Physical Metallurgy of Solder
advertisement

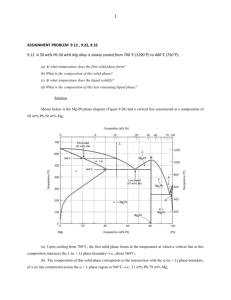
CHAPTER 1 PHYSICAL METALLURGY OF SOLDER SYSTEMS J. F. Roeder, M. R. Notis and H. J. Frost Introduction Man's use of Pb and Sn spans well over a millennium [I]. These metals have been exploited for their low melting points and are particularly useful for soldering applications. Klein-Wassink [2] points out that the Romans used a PbSn alloy for solder joining of pipes. The usefulness of this alloy system has not diminished in the following centuries; today Pb-Sn alloys are used extensively in electronic solder joint applications [2-41. A number of other alloy systems find use in electronic packaging; however, this chapter focuses primarily on the Pb-Sn system and its ternary extensions. This chapter provides a brief overview of the physical metallurgy of solder systems relevant to the issues of mechanical properties and the actual solder joint behavior described in the following chapters of this book. The present discussion is limited to microstructural evolution resulting from solidification and as a result of thermal treatments excluding the effects of stresses induced as a result of thermal cycling. Chapters 3, 5 and 6 describe the significant effects of stress induced by thermal cycling on the microstructure itself. Many of the phenomena occurring in the Pb-Sn eutectic system are not peculiar to that system, and the fundamental aspects may be extended to other eutectic systems of interest. Solidification behavior of the Pb-In system, which contains two peritectic reactions, is also described and contrasted to that of the Pb-Sn system. To illustrate how a solder joint might undergo important thermal fluctuations, consider as an example the hermetic sealing of electronic package lids [5]. A hierarchy of solders with different melting points are employed to produce the final product. A high melting point solder, such as 95Pb-5Sn (wt.%), might be used in the component itself; a solder of lower melting point, such as 85Pb-15Sn, might be used for hermetic sealing; and finally, a solder of even lower melting point, such as eutectic Pb-Sn, might be used to joint the sealed package to the printed circuit board. In this sequence, the joining cycles of the lower melting point solders act as high-temperature heat treatments for the 95Pb-5Sn solder. Service conditions can also involve significant thermal excursions of a cyclic nature; for example, -55" to 125°C in aircraft 161 or involving long hold times during power-on with temperatures reaching up to 80°C [7]. We emphasize that most solder alloys are in high-temperature service from the moment of fabrication to the end of useful life; even room temperature is 65% of the absolute melting point for the eutectic Pb-Sn alloy. Thus, the microstructures of solder alloys can undergo significant changes as they attempt to evolve toward equilibrium in response to thermal conditions. Now that some practical conditions causing microstructural evolution have been identified, the remaining discussion first considers microstructures resulting from solidification and then turns to the various precipitation phenomena that can occur as these structures experience thermal fluctuations. Both continuous (discrete) [8, 91 and discontinuous (cellular) [lo-191 reactions are examined. In Solder Mechanics - A State of the Art Assessment Edited by Darrel R. Frear, Wendell B. Jones and Kenneth R. Kinsman The Minerals, Metals & Materials Society, 1991 Solder Mechanics 2 each case, important effects of the microstructure on mechanical properties are highlighted. Finally, the effect of ternary elements on soldification and precipitation are described. Phase Equilibria and SolidifmationMicrostructures in Binary Solder Systems Pb-Sn SYSTEM The equilibrium Pb-Sn system [71 is characterized by a single eutectic reaction that occurs a t 183OC as shown in Figure 1. Within this system, near equilibrium microstructures resulting from solidification can be divided into three classes based on their bulk composition: eutectic, hypoeutectic, and hypereutectic. Hypoeutectic and hypereutectic alloys refer to those alloys with Sn contents below that of the eutectic composition (61.9 wt.% Sn) and above the eutectic composition, respectively. Binary Pb-Sn solder microstructures have been described recently by Klein-Wassink [2] and by de Kluizenaar [20]. We first consider eutectic solidification, followed by the off eutectic alloys. Weight Percent Tin 0 20 10 , .....,I. , 30 . ., - 40 , 50 , , ,. . .. . , , , , 60 . I , . . 70 , ., ./ ., .. 90 80 I-.! 100 - , 1327 502.C 3 0 - , 0 ...., .. ... Ib , .. . , ..... 20 0 , , ., , $0 d0 Atomic Percent Tin Pb Figure 1 . . . , , . .i0 $0 80 90 100 Sn Pb-Sn equilibrium phase diagram [71. Equilibrium solidification of a Pb-Sn alloy with the eutectic composition follows the eutectic reaction Liquid + a-Pb + P-Sn Chapter 1: Physical Metallurgy of Solder Systems 3 where the homogeneous liquid solution is stable above 183OC and separates into two solid phases, a-Pb (FCC) and P-Sn (BCT), which are stable below 183OC. The microstructure formed by this reaction consists of alternating lamellae of a-Pb (light) and P-Sn (dark) as shown in the backscattered electron image in Figure 2. Each region in which the lamellae are oriented in the same direction is tenned a "colony" (an analogous term to "grain" that describes a region of uniform crystallographic orientation in single-phase alloys). Individual lamellae grow by rejection of solute (Sn for the a-Pb lamellae and Pb for the P-Sn lamellae) from the liquid ahead of one lamella into the liquid ahead of its neighbor as the growth front advances [21]. The interlamellar spacing varies with cooling rate due to a balance of free energy contributions to the driving force for the eutectic reaction: that of redistribution of solute a t the advancing front by diffusion and interfacial energy between lamellae. In practice, slower cooling (where the reaction proceeds more slowly) results in wider interlamellar spacing as shown by the following relationship [21]: where s is the interlamellar spacing and R is the velocity of the reaction front. Colony size also varies with cooling rates due to the relationship of nucleation to undercooling. Faster cooling enhances the number of colony nuclei formed, and therefore colony size increases with slower cooling rates [22]. At sufficiently fast cooling rates, the eutectic structure loses its lamellar character as shown in Figure 3. Figure 2 Backscattered electron Figure 3 micrograph of slowly cooled eutectic Pb-Sn alloy showing a-Pb (light) and P-Sn (dark) lamellae. After de Kluizenaar [201; courtesy of Wela Publications, Ltd. Backscattered electron micrograph of rapidly cooled eutectic Pb-Sn alloy showing a-Pb (light) and P-Sn (dark) non-lamellar structure. After de Kluizenaar [201; courtesy of Wela Publications, Ltd. Both