poor marker of fluid responsiveness. Currently, there are at least three large national and international trials evaluating CVP for sepsis resuscitation, indicating ongoing controversy with its use. GNYHA urges NQF to consider the mounting evidence supporting alternatives to CVP measurement. There may be unintended consequences and harm associated with the use of a central line where it is not required. Including central line insertion as a component of the NQF measure may lead to inappropriate use and compromise patient care in order to meet each bundle element. CVP or Scv02 measurement has not proven to be superior to non-invasive approaches, and is associated with risk of pneumothorax and catheter-associated bacteremia. Recently published data suggest newer 3 methods to assessing preload responsiveness such as bedside echocardiography, non-invasive cardiac output monitors, and measurement of inferior vena cava dimensions will be incorporated into clinical practice with improved predictive value. Columbia University voting comment: On behalf of the NewYork-Presbyterian Hospital, we are grateful for the opportunity to provide comments related to the proposed National Quality Forum (NQF) measure 0500, Severe Sepsis and Septic Shock: Management Bundle, focusing on patients aged 18 and older who present with symptoms of severe sepsis or septic shock. Although we support aims and goals of the Surviving Sepsis Campaign, we have concerns with the NQF measure 0500 as they are currently written. Although the clinical evidence base supports the value of Early Goal Directed Therapy (EGDT), many hospitals find it difficult to adhere to EGDT processes. The most frequent reason has to do with limited emergency department resources to insert central venous catheters as well as measure and monitor central venous pressure (CVP). NewYork-Presbyterian Hospital was one of the hospitals in the GNYHA/United Hospital Fund STOP Sepsis Collaborative.The Collaborative offered the 57 participating hospitals a non-invasive protocol as an alternative to the invasive EGDT approach. This non-invasive protocol provides the option of using ultrasound assessment of responsiveness to intravascular volume administration as an alternative to CVP monitoring. It also gives the option of assessing response to treatment using serial measurements of serum lactate measurement instead of mixed venous oxygen saturation. To date, the STOP Sepsis Collaborative found that the non-invasive approach was the choice selected by hospitals in over 80% of sepsis cases treated. Equivalent reductions in mortality were achieved compared with the sepsis cases monitored with CVP measurements. There is strong and compelling literature to support an alternative to invasive CVP and mixed-venous oxygen saturation monitoring. Jones et al. (2010) successfully tested the hypothesis of non-inferiority between lactate clearance and central venous oxygen saturation as goals of early sepsis resuscitation. The results of this study, which assigned septic patients to one of two protocols an SCV02 group and a lactate clearance group did not demonstrate a significant difference in-hospital mortality. Furthermore, CVP can be a poor marker of fluid responsiveness. Currently there are at least three large national and international trials evaluating the use of CVP for sepsis resuscitation demonstrating ongoing controversy and clinical equipoise with its use. Until the results of these large trials are known, the importance of CVP in sepsis resuscitation is currently unknown. NewYork-Presbyterian Hospital urges NQF to reconsider the mounting evidence that supports alternatives to CVP measurement and consideration of these options before finalizing measure 0500. The use of central lines as an essential element in the composite measure may lead to inappropriate or unnecessary use of central lines, compromising patient care in an effort to meet each element of a bundle measure. Measurement of CVP or central venous oxygen saturation with a central venous catheter has not been demonstrated convincingly to be a superior approach to non-invasive approaches, and is inevitably associated with the risk of pneumothorax and catheter-associated bacteremia. 4 December 28, 2012 National Quality Forum 1030 15th Street NW, Ste. 800 Washington, DC 20005 Re: Member Comment, Measure 0500: Severe Sepsis and Septic Shock Management Bundle The Society of Critical Care Medicine (SCCM) and the Infectious Diseases Society of America (IDSA), as members of the National Quality Forum (NQF), fully support adoption of Measure 0500. SCCM, a codeveloper (with the Henry Ford Hospital System, and in cooperation the Institute for Healthcare Improvement and California Pacific Medical Center) of the measure set, strongly encourages endorsement by the NQF. The bundle provides a framework for performance improvement by delineating immediate clinical interventions that have been shown to reduce mortality even when compliance is modest. Further, adoption will close a measurement gap in direct alignment with the highest national priorities for patient-centered care that improves health outcomes and limits expenses. There is a national imperative to improve the care delivered to septic patients in the United States. Sepsis is associated with mortality rates ranging between 16% and 49%. These rates are more than eight times higher than those observed in patients admitted to the hospital with other diagnoses. [1] From 2007 to 2009, more than 2,047,038 patients with a sepsis-related illness were admitted to medical facilities. [2] A recent report examining data compiled from 15,022 patients in 165 ICUs demonstrated that, over a two-year period, improved compliance with an earlier version of the measure set was associated with a decline in mortality from 37% to 30.4% – and this dramatic improvement in outcome did not reflect universal compliance. It logically follows that application of the bundle to a larger percentage of patients will result in an even greater decline in mortality. [3] Clearly, further extension of compliance with the measure set is an urgent national priority. Thus, there is a pressing need to extend the use of the measures to patients in as many additional healthcare facilities as possible. The incidence of sepsis has increased 83% over the last decade, and two-thirds of the patients affected were older than 65 years. [4] As the population continues to age, the imperative becomes even more acute. Providing improved recognition of septic patients and urgent, reliable delivery of the right care, right now must be a universal priority for all patients within our healthcare system. Measure 0500 exemplifies multidisciplinary care at its best. Early detection and treatment of patients with severe sepsis and septic shock and the universal implementation of the bundle requires the engagement of nurses, physicians, pharmacists, respiratory therapists – indeed, virtually all members of the healthcare team. Septic patients identified in both outpatient and inpatient settings can rapidly progress to develop multiple organ dysfunction. The consequences may be dire. In addition to the high mortality rate, patients who recover from sepsis-induced organ dysfunction may emerge with severe disabilities involving breathing, locomotion, and cognitive function. [5] The adoption of this measure set PDF created with pdfFactory Pro trial version www.pdffactory.com by NQF assures improved recognition of this deadly medical emergency, provides much needed structure to facilitate activity, and delineates specific, potentially lifesaving interventions. After more than six years of data collection and experience with performance improvement in hospitals across the globe, we know that these metrics can be implemented and that their use can save lives. The SSCM and IDSA are in full support of adoption of Measure 0500 and encourage NQF to vote in the affirmative for the sepsis management composite measures. Sincerely, David Relman, MD, FIDSA President, IDSA Clifford S. Deutschman, MS, MD, FCCM President, SCCM References 1. 2. 3. 4. 5. Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. Hospitals, 2009. HCUP Statistical Brief #122, October 2011. Reed K, McBratney S, Thompson D, Nicholas C. Healthgrades emergency medicine in American hospitals study. Health Grades. June, 2010 2011;The First Annual Report(1):1-28. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367-374. Burt CW, McCaig LF, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2005. Adv Data. Jun 29 2007(388):1-15 Iwashyna TJ, Ely EW, Smith DM, et al. Long-term impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787-1794. PDF created with pdfFactory Pro trial version www.pdffactory.com Sean R. Townsend, MD Stern Building Vice President Quality & Safety 2330 Clay St., #301 (415) 600-5770 Phone (415) 600-1541 Fax February 6, 2012 San Francisco, CA 94115 townsesr@sutterhealth.org Kenneth E. Raske President Greater New York Hospital Association 555 W. 57th Street New York, NY 10019 Dear Mr. Raske: I am writing in my capacity as a member of the Executive Committee of the Surviving Sepsis Campaign (SSC). We are in receipt of your draft letter to Ms. Laura Miller, Interim President and Chief Executive Officer of The National Quality Forum (NQF), which was publicly forwarded to GNYHA hospitals regarding Sepsis Measure #0500 currently being considered by the NQF (attached). I would like to sincerely congratulate the GNYHA and the STOP Sepsis Collaborative for the powerful work that you are doing. It is challenging to bring together so many hospitals and collect data on the care of severely septic patients. I applaud your commitment and innovation, and I can see the evident success of your effort. I do have substantial concerns about the content of the letter you have drafted, both in terms of its representation of your results and the representation of the evidence it cites to support your position. In particular, I am most concerned about the appropriateness of using non-published data to challenge the results of published data. As you know, the data from the Stop Sepsis Collaborative is not yet published. NQF sets a high bar for measure developers in proposing measures to be consistent with the evidence. The purpose of peer review is to ensure the integrity of the data in answering key questions. The key issues that sepsis quality improvement data must answer in peer review are: 1) how does one reassure themselves that the cited gains were not the result of identifying less ill patients as screening got better during the course of the collaborative -- that would confound the claimed results irretrievably; and 2) how does one know that there was not an underlying secular trend towards declining mortality in the hospitals due to other interventions such as greater adherence to VAP bundles and CLABSI reductions -- if so, there may be no actual gains attributable to the STOP collaborative. To date, only the SSC data has answered these questions in peer review. We were able to do so statistically taking advantage if the staggered enrollment of patients over calendar time to show that mortality was not decreasing during the course of the intervention and through risk adjustment we demonstrated our patients were as severely ill at the beginning of the trial as afterwards.1 This published data actually supports the intervention that you are most concerned about in your letter, the assessment of central venous pressure (CVP) and central venous oxygenation saturation (ScvO2), a.k.a early goal directed therapy (EGDT). In the letter you state that your primary concern is that “…many hospitals do not adhere to EGDT processes and frequently cite the limitations of emergency department resources,” but I am compelled to respectfully point out that the aim of improvement is not to just endorse strategies hospitals already adhere to as best practice. That would Levy MM, Dellinger RP, Townsend SR, The Surviving Sepsis Campaign: results of an international guidelinebased performance improvement program targeting severe sepsis. Crit Care Med. 2010 Feb;38(2):367-74. 1 1 leave us with few avenues to improve. Moreover, the SSC data comes from more than 200 hospitals, the bulk of which were community facilities that improved their ability to comply with EGDT suggesting hospitals can exceed their current practice and improve. Your draft letter implies that the community hospitals you represent cannot place central lines as required under Sepsis #0500. The published experience of the SSC would stand in against that notion. It may be the case that hospitals and physicians will not place central lines unless compelled by evidence based standards, but it seems less likely that they cannot place lines. Virtually all acute care facilities in the country have the ability to place central lines or PICC lines. This stands in distinction to the strategies that you are recommending in place of central access – the use of ultrasound and echocardiograms to assess volume status. It seems uncontestable that fewer clinicians are trained in the emergency department or in critical care to use ultrasound for volume assessment than to place central lines. Nevertheless, on your website you provide data (reproduced below) indicating that 65% of your hospitals are using the non-invasive technique: : When one further reviews just what this non-invasive technique is, the same slide set indicates that only 38% percent of these patients are receiving ultrasound assessment, but 47% are receiving “empiric fluid loading” without declaring what type of assessment they are actually receiving to determine if the fluid load is adequate: 2 Thus, only a third of the hospitals at best can use ultrasound as the STOP Sepsis Collaborative data suggest and 47% may or may not be assessing volume with any technique. Compared to the universal capability to place central access (a technique for which all emergency physicians or intensivists have at some point been trained), empiric fluid loading or ultrasound assessment would appear to be a challenging strategy to endorse based on the STOP Sepsis Collaborative data. In your draft letter, I notice that you cite Allan Jones’ 2010 paper in JAMA to support your approach of non-invasive monitoring. This paper sought to demonstrate that a strategy of clearing lactate by progressive fluid administration with a drop of 10% or more was equivalent to optimizing the central venous oxygenation (Scv02) in terms of mortality reduction. I wish to clarify that the Jones paper, does not support your conclusions that central venous access is unnecessary. In fact, every patient in the trial received central venous access and had the CVP optimized. The paper specifically notes in the intervention section, “[w]e randomly assigned patients to 1 of 2 resuscitation protocols. The ScvO2 group was resuscitated to normalize central venous pressure, mean arterial pressure, and ScvO2 of at least 70%; and the lactate clearance group was resuscitated to normalize central venous pressure, mean arterial pressure, and lactate clearance of at least 10%.” Thus, the paper in fact supports central access.2 As the Infectious Disease Project Committee at NQF reviewing Sepsis #0500 has considered many times now, CVP may not be the most robust measure of volume status, but no other measure has ever been shown to be better, especially as part of a sepsis resuscitation protocol. We are left with the knowledge that when CVP is used as part of a larger protocol, mortality does decline in the peer reviewed literature.3 Thus, the committee has twice voted in favor of the inclusion of CVP and ScvO2. Lastly, I wish to point out as regards the unintended consequences of central line placement, the best evidence states that consequences that may be fatal occur less than <= 1% of the time (pneumothorax or hemothorax) with central line placement.4 The 7% ARR in mortality with the SSC strategy shown in the Levy 2010 paper (cited above) would It should also be noted that the evidence in the Jones paper has been ranked as inferior to the evidence supporting early goal directed therapy in the only evidence based guidelines published on the topic: Dellinger RP, Levy MM, Rhodes A et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013 Feb;41(2):580-637. 2 3 Please see the original NQF Sepsis #0500 submission section 1c.6. “Quantity of Studies in the Body of Evidence” for 55 citations confirming these findings. Eisen LA, Narasimhan M, Berger JS, Mayo PH, Rosen MJ, Schneider RF, Mechanical complications of central venous catheters. J Intensive Care Med. 2006;21(1):40. 4 3 thus seem to overwhelm the risk of mortality due to line placement. Most of the time even pneumothorax or hemothorax, while severe complications, are not fatal conditions. In conclusion, I believe the right thing for Stop Sepsis and GNYHA to do is to publish the information you are gathering and then present it as part of regular order in the Consensus Standards Development Process at the next review point. The NQF measure development process is designed to consider new evidence at staged intervals as it is available in the literature. I for one would be delighted to see published alternatives to our strategy to make it even stronger. Please do not hesitate to contact me if you should have any questions or comments. Best regards, Sean R. Townsend, M.D. CC: Ann Monroe, M.A., Chair Consensus Standards Approval Committee 4 Appendix D: Measure Evaluation Summary Tables LEGEND: Y = Yes; N = No; H = High; M = Moderate; L = Low; I = Insufficient 0500 Severe sepsis and septic shock: Management bundle Status: Maintenance, Original Endorsement: Oct 24, 2008 Description: This measure will focus on patients aged 18 years and older who present with symptoms of severe sepsis or septic shock. These patients will be eligible for the 3 hour (severe sepsis) and/or 6 hour (septic shock) early management bundle. Numerator Statement: If: A. measure lactate level B. obtain blood cultures prior to antibiotics C. administer broad spectrum antibiotics D. administer 30 ml/kg crystalloid for hypotension or lactate >=4 mmol/L E. apply vasopressors (for hypotension that does not respond to initial fluid resuscitation to maintain a mean areterial pressure >= 65) F. In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate >=4 mmol/L (36 mg/dl) measure central venous pressure