colony size and interlamellar spacing are important factors in determining mechanical properties of the eutectic alloy. It has recently been demonstrated that isothermal fatigue life decreases with increasing colony size [22]. In addition, the tensile strength of unidirectionally solidified eutectic alloys [23] and tensile strength and ductility of randomly solidified eutectic alloys 1221 vary with Solder Mechanics 4 interlamellar spacing. It is therefore important to understand these parameters in the actual solder joint when applying the constitutive models discussed in detail in this book. An additional phase appears in the solidified microstructure of off eutectic alloys. This phase is termed the proeutectic (meaning "before eutectic") phase because it forms on cooling before the eutectic reaction temperature is reached. In hypoeutectic alloys, the proeutectic phase is primarily a-Pb, as shown in Figure 4. Dendrites of the proeutectic phase begin to form when the liquidus line is crossed for the bulk composition of the alloy of interest. The composition of the first solid to form is given by the end of the tie-line opposite that defined by the liquidus temperature for the bulk composition of the alloy [24]. As cooling continues, the composition of the solid phase changes as defined by the solidus end of the tie-line. At bulk compositions above the solubility limit of Sn in Pb (19.2 wt.%), a fraction of liquid remains in the melt when the eutectic temperature is reached. This remaining liquid is then converted to solid phases through the eutectic reaction. The resulting microstructure consists of proeutectic a-Pb surrounded by the eutectic reaction product. In hypereutectic alloys, solidification is directly analogous to hypoeutectic alloys except that the proeutectic phase is P-Sn. Figure 5 shows a typical hypereutectic alloy in the as-solidified condition. Figure 4 Backscattered electron micrograph of as-solidified hypoeutectic Pb-Sn alloy showing proeutectic a-Pb dendrites (light). After de Kluizenaar 1201; courtesy of Wela Publications, Ltd. Figure 5 Backscattered electron micrograph of as-solidified hypereutectic Pb-Sn alloy showing proeutectic P-Sn dendrites (dark). After de Kluizenaar [201; courtesy of Wela Publications, Ltd. Pb-In SYSTEM The equilibrium phase diagram for the Pb-In [25] system differs markedly from that of the Pb-Sn system because it contains two peritectic reactions as shown in Figure 6. Also, the solid solubility for In in Pb is very large (> 50 wt.%). Given the wide range of solubility for In in Pb coupled with the peritectic reaction, Pb-rich alloys generally consist solely of primary Pb dendrites. For more In-rich alloys, such as 50Pb-50In, i t is possible to reach the peritectic reaction Chapter 1: Physical Metallurgy of Solder Systems Liquid + Pb + a with sufficiently rapid cooling. A peritectic reaction product would normally form a n envelope around the primary Pb dendrites. However, in the 50Pb-50In alloy, the predominant constituent in the microstructure is the primary Pb dendrites. In this case, the peritectic reaction product is contained in the pockets of liquid remaining when the solidification process is complete. M e ~ g h tP e r c e n t L e a d 0 350; 10 , ..., In Figure 6 30 20 1 , 40 , , , 50 , , 60 -I1 -- 70 80 gO 100 + . Atomic P e r c e n t Lead Pb Pb-In equilibrium phase diagram L251. MicrostructuralEvolution in the Solid State Discussion thus far has concentrated on as-solidified microstructures. The microstructure of such a n alloy may evolve further toward equilibrium 1261 under service conditions involving elevated temperature holding time or even a t room temperature, which, as pointed out earlier, is a significant fraction of the melting point of the alloys under consideration. Solid-state diffusion a t these temperatures leads to dramatic changes in microstructure. COARSENING The driving force for solid-state microstructural evolution stems from interfacial surface energy. A given microstructure attempts to minimize its total interfacial energy by reducing the interfacial area within it [271. In a single-phase alloy, this leads to grain growth. In the case of a multiphase alloy, the feature sizes of the as-solidified alloy increase and may change character in a process known as Solder Mechanics 6 coarsening. While coarsening occurs in Pb-Sn alloys a t room temperature over extended periods of time, the process is accelerated at elevated temperature due to increased diffision. This is illustrated in Figure 7 reproduced from KleinWassink [2] where a 65Pb-35Sn alloy is shown in the as-cast condition and after an isothermal heat treatment of 300 h a t 155OC. Coarsening also occurs in association with fatigue crack propagation during thermal fatigue. Chapter 5 describes this effect. Figure 7 Light optical micrographs showing coarsening in 65Pb-35Sn alloy: (A) as-cast, (B) after 300 h a t 150°C. The a-Pb phase is dark, P-Sn phase is light. After KleinWassink [21; courtesy of Electrochemical Publications, Ltd. PRECIPITATION Precipitation reactions occur readily in the a-Pb phase in the Pb-Sn system due to its large solubility for Sn at high temperatures. The equilibrium phase diagram in Figure 1shows that the solubility of Sn and Pb is 19.2 wt.% at 183OC. With decreasing temperature, the solubility limit for Sn drops considerably; at 20°C, the solubility limit for Sn is -2 wt.%. Careful inspection of the proeutectic a-Pb dendrites (dark) in the light optical micrograph shown in Figure 8 reveals 0-Sn (light) precipitates dispersed throughout the phase. These precipitates develop because the a-Pb formed during solidification has a composition rich in Sn compared to that which is stable at low temperature. Figure 8 Light optical micrograph of slowly solidified 65Pb-35Sn alloy. a-Pb phase is dark, PSn phase is light. After Klein-Wassink [21; courtesy of Electrochemical Publications, Ltd. Chapter 1: Physical Metallurgy of Solder Systems Precipitation may also occur due to a classical solution treatment and aging sequence [28]. For example, consider an alloy of 5 wt.% Sn. If this alloy is heated above -lOO°C, it crosses the a-(Pb1a)-Pb+P-Snsolvus line and enters the single phase a-Pb region where the alloy becomes single phase, given the required time interval (solution treatment). Rapid cooling results in a supersaturated solid solution a t temperatures below the solvus. During subsequent aging, precipitation of P-Sn occurs to relieve the metastable state of supersaturation. Continuous Precipitation Both continuous (discrete) [8, 91 and discontinuous (cellular) precipitation [lo-191 of P-Sn has been observed in Pb-rich alloys. Frear et al. [9] recently showed that the nature of the precipitation reaction in a 95Pb-5Sn alloy depends upon the cooling rate from the solution treatment temperature. Discrete precipitation is favored under rapid cooling while cellular precipitation was found to occur for slower cooling rates. Discrete P-Sn precipitates are shown in the transmission electron microscope (TEM) image in Figure 9. These precipitates were found t o occupy a (111)type habit plane with the orientation relationship (1ll)pb N ( 0 1 0 ) ~ ~ and [Olllpb // [OO1lsn. Figure 9 Transmission electron microscope bright field image of P-Sn continuous precipitates in a 5Sn-95Pb alloy. Courtesy of Frear et al. [91. The precipitates that often occur in the proeutectic phase upon cooling described earlier (Figure 8) appear to be of the continuous type. This most likely occurs because the Sn content in the dendrites is relatively high, and therefore the a(Pbla)-Pb+P-Sn solvus is crossed a t a correspondingly high temperature on cooling. At higher temperatures, volume (lattice) diffusion is enhanced Solder Mechanics 8 compared to grain boundary diffusion, and discontinuous precipitation occurs via grain boundary diffusion (this is discussed later). Discontinuous Precipitation The precipitation reaction in low-Sn alloys usually proceeds as discontinuous precipitation in which cells of lamellae of the Sn phase nucleate a t the grain boundaries and grow into the matrix. This process, also known as cellular precipitation, has been well studied in Pb-Sn alloys by Turnbull and Treaftis [ l l , 121 and Tu and Turnbull [13-171. Other studies are summarized elsewhere [19, 29-33]. The process begins with nucleation of particles of the Sn phase along the grain boundaries of the original solid solution matrix. These particles typically have a particular crystallographic orientation with respect to one or the other of the parent grains. Tu and Turnbull [I31 reported the same relationship as observed by Frear et al. [9] for continuous precipitation: ( 1 l l ) p b N ( 0 1 0 ) and ~~ [ O l l l p b N [OO1]sn. The Sn particles then grow as a colony of lamellae in the direction of the grain with which they did not have a special orientation relationship, accompanied by the migration of the grain boundary. This behavior evidently minimizes the energy of the Pb-Sn interfaces, and it allows the diffusion required to gather the Sn to occur along the high-diffusivity path of the grain boundary. The discontinuous precipitation process is generally favored over continuous precipitation under conditions in which the availability of the highdiffusivity path is important. This is usually a t relatively low temperatures (compared to the melting temperature) because the ratio of grain-boundary diffusivity to lattice difisivity increases dramatically as the temperature is lowered. The discontinuous precipitation reaction therefore becomes more evident for lower Sn concentrations, because for a given slow cooling rate, the precipitation occurs a t higher temperatures for higher Sn concentrations. For the alloy of Pb-3.5 wt.% Sn the transformation a t room temperature produces an interlamellar spacing that is easily observed optically, and a transformation rate that is slow enough for convenient metallography. Figure 10 shows some cells of Sn lamellae during the early stage of growth. For higher Sn concentrations, the interlamellar spacing is finer, and can become difficult to resolve optically. Figure 11 shows how the cells grow out from grain boundary facets to consume the matrix for the Pb-5 wt.% Sn alloy. Figure 10 Microstructure of Pb-3.5 wt.% Sn aged a t 23'C after solution annealing. The individual Sn lamellae are visible growing out from the grain boundaries in cells. Chapter I : Physical Metallurgy of Solder Systems Figure 11 Micrographs of Pb-5 wt.% Sn alloy aged a t -16°C for various times after solution annealing. At this magnification, the lamellae of the Sn phase are too small to resolve. The series shows how the cells (which appear dark) nucleate a t the grain boundary facets and grow out to consume the matrix. A) Aged 16 hrs, transformed fraction = 5%, B) Aged 30 hrs., transformed fraction = 24%,C) Aged 72 hrs., transformed fraction = 58%, D)Aged 97 hrs., transformed fraction = 85%. The progress of the precipitation transformation is usually described in terms of the Avrami equation that gives the volume fraction transformed f as a function of time t based on random nucleation followed by growth to impingement: where the constant K depends on temperature, and n is an exponent that is typically between 1 and 4 1281. Experimental measurements of K and n allow quantitative calculations of the extent of the phase transformation. Care must be taken, however, to understand how K and n depend on the microstructure and conditions to avoid invalid extrapolations. The kinetic constant K depends on both the cell boundary velocity and the nucleation rate or the density of nucleation sites. The value of n depends on both the nucleation conditions and the growth geometry or dimensionality. For continuous nucleation (at random locations, a t a constant rate per unit of untransformed volume) of spherical regions (cells), with a constant growth rate, we find that n = 4. For simultaneous nucleation (at t = 0, a t random sites), with cell growth as spheres, we find that n = 3. For twodimensional models (growing circles), continuous nucleation gives n = 3, and simultaneous nucleation gives n = 2: one-dimensional models give n = 2 and n = 1,respectively. Lutender [33,341 found n = 2 for alloys with 2.0 to 5.0 wt.% Sn. Solder Mechanics 10 Turnbull and Treaftis [12] found n = 3 for alloys with 6.7 to 10.5 wt.% Sn. The difference between these results is not well explained. It may be due to the difference in composition, or perhaps to a difference in grain size and specimen size. Lutender used bulk samples with a n initial grain size of 0.5 to 1.0 mm. Turnbull and Treaftis used wires that were cast in small pyrex tubes with a n inside diameter of about 0.5 mm. The value of n = 2 is consistent with a onedimensional model in which cells grow out from grain-boundary facets in such a way that their volume increases (on average) only linearly with the distance the cell boundary moves. In general, we must expect K to depend on the grain size because i t should depend on the density of boundary nucleation sites. We may also expect both K and n to depend on the ratio of grain size to sample size, particularly if surface nucleation is important. A quantitative evaluation of the transformation kinetics has been experimentally achieved for a range of compositions. Figure 12 shows this in terms of a timetemperature-transformation curve. The temperature for the maximum transformation rate increases with increasing Sn content. For 3 to 5 wt.% Sn, the maximum rate is near room temperature; for 2 wt.% Sn, i t is a t a lower temperature. For practical purposes, transformation in the 2 wt.% Sn alloy requires many days, but in the 5 wt.