and central venous oxygen saturation G. remeasure lactate if initial lactate is elevated represent processes of care: Numerator statement: Patients from the denominator who received all the following: A, B, and C within 3 hours of time of presentation† AND IF septic shock is present (as either defined as hypotension* or lactate >=4 mmol/L) who also received D and E and F and G within 6 hours of time of presentation. † ”time of presentation” is defined as the time of triage in the Emergency Department or, if presenting from another care venue, from the earliest chart annotation consistent with all elements severe sepsis or septic shock ascertained through chart review. * “hypotension” is defined as systolic blood pressure (SBP) <90 mm Hg or mean arterial pressure (MAP) <70 mm Hg or a SBP decrease >40 mm Hg or <2 SD below normal for age or known baseline. Denominator Statement: Number of patients presenting with severe sepsis or septic shock. Exclusions: A) Patients with advanced directives for comfort care are excluded. B) Clinical conditions that preclude total measure completion should be excluded (e.g. mortality within the first 6 hours of presentation as defined above in 2a1.1). C) Patients for whom a central line is clinically contraindicated (e.g. coagulopathy that cannot be corrected, inadequate internal jugular or subclavian central venous access due to repeated cannulations). D) Patients for whom a central line was attempted but could not be successfully inserted. E) Patient or surrogate decision maker declined or is unwilling to consent to such therapies or central line placement. Adjustment/Stratification: No risk adjustment or risk stratification None Henry Ford Hospital (HFH) encourages the results of this measure to be stratified by race, ethnicity, gender, and primary language, illness severity and have included these variables as recommended data elements to be collected. Level of Analysis: Facility, Integrated Delivery System Type of Measure: Composite Data Source: Electronic Clinical Data, Electronic Clinical Data : Electronic Health Record, Paper Medical Records, Electronic Clinical Data : Registry Measure Steward: Henry Ford Hospital Other organizations: Henry Ford Hospital System(HFHS) California Pacific Medical Center/Sutter Health (CPMC) Society of Critical Care Medicine (SCCM) 6 0500 Severe sepsis and septic shock: Management bundle Infectious Diseases Society of America (IDSA) Institute for Healthcare Improvement (IHI) Surviving Sepsis Campaign (SSC) Ohio State University (OSU) STEERING COMMITTEE MEETING [08/28/2012] Importance to Measure and Report: The measure met the Importance criteria (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-19; M-1; L-0; I-0; 1b. Performance Gap: H-7; M-12; L-1; I-0 1c. Evidence: Y-11; N-5; I-4 Rationale: • There are greater than 750,000 estimated cases of severe sepsis a year in the United States. Additionally, there are an estimated 400,000 ICU admissions for sepsis, approximately 200,000 deaths a year, and at an estimated cost of $17 billion a year. • More than 50 publications have reported improved survival with use of the bundle in the past decade with the vast majority of the studies being observational. Some Committee members noted the lack of randomized controlled trials and they were informed that there are three randomized controlled trials currently ongoing in the U.S., UK and Australia. • Committee members noted that there is some controversy in the field about the need for all of the bundle elements, specifically measuring central venous pressure (CVP). However, only about 15 percent of patients end up needing a CVP line because of the care algorithm in the bundle. • Meta-analyses have shown survival benefit. National and international guidelines have been created for the management of severe sepsis and septic shock based on the data. The recommendations in the guidelines mirror the bundle in this measure. • The developer pointed to the recent GENESIS trial published in the Journal of Intensive Care Medicine of 6000 patients in 11 hospitals throughout the U.S.; hospitals ranging from 100 to 1,000 patients found that meeting the bundle in a prospective, observational cohort resulted in mortality reduction of 14 percent. 2. Scientific Acceptability of Measure Properties: The measure met the Scientific Acceptability criteria (2a. Reliability – precise specifications, testing; 2b. Validity – testing, threats to validity) Initial review: 2a. Reliability: H-1; M-7; L-5; I-7 2b. Validity: NA Rationale: • Committee members asked how the measure clearly distinguishes patients with severe sepsis versus those with septic shock. o Developer response: The key difference is hypotension refractory to fluid administration that requires a vasopressor or a persistent lactate level greater than 4 is septic shock as specified. • After several questions regarding the specifications, NQF staff realized that an attachment containing the data collection tool submitted by the developer had not been provided to the Committee. NQF staff provided the document to the Committee after the meeting. • Committee members questioned whether the inter-rater reliability study of 498 patients in one institution would apply to other institutions. The developer responded that the measure is being used in a variety of health care systems such as Kaiser, Loma Linda University, University of Kansas and Intermountain Health in Utah. NOTE: During the meeting, the Committee decided there was insufficient information included in the submission 7 0500 Severe sepsis and septic shock: Management bundle to determine whether the measure met the reliability criteria. Because the Committee had not been given all of the submitted information and the developer indicated additional data on reliability testing could be provided, the Committee agreed to revisit this measure. Additional information was provided to address the questions on reliability. After Review of all Submitted Information and Additional Information Addressing Reliability via Email: 2a. Reliability: H-5; M-11; L-1; I-0 2b. Validity: H-1; M-14; L-2; I-0 Rationale: • The term ‘broad spectrum antibiotics’ is not defined. This could potentially be problematic for a data abstractor to precisely, accurately and reproducibly identify antimicrobials that will satisfy the measure. A Committee member noted that the term ‘broad spectrum antibiotics’ was not used in the reliability testing results, instead, the term ‘timely antibiotics’ was used, which seemed to be more specific to measure Developer response: The surviving sepsis campaign defined "broad spectrum antibiotics" as those with both Gram positive and Gram negative bacterial coverage. The rationale for antibiotic selection is further discussed in the 2004 and 2008 sepsis guidelines publications. Credit for timely antibiotics was assigned in the data set used for the analyses only if both species were covered. The ICD-9 diagnostic codes to identify the denominator were thought to be appropriate. The measure was tested both at the data element and measure score levels for reliability. For validity the measure was only tested at the measure score level. o • • • In review of the validity testing, a Committee member noted that measuring central venous pressure (CVP) and central venous oxygen saturation (ScvO2) were not a part of the validity testing. • Committee members noted that the validity testing indicated that after adjusting for baseline characteristics, only administration of broad spectrum antibiotics and obtaining blood cultures before their initiation were associated with lower hospital mortality. • The question of whether the sepsis bundle as a whole should be incorporated versus specific validated elements of the bundle (e.g., antibiotic selection and timing) was discussed. Though a few members supported individual measure, the majority support the bundle. The question of how the specifications indicate accountability was raised. A member commented that time zero is triage for time limited Emergency Department (ED) therapies. If a patient presents to the ED triage and does not qualify as severe sepsis or septic shock but develops it later, would the hospital and/or physician be held accountable? Another accountability example was if a patient presents to the ED with pneumonia without severe sepsis or septic shock, and 4 hours later the patient becomes hypotensive, would the ED physicians and/or hospital be held accountable for not providing care over a timeline that had elapsed once the patient developed symptoms? Although unit and ICU time zero is based upon when the patient is diagnosed, in the ED it is time of triage which may or may not be the time at which the patient developed symptoms. The Committee member questioned how it would be reconciled. o Developer response: The patient is somewhere on the natural trajectory of becoming septic regardless of the point of presentation. If the patient who becomes hypotensive or has a high lactate does so in the ED, the reason for presentation to the ED is severe sepsis or shock. • 8 0500 Severe sepsis and septic shock: Management bundle Likewise, the patient who presents with septic physiology on the floor and becomes hypotensive there after an initial admit for something else need to have time to start the clock. In both instances, we are relying on the presence of key features of severe sepsis or shock to make the attribution. Specifying triage time in the ED is not only reasonable since that is most likely what occasioned their visit to the ED, but also provides a standard time. The evidence in the literature also is consistent with picking triage time on this basis. There is less certainty with the floor patient, but again, a proper review yields the time that all the key features were first present. Thus, while there may be some admitted variability between the wards and the ED time of presentation in terms of precision, both are accurate for purposes of measurement. The data in the reliability and validity sections of the NQF submission accept this loss of precision in favor of accuracy. The evidence and data cited demonstrate a high degree of reliability at the level of a performance measure even with this known variability. Thus, we do not need to view it as a threat to reliability. According to the RAND paper, these very high scores on the signal-tonoise reliability indicator actually mean that meaningful comparisons can be drawn in performance using this metric “as is” even with some known variability. 