% Sn alloy a t 20' to 40°C, i t is completed within one day. A more fundamental treatment could be derived from measurements of the cell boundary velocity as a function of composition and temperature, coupled with a quantitative model of the nucleation behavior. A series of papers [lo, 35-42] have attempted to construct theoretical models to predict cell boundary velocity and the interlamellar spacing. Zener's early treatment [35] of the eutectoid transformation (such as austenite to pearlite in steels) first recognized that the available chemical-driving free energy must be spent on both the creation of new interfaces between the lamellae of the new phases, and on driving the diffusive fluxes to achieve the phase separation. On this basis, he derived a n expression for the velocity of the transformation front v as a function of the interlamellar spacing S of the form v = (kinetic constants) - I--- ( Sgin) where S,;, is the minimum spacing that could be obtained if all the driving free energy went into interface creation. The maximum velocity occurs for a particular spacing (at S = 2 Sminin the equation shown), but there are other combinations of velocity and spacing that are admissible solutions. Turnbull [lo] adapted Zener's theory for the case in which the diffusion is limited to the grain boundary or interphase interface, as is expected in dilute Pb-Sn alloys. In this case, a n addition factor of (11s) must be included in the equation for velocity. Cahn [36] pointed out that the relationship between velocity and spacing depends on another kinetic parameter that describes the mobility of the boundary of the reaction. The driving free energy must also supply the free energy that is dissipated in the diffusive process of boundary migration, in addition to that dissipated in diffusion along the boundary or stored in interlamellar interfaces. Finally, there is a component of the driving free energy that is not dissipated by the process, but is stored in the supersaturation that remains in the phases produced by the precipitation. Hillert [38-401 has developed more detailed models of the process based on the four components of free energy loss or storage. In his Chapter 1 : Physical Metallurgy of Solder Systems 11 treatment, there remains a range of permissible velocity and spacing values. The minimum spacing is given in Zener's treatment. Sundquist [41] showed that a maximum spacing can be estimated by considering that if two neighboring lamellae become too widely spaced, a new lamella can nucleate between them. Solorzano and Purdy [421 propose that the optimal spacing is that for which the rate of dissipation of free energy is a maximum. This is generally a different spacing from that which produces the highest velocity, but should be related. As a general conclusion here, we do not expect that any theoretical treatment will be sufficiently simple and convenient to give cell boundary velocity as a function of temperature and composition, and we must depend on experimental observations. 50%TRANSFORMATION TIME (HOURS) Figure 12 Time required for a 50% transformation for the precipitation reaction in Pb-Sn alloys of various compositions. The curves for 2 to 5 wt.% Sn are from metallographic observations [341. Those for 6 and 9 wt.% Sn are taken from the resistivity measurements of Borelius e t al. 1531. Early studies of the cellular precipitate structure 111, 291 discovered that the average spacing between Sn lamellae decreased with increasing Sn concentration and decreasing precipitation temperature. This is certainly the expected behavior for a diffusion-controlled phase transformation, and was confirmed by Frost et al. [18,34,43]. Figure 13 shows the variation in microstructure produced by different precipitation temperatures. Figure 14 shows that the reciprocal of interlamellar spacing decreases approximately linearly with increasing temperature. The interlamellar spacing can be an important element of the microstructural state because it can strongly affect the flow stress of the alloy. The precipitation process removes Sn from the Pb matrix and therefore reduces a strengthening component due to the solid solution. Solder Mechanics 12 However, the precipitation adds a strengthening component, analogous to grain size strengthening, which is probably proportional to the inverse square-root of the interlamellar spacing. Speich [44] found that relationship for the hardness of Fe-30 Ni-6 Ti, and Nakase and Bernstein [451 found i t for the yield strength of various pearlitic steels. When the interlamellar spacing of Pb-Sn is smaller than about 1 pm, the precipitate structure strengthening outweighs the loss of solid solution strengthening and becomes significant [34]. Dissolution Behavior When the temperature is raised above the solvus temperature, the precipitate structure begins to dissolve. This process may be as complicated as the precipitation. One possible mechanism is the direct diffusion of the Sn into the Pb phase. Another mechanism is the retraction of the cell boundaries as a reversal of the cellular precipitation process, as shown in Figure 15. The grain boundaries here provide a fast diffusion path for the dispersal of the Sn into the Pb phase. This process is dominant a t the relatively lower temperatures only slightly above the solvus. Higher temperatures result in faster dissolution, both because of the temperature dependence of the diffusion coefficients and because the thermodynamic driving force for dissolution increases with temperature. The apparent activation energy for the process is therefore not directly comparable to the activation energy of the rate-limiting diffusion process. Figure 13 Scanning electron micrographs of the Pb-5 wt.% Sn alloy showing the variation of interlamellar spacing with the aging temperature at which the cellular precipitation took place. Aged at: A) 40"C, B) 23'C, C) O'C and D)-20'C. Chapter I : Physical Metallurgy of Solder Systems TEMPERATURE ("C) Figure 14 Reciprocals of interlamellar spacings a s a function of transformation temperature, for various Sn concentrations. Data for 2.0, 3.0, 3.5 and 5.0 wt.% Sn are from Lutender [331. Data for 4.1 wt.% are from Liu and Aaronson [291. Data for 6.7, 7.5,g.O and 10.5 wt.% Sn are from Turnbull and Treaftis [Ill. Figure 15 Scanning electron micrographs showing the progressive dissolution of the precipitate structure in the Pb-3.5 Sn wt.% alloy a t various annealing times a t 90°C [431. The precipitation was formed during aging a t 23°C. The regions containing Sn precipitates appear light. Each micrograph shows nearly the same region, but a t a slightly different depth because of the microtoming and etching needed for each photograph. At later times, there is evidence of spheroidization. Aged at: A) 38 hr. 90"C, B) 89 hr. 90"C, C) 205 hr. 90°C and D) 370 hr. 90'C. 14 Solder Mechanics Tu and Turnbull [16] observed that dissolution by cell boundary retraction would occur even a t temperatures slightly below the solvus. (The effect extends to about 10°C below the solvus.) One driving force for this retraction is the surface energy of the interfaces between the Pb and Sn phases. Frost et al. [431 have reported that the rate of dissolution by cell boundary retraction increases with smaller interlamellar spacings. This effect results both from shorter diffusion distances required to disperse the Sn and from the additional driving force for retraction provided by the interphase interfaces. The dissolution rate therefore depends on the temperature a t which the precipitate structure initially formed. The rate of cellular dissolution was described by Tu and Turnbull [16] in terms of the velocity of the cellular interface v, which depends on the interlamellar spacing S, and the grain boundary thickness and diffusion coefficient 6Db. A related formulation is given by Gupta and Nakkalil [46,47], based on the formulation for cellular precipitation of Peterman and Hornbogen [48]: In this formula, k is the ratio of the solute concentration of the matrix obtained as a result of the cellular dissolution to the solute concentration in the boundary (the segregation ratio), and AGd is the driving force available for dissolution. R is the gas constant and T is temperature. (The sign of AGd must be negative for the dissolution velocity to be positive.) Part of the driving force is due to the chemical free energy change AGdC when the depleted a phase and the P phase transform into a more concentrated a phase. Another component of the driving force is due to the release of the surface free energy of the alp interface released during dissolution. The total driving force is then: where y is the surface free energy and Vm is the molar volume. In this formulation, we see that the total driving force AGd can be negative (implying dissolution) even if the chemical driving force AGdC is positive, because of the driving force for dissolution produced by the interfaces. This agrees with the observation of Tu and Turnbull that dissolution by cell boundary retraction may occur a t temperatures slightly below the solvus. We also see that the dependence of velocity on interlamellar spacing is more complicated than just the inverse spacing squared. The chemical driving force available for dissolution may actually be a complicated matter. Cellular precipitation cannot produce exactly the equilibrium compositions for the given precipitation temperature, because exact compositional equilibrium cannot be reached in a finite time. The Pb matrix is supersaturated with Sn, the Sn is slightly supersaturated with Pb, and the degree of supersaturation should depend on the precipitation temperature. The dissolution therefore requires less diffusion than i t would for compositions that were in equilibrium solvus a t the precipitation temperature. Such variations in Chapter I : Physical Metallurgy of Solder Systems 15 composition should have pome effect on dissolution rates, but probably less effect than variations in the interlamellar spacing. During the process of dissolution by cell boundary retraction, there is also an evolution in the structure of Sn lamellae within the cells. The Sn phase actually occurs as blades with numerous rifts and holes instead of complete plates [13]. The lamellae are therefore subject to instabilities due to capillarity effects that Pb to breakup of the lamellae and spheroidization. This spheroidization was observed by optical metallography [33, 34,491, as shown in Figure 16. During the spheroidization process, there is also some dissolution of the Sn phase by lattice diffusion directly into the Pb matrix. For temperatures near the solvus a t low Sn concentrations, the lattice diffusion is so slow that direct dissolution by lattice diffusion is not completely accomplished before the cellular dissolution is nearly complete. Cellular dissolution by cell boundary migration continues after the spheroidization has become prominent, but the boundaries appear to slow down. Spheroidization should reduce two components of the driving force for the cell boundary migration. It reduces the area of lamella-matrix interface, and i t also disperses some Sn into the Pb matrix, thereby reducing the chemical driving force for cell boundary migration. Figure 16 Microstructure of Pb-5 wt.% Sn after precipitation a t room temperature, followed by annealing at 100°C for 64 h [491. Most of the precipitate structure has undergone cellular dissolution; in those regions that have not, the lamellar Sn precipitates have undergone spheroidization. The cellular dissolution process can also be analyzed in terms of the Avrami transformation equation (Equation 21, in which f is taken to be the fraction in which the cellular precipitate has dissolved. Stone [49] found that for dissolution, the exponent n was about 1. This is the same as Gupta [46] found for a Cu-In alloy, and Sulonen [50] found for Cu-Cd alloys. It is distinctly less than the n = 2 observed for precipitation. The value n = 1is consistent with a model of onedimensional growth from a constant number of initial nuclei. In this context, the Avrami transformation equation implies that the cell boundaries that moved to allow the precipitation are evidently immediately available for reversed 16 Solder Mechanics migration to produce dissolution, without any (random) waiting or incubation time for particle nucleation or cell initiation. Discontinuous Coarsening Just as the precipitate microstructure is unstable to coarsening and spheroidization during dissolution above the solvus, i t is also unstable below the solvus. The initial cellular precipitation process cannot produce a microstructure that is completely a t equilibrium. There must be some free energy of the interfaces between the two phases, and the compositions of the two phases will deviate from their equilibrium values. These factors provide a driving force for evolution towards a coarser microstructure. If the Sn were distributed in small spherical particles, then those particles should coarsen in the usual manner. Frear et al. [9] report a decrease in hardness during aging, which is directly attributable to such coarsening. If the Sn were distributed in planar lamellae, without imperfections, then coarsening might occur in a discontinuous fashion by the passage of interfaces that consumed the fine lamellae and left behind coarse lamellae. Although the process of discontinuous or cellular coarsening has long been recognized [48, 511, i t has not yet been quantitatively studied in Pb-Sn alloys. Fournelle [52] has successfully modeled the kinetics of the process, including both the chemical and the interfacial driving forces. The velocity of the coarsening front depends on both the new interlamellar spacing and the previous interlamellar spacing. It therefore must depend upon the temperature of the initial precipitation process. If the precipitation