3. Usability: H-1; M-15; L-1; I-0 (Meaningful, understandable, and useful to the intended audiences for 3a. Public Reporting/Accountability and 3b. Quality Improvement) Rationale: • This measure is currently in wide use for public reporting and quality improvement by Kaiser Permanente, Surviving Sepsis Campaign, Catholic Healthcare West, Intermountain Healthcare and Sutter Healthcare. • Highmark has been using the measure in its pay for performance program for the past two years. They initially had some data collection issues been those were soon resolved. • The University of Kansas is currently using the measure in their EHR with real-time notifications. 4. Feasibility: H-1; M-10; L-6; I-0 (4a. Clinical data generated during care delivery; 4b. Electronic sources; 4c.Susceptibility to inaccuracies/ unintended consequences identified 4d. Data collection strategy can be implemented) Rationale: • The measure requires chart review and manual abstraction. • The measure still has elements that may not be captured completely by EHR. The amount of data that needs to be collected may be overwhelming for facilities trying to work on improving outcomes for sepsis. Some of the individual elements may be helpful for internal monitoring within the institution to evaluate improvement over time. 5. Related and Competing Measures • No related or competing measures noted. Steering Committee Recommendation for Endorsement: Y-13; N-4 6. Public and Member Comment General Support for the Measure • Three NQF members submitted comments in support of the measure noting that the developer had 9 0500 Severe sepsis and septic shock: Management bundle responded to questions from the Steering Committee. One commenter stated that “[the] steering committee questioned whether the sepsis quality measure addressing a bundle should be endorsed versus specific validated elements of the bundle. The SS Campaign noted that by making the bundles standard practice, there is elimination of piecemeal or chaotically applied standards for sepsis care that exist in many clinical environments today.” One supportive comment suggested that implementation may difficult with claims data. Lack of Evidence for the Central Venous Pressure (CVP) Measure Component • A commenter noted that “While we recognize that the SSC recommends central venous pressure monitoring (an unreliable and seldom followed parameter), both it and measuring central venous oxygen saturation are only supported by one single center clinical trial (as such limited evidence supports its use).” • ACEP states that “ACEP has serious concerns surrounding the lack of evidence for measuring CVP as a surrogate for intravascular volume. “ “The measure developers have now cited five additional studies in which multivariate logistic regression demonstrated no independent effect on mortality in patients who achieve CVP targets versus patients who do not. (Castellanos-Ortega 2010, Nguyen 2007, Jeon 2012, Levy 2010, Cannon 2010).” • A commenter suggested that “There may be the unintended consequence of increasing the use of central lines in situation where they may actually not be needed and potentially causing harm by their placement (bleeding pneumothorax, pain) or causing infections. By including this single item in the composite measure may encourage the over utilization of central line placement specifically not to fail the measure rather than taking care of the patients best interests.” Committee Response: The developer indicated that when the central venous pressure (CVP) component is utilized as part of the bundle, there is a decrease in mortality. Some members of the Committee did agree that there may be limited evidence for CVP use; however, the Committee concluded that use of the bundle as specified with CVP demonstrated reduction in mortality. Lack of Evidence for Blood Culture prior to Antibiotics Element • A commenter stated that “The whole point is that the patients receive broad spectrum antibiotics not that they are timed prior to antibiotic administration. The theoretical concern about sensitivities should not trump actual administration of those antibiotics. If not eliminated than perhaps altering the wording to simply state; “obtaining appropriate cultures” which would allow simplicity and more flexibility in the actual abstraction process. Having to identify the time of antibiotic administration along with the time of collection of cultures adds significantly to the burden and complexity of the abstraction process. Theoretically this may seem important but does the act of obtain blood cultures or any culture prior to the administration of antibiotics actually have any effect on outcomes?” • A commenter states that “Often time’s patient present to the ED with normal vital signs then decompensate and meet criteria of sepsis. Including the initial time of presentation as the start time may not reflect patient’s condition adequately. This ambiguity of utilizing different criteria of time of presentation based on location, calls into question the measure reliability.” Committee Response: The Committee concluded that blood cultures remain important for adjusting antibiotic coverage in patients with severe sepsis and reduced response to treatment and that the bundle of care processes 10 0500 Severe sepsis and septic shock: Management bundle are related to patient outcomes. The Committee determined that the measure met the evidence criteria (Y-12; N0; I-2). Reliability of Triage being Time Zero for ED Patients and the Impact of ED Length of Stay • Another commenter suggests that “Many ED patients will present with uncomplicated pneumonia, urinary tract infection, or cellulitis only to meet the criteria for severe sepsis/septic shock hours later. If the measure calls for early goal directed therapy within three hours of triage, but the patient does not meet criteria for severe sepsis or septic shock until four hours later, then even if all required interventions are completed within an hour, the hospital will fail on this measure as currently specified. That type of measurement does not differentiate hospitals based on the quality of care provided, but rather on the ED length of stay. If used for accountability as specified, this measure could cause the unintended consequence of penalizing large volume and safety net hospitals.” • Another commenter argued that “Time-based measures that potentially start the clock ticking prior to patients meeting the defining criteria of the syndrome in question have to be recognized as invalid. The developers responded that ED patients with infections are “somewhere on the natural trajectory of becoming septic regardless of point of presentation.” Statements such as this encourage overly aggressive treatment for patients who do not initially meet criteria for severe sepsis/septic shock due to provider concern of being deemed retrospectively “non-compliant” should the patients’ condition subsequently change. The developers state “if the patient who becomes hypotensive or has a high lactate does so in the ED, the reason for the presentation to the ED is severe sepsis or shock.” While this is true in cases where criteria are met at triage, it’s absolutely not the case for those who only do so hours later. Patients present with chief complaints (which are often non-specific), not diagnoses.” Committee Response: There was significant discussion on the post-comment call regarding the reliability of triage being time zero for Emergency Department (ED) patients and the impact of the ED length-of-stay. Some Committee members did agree that certain elements of the measure may be related to hospital situations (beds, changing clinical status) that are out of the control of the provider. The Committee reconsidered their evaluation of reliability and determined it meets the reliability criteria at moderate to high. Feasibility of Abstracting the Composite Measure • A commenter noted that “This new composite is far too complex for implementation as a potential accountability measure. Furthermore, all of the data elements and time stamps required to calculate this measure are not readily available discrete fields from existing electronic sources making it a significant burden on hospitals to sort and collect this data.” Committee Response: Committee members discussed the data collection burden for the input of multiple data points and the timestamps. Some members were less concern due to the large number of hospitals who are currently collecting the data for the measure. The Committee reconsidered their evaluation of this criterion and rated feasibility as moderate. Re-vote following Public and Member Comment Following the Public and Member Comment period of the addendum report, the Committee decided to re-vote on whether the measure met the NQF criteria for endorsement. 