and coarsening temperatures are allowed to be different, the general formulation becomes complicated. In any event, we expect that the cellular coarsening process would be much slower than the cellular precipitation (for a given temperature), and will therefore become important only for extended dwell times a t intermediate temperatures below the solvus. It probably accounts for the overaging (softening aRer several months) a t room temperature reported, for example, by Frost et al. [34]. MICROSTRUCTURES FAR FROM EQUILIBRIUM Superplastic behavior of the Pb-Sn eutectic has received sigriificant attention [54571. When the eutectic Pb-Sn alloy is in the superplastic state, it exhibits unusually large ductilities: up to 4850% elongation in tensile testing [56]. Improved fatigue behavior might be expected with such a ductile alloy. Chapters 3 and 6 discuss this in detail. Unfortunately, the Pb-Sn eutectic alloy must be heavily cold worked to induce the recrystallization necessary to produce the required small grain size (< 1 pm) [58] to obtain a superplastic condition. During discussion a t the workshop, i t was further suggested that rapid cooling might be used to produce a small grain size. - Like superplasticity, rapid cooling of Pb-Sn alloys has received much attention [59-621. It has been shown [61,621 that the eutectic behavior of the equilibrium system gives way to a peritectic reaction involving a metastable Pb phase ( a l ) under rapid cooling. These investigations have generally involved the use of small droplets of alloy that can be cooled from the melt a t rapid enough rates t o induce sufficient undercooling to form the metastable a1 phase. The question as to whether sufficiently small grain size of the eutectic alloy could be achieved by Chapter 1: Physical Metallurgy of Solder Systems rapid cooling, without cooling too fast and causing the formation of the metastable a1 phase, remains open. It seems likely that such a cooling rate would be difficult to apply to electronic packages due to the thermal shock it would impart. Ternary Element Additions The effects of third element additions to binary solder systems, such as for Pb-Sn solders, include Reactions between binary solders and substrates (which provide the third element) and which form intermetallic layers by reactiondiffusion. (These are described in Chapter 2). Modification of melting-solidification temperatures and wetting characteristics by third element addition, and which may therefore be of significant importance to aid in manufacturing. Formation of distributed third-phase intermetallics as a consequence of third element additions, and which could affect a broad range of mechanical properties, strength, creep behavior and fracture. In the latter case, although significant ternary effects are expected, these effects have not been clearly elucidated-no definitive work on the effect of distributed intermetallic phases upon mechanical properties of solders has been performed The most studied Pb-Sn-X ternaries include Pb-Sn-Cu and Pb-Sn-Au because of bonding of Pb-Sn solders to Cu printed circuit boards or to Au-plated Si devices: Pb-Sn-Ag because of its use as a ternary solder alloy system and Pb-Sn-Bi because of its low ternary eutectic melting temperature. A useful summary of information on ternary Pb-Sn-X systems is given by Steen and Becker [63] and is included in modified form in Table 1. It is convenient to categorize the various PbSn-X ternary systems according to the proximity of the ternary eutectic ET with respect to the binary Pb-Sn eutectic (EB). Table 1. Relationship of Ternary Pb-Sn-X to Binary Pb-Sn ET near EB (wt.%) ET distant from EB No ET The amount (volume fraction) and morphology of the third phase may be estimated from a knowledge of phase equilibria and surface energies for each of 18 Solder Mechanics the phases encountered. Thus, with reference to the schematic ternary diagram shown in Figure 17, a t the eutectic liquid composition, and the three solid eutectic phases in equilibrium with x, y, and z, then Because x and y are closest to the A and B corners respectively, they may be considered as the solid solution limits of the binary A-B (for example, Pb-Sn) system, and z may be considered as the composition of the third component phase (for example, AggSn and AuSnq). As the equilibrium liquid eutectic composition moves closer to the A-B binary side of the diagram, or as the position of the third component phase moves further away from the A-B binary ( the intermetallic phase contains a lesser amount of A or B), then the % z will decrease. Figure 17 Schematic demonstrating the equilibrium tie triangle for the four-phase eutectic reaction X + Y + Z. Many intermetallic phases tend to be faceted, elongated, and needle-like, platelike or blade-like in shape, because of the large directional anisotropy in their surface energies and growth rates as related to their crystal structures [64]. In addition, third element impurities commonly lead to segregation a t the liquidsolid interface during solidification which in turn can lead to cellular interfaces and dendritic growth. Therefore, depending upon the specific system and solidification conditions, the morphology of the intermetallic phases can range from rounded-dendritic to faceted-elongated. The effects of third element impurities on surface energies as related to wetting behavior are discussed by Steen and Becker [63]. They propose that the wetting force is lowered most strongly by (1)impurities which have eutectics very close to Chapter 1: Physical Metallurgy of Solder Systems the binary solder eutectic (for example, As, Fe, Ni) and which tend to form intermetallics that are present in the liquid during soldering, and (2) impurities that tend to enhance oxidation (for example, Al, In, Sn). At the same level of addition, Cu, Ag and Au all have lesser effects on wetting force than those mentioned above. Carol1 and Warwick [651 indicate that addition of Bi(0-4 wt.%) or Sb(0-5 wt.%) reduce the surface tension of 60Sn-40Pb, while Cu(0-0.6 wt.%), Ag(0-4 wt.%) and P(0-0.013 wt.%) increase surface tension. For the cases of Bi and Sb versus Cu and Ag, this effect appears related to the difference in segregation behavior of these elements a t the Sn-Pb solder interface. A short summary of recent work related to phase equilibria and microstructure for each of the ternary Pb-Sn-X systems follows. Table 2 also contains this information. (Pb-Sn) - Ag: A recent summary of this system, but without figures, has been given by Petzow and Effenberg [66]. The liquidus surface is given in Hofmann [67]. The ternary eutectic has been determined by Earle [68] to be a t 1.35 wt.% Ag, 62.5 wt.% Sn, and 36.15 wt.% Pb, and 178°C;the ternary eutectic phases are AggSn, Pb and Sn. Tarby and Notis [691 have performed a thermodynamic analysis to evaluate the lowering of the Pb-Sn eutectic temperature, which was found to decrease by 3.3OC by the addition of 1wt.