11 0500 Severe sepsis and septic shock: Management bundle Importance to Measure and Report: The measure met the Importance criteria (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-13; M-1; L-0; I-0; 1b. Performance Gap: H-5; M-9; L-0; I-0 1c. Evidence: Y-12; N-0; I-2 2. Scientific Acceptability of Measure Properties: The measure met the Scientific Acceptability criteria (2a. Reliability – precise specifications, testing; 2b. Validity – testing, threats to validity) 2a. Reliability: H-1; M-11; L-2; I-0 2b. Validity: H-0; M-14; L-0; I-0 3. Usability: H-0; M-12; L-1; I-1 (Meaningful, understandable, and useful to the intended audiences for 3a. Public Reporting/Accountability and 3b. Quality Improvement) 4. Feasibility: H-0; M-8; L-5; I-1 (4a. Clinical data generated during care delivery; 4b. Electronic sources; 4c.Susceptibility to inaccuracies/ unintended consequences identified 4d. Data collection strategy can be implemented) Steering Committee Recommendation for Endorsement: Y-11; N-3 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia Status: Maintenance, Original Endorsement: Jul 31, 2008 Description: Percentage of patients aged 18 years and older with a diagnosis of hepatitis C seen for an initial evaluation who had HCV RNA testing ordered or previously performed Numerator Statement: Patients for whom HCV RNA testing was ordered or previously performed Denominator Statement: All patients aged 18 years and older with a diagnosis of hepatitis C seen for initial evaluation Exclusions: Documentation of medical reason(s) for not ordering or performing HCV RNA testing Documentation of patient reason(s) for not ordering or performing HCV RNA testing Adjustment/Stratification: No risk adjustment or risk stratification None We encourage the results of this measure to be stratified by race, ethnicity, gender, and primary language, and have included these variables as recommended data elements to be collected. Level of Analysis: Clinician : Group/Practice, Clinician : Individual, Clinician : Team Type of Measure: Process Data Source: Administrative claims, Electronic Clinical Data, Electronic Clinical Data : Electronic Health Record, Electronic Clinical Data : Laboratory, Electronic Clinical Data : Registry Measure Steward: American Medical Association - Physician Consortium for Performance Improvement (AMAPCPI) Other organizations: American Association for the Study of Liver Diseases, American Gastroenterological Association Institute STEERING COMMITTEE MEETING [08/28/2012] Importance to Measure and Report: The measure does not meet the Importance criteria 12 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-16; M-4; L-0; I-0; 1b. Performance Gap: NA 1c. Evidence: Y-3; N-8; I-9 Rationale: • Hepatitis C affects a large portion of the baby boomer population. Recently CDC recommended that all adults born from 1945 to 1965 receive hepatitis C screening. More patients with chronic HCV will be identified. • More people died in 2007 from hepatitis C than HIV. • Hepatitis C is a highly prevalent condition with a large health impact. However, there was no evidence provided that this test is not being done. • The Committee noted that there is little to no disparities data available for hepatitis C for the individual performance measures, though minorities are over-represented in the population of patients with HCV • Studies on long term benefit or treatment, which results from the test, are all observational except one, and do not look at long term benefits/harms. • A body of evidence does exist, but weakly addressed in the measure submission. The measure defaults to AASLD guidelines that were based on data and rated IB and 1A. Consistency was not addressed. Additional information provided by PCPI included a meta-analysis of 31 studies and all are consistent with an overall estimate of 15 to 20 percent of people who become infected with hepatitis C who clear the virus. Thus, this test is important in differentiating whether or not people have resolved infection or chronic infection. • Committee members asked about the evidence that it is important to know whether the patient is viremic if they are not candidates for treatment. Others noted that it is important to other aspects of care such as avoiding alcohol, vaccination, counseling regarding transmission and remaining engaged in care. • The Committee discussed the need for evidence for a standard assessment measure. NQF staff advised the Committee that CSAC has discouraged assessment measures that are essentially a standard of care. • Some Committee members concluded that the question regarding the timing of the testing and whether or not the initial time is appropriate and beneficial to patient outcomes, particularly in view of measure 0584: Hepatitis C: Viral load test which is testing before therapy. • The Committee elected not to make an exception for the evidence criteria. 6. Public and Member Comment • CDC does not support (encourage recommendation). CDC has recommended prompt RNA confirmation of Hepatitis C without regard to the intent to provide antiviral treatment (Recommendations for Prevention and Control of Hep C Virus (HCV) Infection and HCV-Related Chronic Disease MMWR October 16, 1998 / 47(RR19);1-3 9; Recommendations for the Identification of Chronic Hep C Virus Infection Among Persons Born During 1945–1965 August 17, 2012 / 61(RR04);1-18). CDC does not agree that such testing is performed so regularly that it can be regarded as “standard of care”. We recognize that data in the NQF report demonstrate substantial adherence to the recommendation: “CMS Physician Quality Reporting Initiative: Scores on this measure: 95.86% is the aggregate performance rate in the total patient population (N = 1,610) and 95.84% is the mean performance rate of TIN/NPI’s 10th percentile: 87.50% 25th percentile: 100.00% 50th percentile: 100.00% 13 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia 75th percentile: 100.00% 90th percentile: 100.00% The inter-quartile range (IQR) provides a measure of the dispersion of performance. The IQR is 0.00 and indicates that at least 50% or more of physicians have performance on this measure at 100.00%. The bottom 10% of physicians are performing at or below 87.50%. Source: Confidential CMS PQRI 2009 Performance Information by Measure. TAP file.” However, such data may not be representative at all. There are other reports that indicate there is substantial performance gap: Of 20,285 reports of HCV infection received by CDC from state/local surveillance programs in 2006-2007, a total of 10,834 (47.6%) reports had no positive result for HCV RNA. Klevens RM, Miller J, Iqbal K, Thomas A, et al. The Evolving Epidemiology of Hepatitis A in the United States: Incidence and Molecular Epidemiology from PopulationBased Surveillance. Arch Intern Med. 2010;170(20):1811-1818. CDC recently reviewed electronic health records of >1,652,055 adult patients seen from January 2006 through December 2010 at 4 integrated healthcare systems in Detroit, Michigan; Danville, Pennsylvania; Portland, Oregon; and Honolulu, Hawaii were collected and analyzed. Of 9086 patients with a positive HCV antibody test, 3428 (37.7%) had no documented follow-up HCV RNA testing in the electronic database.” MoormanAC, Gordon SC, Rupp et al. Baseline Characteristics and Mortality Among People in Care for Chronic Viral Hepatitis: The Chronic Hepatitis Cohort Study. Clin Infect Dis.2012 Oct 19. [Epub ahead of print]. A poster presentation from the 2012 IDSA meeting demonstrated a decline in the documentation of HCV viremia from 73% to 63%: “Quality of Hepatitis C care at an urban tertiary medical center” IDSA San Diego Oct 17-21 2012; Sabrina A. Assoumou MD, Wei Huang MA, Benjamin P. Linas, MD MPH. • The majority of SC members determined that the requirement for evidence was not met. However, a few SC members recognized the importance of the measure and discussed the indirect evidence linking the process to the outcome. Additional information provided by the Work Group included a meta-analysis of 31 studies that found a consistent overall estimate of 15 to 20 percent of people who become infected with acute Hepatitis C will clear the virus. The absence of confirmatory viral testing may then leave these 15 to 20 percent of patients with the mistaken belief that they have chronic Hepatitis C, subjecting these patients to unnecessary anxiety and other harms. The remaining viral positive patients could benefit from the additional counseling for their own and for transmission risk, as mentioned by SC members, namely avoiding alcohol, getting vaccinated, and providing counseling regarding transmission and remaining engaged in care. Thus, this test is critically important in differentiating whether or not people have resolved infection or are currently infected with HCV, regardless of whether antiviral treatment is contemplated. The SC was also concerned that little evidence was provided to demonstrate opportunity for improvement and that, like most assessment measures, it represents the “Standard of Care” and does not warrant a performance measure. However, additional evidence provided by the CDC, Boston Medical Center and the Cleveland VA Medical Center below shows that a substantial performance gap remains, illustrating that in practice, confirmatory testing after initial HCV antibody testing is NOT being done often enough to constitute “Standard of Care.” Of 20,285 reports of HCV infection received by CDC from state/local surveillance programs in 2006-2007, a total of 10,834 (47.6%) reports had no positive result for HCV RNA.1 CDC recently reviewed electronic health records of >1,652,055 adult patients seen from January 2006 through December 2010 at 4 integrated healthcare systems in Detroit, Michigan; Danville, Pennsylvania; Portland, Oregon; and Honolulu, Hawaii. Of 9,086 patients with a positive HCV antibody test, 3,428 (37.7%) had no documented follow-up HCV RNA testing in the electronic database.2 A study conducted at Boston Medical Center of CMS-defined HCV quality indicators, comparing data from 2005- 14 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia 2007 to 2008-2011, revealed a decline in the confirmation of HCV viremia from 73% to 63%.3 Members of the Department of Medicine at Louis Stokes Cleveland Department of Veterans Affairs Medical Center in Cleveland, OH found similar rates of testing in their study and included additional information in their conclusions related to implications. They looked at ~400 people who lacked HCV nucleic acid amplification technology (NAT) testing to characterize behaviors in response to patients who have a positive HCV antibody (ab) test but lack viral confirmatory testing. Below are their findings: 1. 31% of patients with a positive HCV ab test, never had that result acknowledged by a medical provider (HCV ordering or other provider), resulting in missed opportunities for follow-up liver care and Hepatitis C treatment.4 2. In 251 instances, the positive HCV ab test was acknowledged by the ordering provider, and despite the lack of viral NAT, these providers took actions that indicated they believed patients had chronic Hepatitis C.4 These actions included addition of the ICD-9 diagnosis for chronic Hepatitis C to the patient’s problem list, ordering serial liver function tests, ordering HAV/HBV vaccinations, etc. Interestingly, very few providers ordered confirmatory NAT in response to the positive HCV ab. 3. In the cases where HCV was entered into the patient’s problem list in the EMR, this unconfirmed diagnosis was “perpetuated” by future medical providers that the patient saw in 85% of instances.4 While this data is not randomized, nor does it contain a control group, it highlights some of the misconceptions about HCV diagnosis amongst general medical providers and mental health providers that may order HCV ab tests as part of their practices. Unconfirmed diagnoses of HCV can lead to stigmatization, receipt of unnecessary medical interventions, and avoidance of important medical interventions (e.g., statin use). This may be even more impactful as the CDC’s birth cohort screening recommendations trigger more screening. Based on all available evidence, our Hepatitis C Expert Work Group agrees that this measure is of great value. Ultimately, by not recommending Measure #0393, there will be no NQF-endorsed measure to promote use in national measurement programs. We hope that these explanatory comments better clarify the importance of confirming Hepatitis C viremia after initial testing for the HCV antibody to confirm a diagnosis of HCV infection. We respectfully request that the SC reconsider recommending this valuable measure to improve the quality of care provided to patients with Hepatitis C. References: 1 Speers S, Klevens RM, Vonderwahl C, Bryant T, Daniloff E, Capizzi J, Poissant T, Roome A. Electronic matching of HIV/AIDS and hepatitis C surveillance registries in three states. Public Health Rep. 2011 May-Jun;126(3):344-8. 2 Moorman AC, Gordon SC, Rupp et al. Baseline Characteristics and Mortality Among People in Care for Chronic Viral Hepatitis: The Chronic Hepatitis Cohort Study. Clin Infect Dis. 2012 Oct 19. [Epub ahead of print]. 3 Sabrina A. Assoumou MD, Wei Huang MA, Benjamin P. Linas, MD MPH. [Poor] Quality of Hepatitis C care at an urban tertiary medical center. Study conducted at Boston Medical Center. Outcomes: Centers for Medicare & Medicaid (CMS)-defined HCV quality indicators introduced in 2008: HCV RNA testing, Genotype testing, Hep A & Hep B vaccinations. Poster presentation from the Infectious Diseases Society of America (IDSA) meeting, 2012. 4 Yang Liu, BA, Renee H. Lawrence, PhD, Brook Watts, MD, Yngve Falck-Ytter, MD, Amy Hirsch, PharmD. Understanding the Care Gap and Missed Opportunities for Hepatitis C Confirmatory Viral testing. Poster presentation from the Society of General Interal Medicine (SGIM) meeting, 2012. Committee Response: The Committee agreed that the comments had merit. The purpose of viral load testing is to identify those individuals who need to be linked to a provider who is able to provide counseling for their hepatitis C and potential treatment and to differentiate from the individuals who have resolved the infection. Avoiding inappropriate intervention in 15-20 percent of patients that spontaneously resolve the Hepatitis C infection is important. The Committee agreed to reconsider the measure. The measure developer is encouraged to update the 15 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia measure submission with all relevant information for the Committee to consider. The Committee will evaluate the measure on the December 5 conference call. The final recommendation will be included in the addendum to the main report and has been removed from this current report. Re-vote following Public and Member Comment Following the Public and Member Comment period of the draft report, the Committee decided to reconsider the measure. Importance to Measure and Report: The measure met the Importance criteria (1a. High Impact: 1b. Performance Gap, 1c. Evidence) 1a. Impact: H-5; M-8; L-0; I-0; 1b. Performance Gap: H-7; M-6; L-0; I-0; 1c. Evidence: Y-13; N-0; I-0 Rationale: • CDC received 20,285 reports of HCV infection from state and local surveillance programs in 2006-2007, 47 percent of those reports had no positive result for HCV RNA. • A study conducted at Boston Medical Center showed a decline in the confirmation of HCV viremia from 73 percent (2005-2007) to 63 percent (2008-2011). 2. Scientific Acceptability of Measure Properties: The measure met the Scientific Acceptability criteria (2a. Reliability – precise specifications, testing; 2b. Validity – testing, threats to validity) 2a. Reliability: H-4; M-9; L-0; I-0 2b. Validity: H-3; M-10; L-0; I-0 Rationale: • The measure was only tested in EHRs. • The kappa for the measure result comparing the automated results from the EHR and the visual inspection of the record was 0.948. • The measure was assessed using face validity (an expert panel of 22 members) with a mean rating of 4.92 out of 5. 3. Usability: H-4; M-9; L-0; I-0 (Meaningful, understandable, and useful to the intended audiences for 3a. Public Reporting/Accountability and 3b. Quality Improvement) Rationale: • This measure has been in used in PQRS since 2008 though not publicly reported. 