% Ag; this value was in good agreement with experimental determinations. Figure 18 shows the microstructure of a eutectic Pb-Sn solder with 2 wt.% Ag addition. This alloy is slightly Ag-rich with respect to the ternary eutectic composition, and primary-phase AgaSn is observed in the alloy. Surrounding the AggSn primary-phase is a "halo-like" region (Figure 18B) which is depleted by Ag. Due to non-equilibrium cooling and the presence of the primary AggSn, only the Pb-rich and Sn-rich phases are nucleated by the pre-existing phase. The true ternary eutectic solidification microstructure of three solid phases growing cooperatively is observed only outside this "halo-like" region. Thwaites [70] has used deep-etching studies to show that the AggSn phase can appear as thin rectangular platelets; therefore the two-dimensional images shown in Figure 18 can be misleading concerning the true morphology of the third intermetallic phase in a ternary microstructure. Hoyt [71 indicates that a 2 wt.% Ag addition provides both increased solder strength and refined eutectic structure, and more recently, Hwang and Vargas [72] have noted superior creep resistance for Ag-containing solders. (Pb-Sn) - Au: This system has been reviewed recently by Prince, Raynor and Evans 1731. Of the Pb-Sn-X systems studied in the literature, this is by far the most complex, and only the most pertinent reactions are listed here. There is a ternary eutectic (Pb) + (Sn) + AuSnq (near the Pb-Sn binary eutectic) a t 177OC with composition 3.0 wt.% Au, 32.5 wt.% Pb, and 64.5 wt.% Sn. There is a second ternary eutectic (Pb) + AuSn + AuPbg at 211°C, very close to the Pb-AuPb3 binary eutectic. Because of the considerable importance of this ternary system for a number of manufacturing processes, i t is of interest to understand the effect of changing the Au concentration with respect to a eutectic Pb-Sn solder. The liquidus surface in the Pb-Sn-Au system near the binary Pb-Sn eutectic, showing solidification paths 20 Solder Mechanics for 60Sn-40Pb solder with 1wt.% and 5 wt.% Au is shown in Figure 19A. For 1 wt.% Au, the primary crystallization is Pb, followed by the Pb-Sn three-phase reaction line, and ending a t the ternary Pb-Sn-AuSnq ternary eutectic. For 5 wt.% Au, the primary crystallization of Pb gives way to the Pb-AuSnq three-phase reaction line, and ends a t the ternary eutectic. The AuSnq which is produced by the three-phase reaction is much coarser than that produced in the ternary eutectic reaction; this is shown in Figure 19B. The finer Pb-Sn-AuSnq ternary eutectic microstructure appears towards the top of this figure. Figure 18 Backscattered electron (A), wave length dispersive spectroscopy (WDS)-Ag X-ray (B), WDS-Pb X-ray (C), and WDS-Sn X-ray (Dl images of a eutectic Pb-Sn alloy with 2 wt.% Ag. (Pb-In) - Au: SGTE (Scientific Group Thermodata Europe) has performed an assessment of thermodynamic data for the condensed phases in the Au-In-Pb ternary system. Experimental work on this system has recently been reviewed by Prince, Raynor and Evans [73]. Most of the ternary field involving reactions with the liquid is dominated by a liquid-liquid immiscibility region. There is a ternary eutectic near the Pb corner, (Pb) + AuIn + AuPbg a t 207.5%. (Pb-Sn) - Bi: The most recent evaluation of this system is given by Osamura [74]. The lowest temperature reaction involving the liquid is the ternary eutectic a t 50 wt.% Bi, 32.5 wt.% Pb, and 17.5 wt.% Sn a t 96'C. There are two ternary peritectic reactions in the system; the one closest to the Pb-Sn binary eutectic is a t 32.6 wt.% Bi, 40.8 wt.% Pb, 26.6 wt.% Sn at 137.q°C. (Pb-Sn) - Cd: This is a simple system with a ternary eutectic a t 18 wt.% Cd, 32 wt.% Pb, and 50 wt.% Sn, and 145°C [75]. Savintsev et al. [76] have studied interdiffusion reactions a t 160°C and give an isothermal section a t that temperature. Major and Rutter [771 have performed directional solidification studies of the ternary eutectic and have examined the crystallographic Chapter 1: Physical Metallurgy of Solder Systems 21 orientation relations between the solid-phases, as well as the nature of the liquidsolid interface morphology. They noted, as have previous investigators, the unusual morphology of this ternary eutectic (Figure 20) which has layers of Sn (dark phase), and Pb-rich solid solution (white phase) on either side of essentially pure Cd (grey phase), producing an ABCBA layering. Figure 19A The Pb-Sn-Au liquidus surface near the binary Pb-Sn eutectic. Figure 20 Figure l9B Backscattered scanning electron micrograph of Pb-6OSn Solder with 5 wt.% Au addition showing primary Pb (lower right), AuSn4 (dark blades)-Pb three-phase reaction products (middle) and Pb-Sn-AuSn4 ternary eutectic product (top). Note the large difference in size of the AuSn4 product formed in the two different reactions. Backscattered scanning electron micrograph of Pb-Sn-Cd aIloy a t the ternary eutectic composition. [Pb-Sn) - Cu: The system has been reviewed by Chang et al. [78]. As in the Pb-SnZn system, there is a miscibility gap in the Pb-Cu binary system which extends significantly into the ternary system. Marcotte and Schroeder [79] report a ternary eutectic, (Pb) + (Sn) + Cug Sng, to occur a t 182°C (1°C below the binary Pb- 22 Solder Mechanics Sn eutectic) a t a calculated composition of 0.16 wt.% Cu, 38.08 wt.% Pb, and 61.76 wt.% Sn. Figure 21 shows the CugSng phase as dendrite inclusions in a Pb-Sn solder used for joining the shaft and base of an ancient Roman bronze (Cu-Sn-Pb) candelabrum. Figure 21 Scanning electron micrographs of Cu6Sn5 dendrite inclusions in a Pb-Sn solder used for joining parts in an ancient Roman bronze (Cu-Sn-Pb) candelabrum. (A) secondary electron image (B) wave length dispersive x-ray dot map for Cu. (Pb-Sn)- In: There is no ternary eutectic in this system [80]. The most recent study in this system is that of Evans and Prince 1811. They indicate the presence of two quasi-peritectic invariant reactions with that closest to the Pb-Sn binary eutectic to occur a t 59.4 wt.% Sn, 35.3 wt.% Pb, 5.3 wt.% In and a t 171°C. (Pb-Sn) - Pt: No phase diagram for the Pb-Sn-Pt is known to the authors, but Meagher and Bader [82] have examined reaction-diffusion in this system. The only intermetallic phase observed during solid-state diffusion studies was PtSn4. (Pb-Sn) - Sb: This system has been reviewed by Osamura [83]. There is a quasiperitectic reaction which occurs near the Pb-Sn binary eutectic a t 36.5 wt.% Pb, 60.5 wt.% Sn, and 3 wt.% Sb, a t 191°C. There is also a ternary eutectic near the Pb corner a t 85 wt.% Pb, 11.5 wt.% Sb, and 3.5 wt.% Sn, a t 240°C. (Pb-Sn) - Zn: There is a miscibility gap in the Pb-Zn binary system which extends significantly into the ternary system. There is a ternary eutectic a t 24 wt.% Pb, 71 wt.% Sn, and 5 wt.