4. Feasibility: H-6; M-7; L-0; I-0 (4a. Clinical data generated during care delivery; 4b. Electronic sources; 4c.Susceptibility to inaccuracies/ unintended consequences identified 4d. Data collection strategy can be implemented) Rationale: • This measure is specified for use in EHRs. 5. Related and Competing Measures 16 0393 Hepatitis C: Testing for chronic hepatitis C – Confirmation of hepatitis C viremia • No related or competing measures noted. Steering Committee Recommendation for Endorsement: Y-13; N-0 17 1 Headquarters 500 Midway Drive Mount Prospect, Illinois 60056-5811 USA Main Telephone +1 847 827-6869 Customer Service +1 847 827-6888 Facsimile +1 847 827-6886 Email info@sccm.org www.sccm.org April 19, 2013 NQF Board of Directors National Quality Forum 1030 15th Street NW Suite 800 Washington, DC 20005 Dear Directors: I understand that one appeal with seven co-signatories was received during the appeal period for Infectious Diseases #0500, Severe Sepsis and Septic Shock: Early Management Bundle (#0500). I appreciate the opportunity to address the concerns of the appellants on behalf of the Society of Critical Care Medicine (SCCM), a co-founder of the Surviving Sepsis Campaign (SSC) with support from the measure developers. Support also comes from several important, noteworthy cosignatories listed in this communication. Some of the endorsers represent large hospital systems that are already implementing these measures to benefit the patients and families they serve. Together we endeavor to represent the overwhelming coalition of interests assembled during the Consensus Measures Development Process. As you know, the Consensus Measures Development Process is designed to call for input and carefully consider the interests of stakeholder groups from across the healthcare industry. To that end, we wish to begin by noting that in member voting the measure has secured the support of: · · · · · · · 100% of the Consumer Measure Council 100% of the Health Plan Measure Council 82% of the Health Professional Measure Council 100% of the Purchaser Measure Council 100% of the QMRI Measure Council 100% of the Supplier/Industry Measure Council 29% of the Providers Measure Council The measure had an overall Member Council approval rating of 87%. This consensus is more than sufficient grounds to continue to endorse the measure despite the concerns raised by the appellants. Clarifying the value of the measure to this group, however, would be beneficial to develop further consensus. We think this letter will explain why the appellants’ suggestions cannot be adopted at this time. We believe that the appellants’ unfamiliarity with the Consensus Measures Development Process PDF created with pdfFactory Pro trial version www.pdffactory.com 2 has led them to prematurely request consideration of strategies that cannot be simply inserted into the process now. We will address those issues here and defer to Dr. Rivers and Dr. Townsend to address the specific scientific arguments presented in the appeal in a separate letter. 1. The appellants misunderstand the Consensus Measures Development Process and recommend a suite of ideas that could not simply be adopted by the Board without proper review for scientific acceptability, usability, feasibility, reliability, and validity – a standard the therapies they recommend could not meet in any case because they have never been tested in performance improvement. The appellants seek to appeal the measure asserting, in part, that a host of new strategies to monitor volume status exists and that they are being tested now in clinical trials such as ARISE, PROMISE, and PROCESS. While the outcomes of these trials are unknown, the Consensus Measures Development Process has demonstrated that: 1) a performance gap exists now as regards sepsis therapies; 2) a scientific basis for the therapies in #0500 exists; 3) when tested in performance improvement trials, the measure itself can be applied reliably; 4) when tested in performance improvement trials, the measure itself can be applied with validity; and 5) in 210 hospitals of all types and sizes across the country, the measure is usable and feasible. The trials underway could show a benefit to the modalities the appellants prefer in severe sepsis and septic shock. However, the time course to publish mature clinical science from these trials in the literature is not short. Once the trials conclude—which they have not—the primary data must be analyzed, papers must be written for submission to journals, peer review must be completed, revisions to manuscripts will be required. This process may take 1-2 years from the conclusion of the ongoing trials. Even after publication, left wholly unconsidered by the appellants is that the clinical science will then need to be incorporated into new measures for testing, followed by a prolonged period of performance improvement and, if the resulting data show the metric is sound, it may then be ready to be brought before NQF. The burden for measure development is high. The hypothetical new measure must demonstrate that it is: 1) reliable in performance; 2) valid as a measure; 3) usable and feasible in hospitals. Four years were needed to generate the data that the SSC relies on in advancing #0500. The appellants must expect years of meticulous data collection, analyses, and efforts to publish those results as a precursor to establishing a new valid and reliable performance measure. To wit, #0500 itself was nearly rejected before the SSC was able to bring data on the reliability and validity of the measure to bear. Therefore, the appellants’ request that alterations be made in the start time (“time zero”) of the measure, that some items simply be deleted or, in the alternative, changed to read that “the resuscitation is objectively monitored using a method such as lactate clearance, ScvO2 monitoring or CVP monitoring,” cannot be done. These suggestions speak to an PDF created with pdfFactory Pro trial version www.pdffactory.com 3 entirely different measure from #0500 that would require its own supporting data to enter the Consensus Measures Development Process. An estimate of up to 5 years to develop such a performance metric is not unreasonable. Case in point: one of the therapies that the appellants wish to see available, “lactate clearance,” was first published in 2010 after a randomized control trial established its efficacy.1 However, 3 years later no data exist on using this therapy as a performance metric, and it therefore cannot demonstrate the requirements that the Consensus Measures Development Process demands. Were the appellants to be successful in overturning #0500, the demonstrated performance gap would continue and the proven adverse impact on patients would continue unchecked. 2. The present measure allows for innovation, testing, and development of new strategies, but preserves a basic public health function that any competing strategy cannot fulfill. While any competing measure is far off in terms of fulfilling the requirements for the Consensus Measures Development Process, the present measure represents no adverse impact on the appellants and represents a floor of measurement, not a ceiling. As endorsed by the Board, #0500 does not restrict providers from trial of therapies they may consider to be better. Providers are free to use any other method of volume assessment in addition to the placement of a central venous catheter. The placement of the catheter itself in these severely ill patients should not be controversial: nearly all patients with the underlying condition, sepsis-induced hypoperfusion (hypotension, lactate > 4 mmol/dL), will need to have a central venous catheter placed early in their therapy, either due to the likely need to administer vasopressors or to provide multiple simultaneous therapies. When placed, the catheter is almost always positioned in the superior vena cava to prevent further infection in these already-infected patients. For example, in the trial the appellants cite regarding use of the novel therapy “lactate clearance,” all patients received central venous catheters. Thus, assessing central venous pressure (CVP) is but one more variable for providers’ consideration. While the appellants are concerned that there are no targets specified for CVP or central venous oxygen assessment (ScvO2), this permits the provider to interpret the data alongside any other information gathered. The provider may still assess, via minimally invasive or non-invasive technique, other estimates of volume status. We encourage this strategy. In the meantime, patients should not be deprived proven therapies embodied in #0500. 1 Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;24;303(8):739-746. PDF created with pdfFactory Pro trial version www.pdffactory.com 4 Considering #0500 as a basic public health safeguard is essential and its approach is utilitarian. The developer has been successful in demonstrating that a large number of hospitals can apply this measure. Every hospital in the country, at least in principle, has the capacity to secure central venous access.2 It is equally important to consider that the other volume assessment modalities entertained by the appellants (“bioimpedence, serial ultrasonographic assessments”) meet no such standard. Only some large community hospitals or academic medical centers will have the equipment available. Even those hospitals have not demonstrated the operational capacity to deploy the therapies within the first hours of care. The appellants cannot show the Board that the 5000 hospitals across the country have such equipment ready to use. On the other hand, every hospital in the country at least has a central line kit in supply. Therefore, a successful appeal would remove the floor standard set by #500 as a public health safeguard and replace it with either nothing or wholly uncertain care. 3. The Consensus Measures Development Process is designed to handle complexity in measure development and ensure a proper result. Once a measure is developed, a “measure steward” is identified. That steward must submit yearly reports on the measure. In this instance, the SSC and its representatives have served this role. The steward may initiate an “ad hoc review” at anytime in the event that credible evidence emerges that challenges the measure or requires an update of the measure. In addition, any party (including the appellants) can also request an ad hoc review at any time to have new evidence considered under the standards of the Consensus Development Measures Process. Beyond those safeguards, the measure will be comprehensively reviewed every 3 years and competing measures will be evaluated for readiness for deployment under NQF’s Consensus Measures Development Process Measure Maintenance Program. Given the long gap (years) between the emergence of the clinical trials establishing the value of the newer modalities and conducting clinical performance initiatives that will provide the foundation for a new valid and reliable measure, these standard windows for review are entirely appropriate to address any needed revisions in the current measure. 2 For those critical access hospitals that may be unable to comply due to a lack of available skilled providers, third party payers (such as CMS) typically exclude critical access facilities from financial penalty for value-based purchasing metrics. PDF created with pdfFactory Pro trial version www.pdffactory.com 5 The SSC is a trusted steward and reviewer of available evidence as the only guidelines that rank the quality of the evidence in the literature were advanced by the SSC. Those guidelines have always been developed with the inclusion of most of the appellants. The SSC will gladly work with these groups again in the event that the literature changes and requires an update of this measure. This spirit of cooperation among the societies has been critical to bringing the present measure forward as a mature measure with sufficient data to justify its deployment. 4. The appeal does not meet NQF threshold standards for consideration because the appellants have not demonstrated a direct and material adverse impact on their interests as required by the appeal rules. The appellants have not shown a direct and material adverse impact to adoption of the measure. The letter states only that the appellants have concerns that the use of a central venous catheter would have “unintended deleterious effects,” but these deleterious effects are never specified. The letter contends that central venous catheter-guided fluid management, and CVP in particular, have not been demonstrated to be superior to other methods, that targets are not specified, and that there is allegedly equipoise on the question of optimal volume assessment and fluid management. None of these concerns demonstrates a “direct and material harm” to the appellants. At best, these concerns suggest the therapies in #0500 are “not superior” to other therapies. Again, the measure is a floor, not a ceiling. Thus, there is no adverse impact to the appellants under the NQF rules for appeals.3 5. Several of the appellants have already endorsed the measure exactly as specified as a bundle in their endorsement of the 2012 Surviving Sepsis Campaign Guidelines in February of this year. We respect the rights of the appellants to appeal for modification of the measure. We suspect that they do not understand that a successful appeal will result in non-adoption of any measure, not in modifications along the lines they suggest. The appellants write that they “strongly support the development of performance measures targeting severe sepsis and septic shock” and several are co-sponsors of the 2012 Surviving Sepsis Campaign Guidelines. Co-sponsorship means that their organization has endorsed the evidence-based rankings in the guidelines. The guidelines 3 In addition, the appellants represent provider organizations. The measure is a hospital-level measure. The NQF standard of direct adverse impact is therefore not present inasmuch as any theoretical (not actual) harm would be indirectly related to the provider and directly related to the hospital. PDF created with pdfFactory Pro trial version www.pdffactory.com 6 paper specifically includes the composite measure elements specified in #0500 verbatim.4 To conclude, the appellants seek modification rather than appeal; however, the appellants have not (and cannot at this time) show sufficient data to accept their proposed modifications through the Consensus Measures Development Process. 6. The issues raised by the appellants have been raised already during the proper proceedings in the Consensus Measures Development Process and rejected. The appellants join the Consensus Measures Development Process late with regard to their concerns; however, this does not mean that each of the issues raised in their letter has not already been addressed. Specifically, the Infectious Diseases Steering Committee twice voted on the scientific acceptability of the evidence and twice approved the measure. Issues about central venous pressure monitoring were duly considered. Even after approval in the Infectious Diseases Steering Committee, the Greater New York Hospital Association (GNYHA) raised these scientific issues again at the level of the Consensus Standards Approval Committee. The issues they raised, which substantially mirror those that the appellants now raise, were turned aside because the evidence basis exists to support the measure the Board has endorsed.5 Continuing to raise the issue is not productive for a measure that has been scientifically validated and that remains statistically reliable and valid. Finally, abandoning #0500 through a successful appeal would have measurable consequences. The SSC data demonstrate when more than 200 hospitals engage in this composite measure, a 7% absolute risk reduction (ARR) in mortality is achieved.6 Conservative estimates suggest there are 750,000 cases of severe sepsis or shock annually, with an average mortality rate of 29% and resulting in 217,500 deaths annually.7 The 7% ARR as shown by the SSC would therefore save 15,225 lives each year if all hospitals were accountable to this metric. Stated differently, #0500 stands to save 1,269 lives per month if this measure becomes a national standard. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. 4 5 Please see Dr. Rivers’ and Dr. Townsend’s peer review of the available unpublished GNYHA data in their separate letter of reply. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367-374. 6 Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. 7 PDF created with pdfFactory Pro trial version www.pdffactory.com 7 In conclusion, for the reasons set out above, SCCM, the measure developers, the cosignatories on this letter, and leadership from the Surviving Sepsis Campaign continue to support #0500. We believe the Member Councils support the measure for the same reasons. This basic public health measure stands to advance care for thousands of patients who develop severe sepsis each year and provides a floor of standards for their care while not prohibiting other efforts. Thank you for your continued consideration. Sincerely, Carol L. Thompson, PhD, ACNP, CCRN, FCCM President, Society of Critical Care Medicine Professor, Critical Care Nursing University of Tennessee Health Science Center Memphis, Tennessee Cosignatories Adrienne Kirby, PhD, FACHE President and Chief Executive Officer Cooper University Hospital Camden, New Jersey Jean-Daniel Chiche President, European Society of Intensive Care Medicine (Co-founder organization Surviving Sepsis Campaign) Reanimation Medicale – Hospital Cochin Paris, France PDF created with pdfFactory Pro trial version www.pdffactory.com 8 Michelle B. Schreiber, MD Associate Chief Quality Officer Senior Vice President Clinical Transformation and Clinical Information Technology Integration Henry Ford Health System Detroit, Michigan David A. Relman, MD President Infectious Diseases Society of America Arlington, Virginia Jed Weissberg, MD SVP, Hospitals, Quality and Care Delivery Excellence Kaiser Foundation Health Plans and Hospitals Kaiser Permanente Oakland, California Martin E. Doerfler, MD SVP, Clinical Strategy and Development Center for Learning and Innovation North Shore LIJ Health System New Hyde Park, New York Gordon C. Hunt, MD, MBA Senior Vice President/Chief Medical Officer Sutter Health Sacramento, California PDF created with pdfFactory Pro trial version www.pdffactory.com April 22, 2013 NQF Board of Directors National Quality Forum 1030 15th Street NW Suite 800 Washington, DC 20005 Dear Directors: On behalf of the Institute for Healthcare Improvement (IHI), I am writing in regards to Infectious Diseases #0500, Severe Sepsis and Septic Shock: Early Management Bundle (#0500). It is my understanding that the Society of Critical Care Medicine (SCCM) and co-signatories have submitted a letter to NQF concerning an appeal raised by seven co-signatories. IHI agrees with SCCM that #0500 is an important bundle that will improve the care and outcomes of patients who develop severe sepsis. Consequently, IHI is pleased to join SCCM, its cosignatories, and other individuals and stakeholders in lending its support of #0500. Sincerely, Don A. Goldmann, M.D. Chief Medical and Scientific Officer, Institute for Healthcare Improvement Clinical Professor of Pediatrics, Harvard Medical School Professor of Immunology and Infectious Diseases, and Epidemiology, Harvard School of Public Health