% Zn, a t 177OC [75]. Chapter I : Physical Metallurgy of Solder Systems 23 Table 2. Information on Ternary Systems Sn-Pb-X Ternary Element Amount in Ternary Eutectic (wt.%) xE Bi In Amount in Peritectic (wt.%) Eutectic (Peritectic Temperature) OC 32.6 5.0 2.5 Cd Zn Au Mg 22.0 5.0 3.0 1.5 137 171 189 145 177 177 170 Ag Cu Al Ni Fe As Te 1.35 0.3 0.08 -0.01 -0.01 -0.01 -0.005 -0.005 -0.001 -0.001 178 183X 183X 183X 183X 183X 183X 183X 183X 183X Sb Se S P Solid Solubility Sn(150°C) (wt.%) Pb(150°C) (wt.%) 10 10 4 1 0.4 0.3 0.02 0.05 0.006 <0.01 0.01 0.001 <0.01 <0.05 0.02 <0.0001 ? 20 70 3.5 5 0.005 0.03 2.5 0.05 0.007 <0.01 0.01 0.0002 0.04 ? ? <0.0001 ? Ternary Phase SbSn Cd Zn AuSnq Mg2Sn Ag3Sn CugSng A1 NigSnq FeSn2 As3Sn4 TeSn SeSn SnS Sn4P3 ? denotes solid solubility not known exactly, but very low. denotes ternary eutectic temperature not known exactly but probably negligibly different from that of the binary Sn-Pb eutectic. X summary The preceding discussion has shown that the behavior of the Pb-Sn system, which a t first glance appears deceptively simple, is actually quite complex. For example, i t was shown that a microstructure of alterhating a-Pb and P-Sn lamellae can occur by very different processes (eutectic solidification versus discontinuous precipitation). Significant advancements have been made in understanding the physical metallurgy of this system as a result of many years of research. However, there still exists opportunity for improved understanding in many areas, including discontinuous phenomena and ternary element effects in solidification and precipitation. In particular, the development of continuous cooling curve diagrams would aid in designing joint dimensions from a microstructural point of view. Finally, the microstructures of electronic packaging solder joints should be incorporated in a meaningful way into lifetime prediction models. References 1. R. F. Tylcote, History of Metallurgy, Institute of Metals, London, England, 1976. Solder Mechanics 24 R. J. Klein-Wassink, Soldering i n Electronics, Electrochemical Publications, Ayr, Scotland, 1989. R. R. Tummala and E. J. Rymaszewski, eds., Microelectronic Packaging Handbook, Van Nostraud Reinhold, New York, 1989. C. J. Thwaites, Int. Met. Rev., 1984, vol. 29, pp. 45-74. C. A. Mackay and S. W. Levine, IEEE Trans., 1986, vol. CHMT9, pp. 197-201. Military Standard MIL-STD-883C. I. Karakaya and W. T. Thompson, Bull. Alloy Pha. Dia., 1988, vol. 9, pp. 144-152. D. R. Frear and J. W. Morris, Jr., Proc. 43rd Annual Meeting EMSA, G. W. Bailey, ed., 1986, vol. 43, pp. 342-343. D. R. Frear, J. B. Posthill, and J. W. Morris, Jr., Met. Trans., 1989, vol. 20A, pp. 1325-1333. D. Turnbull, Acta Met., 1955, vol. 3, pp. 55-63. D. Turnbull and H. N. TreaRis, Trans. AIME, 1958, vol. 212, pp.33-39. D. Turnbull and H. N. Treaftis, Acta Met., 1955, vol. 3, pp. 43-54. K. N. Tu and D. Turnbull, Acta Met., 1969, vol. 17, pp. 1263-1279. K. N. Tu and D. Turnbull, Acta Met., 1967, vol. 15, pp. 369-376,. K. N. Tu and D. Turnbull, Acta Met., 1967, vol. 15, pp. 1317-1323. K. N. Tu and D. Turnbull, Met.Trans., 1971, vol. 2, pp. 2509-2515. K. N. Tu, Met. Trans., 1972, vol. 3, pp. 2769-2776. H. J. Frost, P. R. Lavery, and S. D. Lutender, Proc. 3rd Annual Electronic Packaging and Corrosion in Microelectronics Conf , 1987, vol. 3, pp. 259-267. N. I. Afanas'ev and T. F. Yelsukova, Phys. Met. Metall., 1982, vol. 53, pp. 129-134. E. E. de Kluizenaar, Soldering and Surf. Mount Tech., 1990,vol. 4 , pp. 27-38. B. Chalmers, Principles of Solidification, John Wiley, New York, 1964. V. Raman and T. C. Reiley, Mat. Sci. and Engr. A , accepted for publication. Chapter 1: Physical Metallurgy of Solder Systems P. S. Bassi, N. K. Sharma, J. S. Chawla, and M. K. Sharma, Crystal Res. and Technol., 1983, vol. 18, pp. 637-640. F. N. Rhines, Phase Diagrams i n Metallurgy, McGraw-Hill, New York, 1956. J. P. Nabot and I. Ansara, Bull. Alloy Pha. Dia., 1987, vol. 8, pp. 246-295. J. A. Eady and W. C. Winegard, Can. Met. Quat., 1971, vol. 10, pp. 213-214. J. W. MarSn and R. D. Doherty, Stability of Microstructure i n Metallic Systems, Cambridge Univ. Press, Cambridge, 1976. D. A. Porter and K. E. Easterling, Phase Transformations i n Metals and Alloys, Van Nostrand Reinhold, New York, 1981. Y. C. Liu and H. I. Aaronson, Acta Met., 1968, vol. 16, pp. 1343-1358. P. A. Varkey and A. R. K. L. Padmini, Acustica, 1983, vol. 52, pp. 290-295. F. S. Osorio, Morphology of the Cellular Precipitation Reaction in a Pb-3.0 wt.% S n Alloy, Ph.D.Thesis, University of Missouri-Rolla, 1973. S. A. Hackney and F. S. Biancaniello, Scripta Met., 1987, vol. 21, pp. 371-376. S. D. Lutender, Micrographic Study of the Precipitation and Dissolution Kinetics of Selected Pb-Sn Alloys, M.E. Thesis, Dartmouth College, 1986. H. J. Frost, R. T. Howard, P. R. Lavery and S. D. Lutender, IEEE Trans. on Components, Hybrids and Manufact. Tech., 1988, vol. 11, pp. 371-379. C. Zener, Trans AIME, 1946, vol. 167, pp. 550-595. J. W. Cahn, Acta Met., 1959 vol. 7, no. 1, pp. 18-28. H. I. Aaronson and Y. C. Liu, Scripta Met., 1968, vol. 2, pp. 1-8. M. Hillert, The Role of Interfaces i n Phase Transformations, The Mechanism of Phase Transformations i n Crystalline Solids, Institute of Metals, London 1969, pp. 231-247. M. Hillert, Met. Trans., 1972, vol. 3, pp. 2729-2741. M. Hillert, Acta Met., 1982, vol. 30, pp. 1689-1696. B. E. Sundquist, Met. Trans., 1973, vol. 4, pp. 1919-1934. I. G. Solorzano and G. R. Purdy, Met. Trans., 1984, vol. 15A, no. 6, pp. 1055-1063. Solder Mechanics 26 H. J . Frost, R. T. Howard and G. J. Stone, Microelectronic Packaging Technology, Materials and Processes, W.T. Shieh, ed., ASM International, 1989, pp. 121-127. G. R. Speich, Trans. AIME, 1963, vol. 227, pp. 754-762. K. Nakase and I. M. Bernstein, Met. Trans., 1988, vol. 19A, pp. 2819-2829. S. P. Gupta, Mat. Sci.and Eng'g., 1986, vol. 83, pp. 255-267 R. Nakkalil and S. P. Gupta, Acta Met., 1987, vol. 35, no. 8, pp. 2157-2165. J . Peterman and E. Hornbogen, 2. Metallkde., 1968, vol. 59, pp. 814-822. G. J . Stone, Dissolution in Pb-Sn Alloys, Master's Thesis, Dartmouth College, 1989. M. S. Sulonen,Acta Met., 1960, vol. 8, no. 10, pp. 669-676. J. D. Livingston and J. W. Cahn, Acta Met., 1974, vol. 22, pp. 495-503. R. A. Fournelle, Acta Met., 1979, vol. 27, pp. 1135-1155. G. Borelius, F.Larris and E. Ohlsson, Arkiv For Matematik, Astronomi och Fysik, 1944, vol. 31A, no. 10, pp. 1-19. D. H. Avery and W. A. Backofen: Trans. Quart. ASM, 1964, vol. 57, p. 980. H. E. Cline and T. H. Alder, Trans. AIME, 1967, vol. 239, pp. 710-714. M. M. J. Ahrned and T. G. Langdon, Met. Trans., 1977, vol. 8A, pp.1832-1833 A. Juhasz, P. Tasnadi, P. Szaszvari, and I. Kovacs, J. Mat. Sci., 1986, vol. 21, pp. 3287-3291. R. W. Hertzberg: Deformation and Fracture Mechanics of Engineering Materials,, John Wiley and Sons, New York, 1976. 59. J. H. Perepezko, Mat. Sci. and Eng., 1984, vol. 65, pp. 125-135. 60. M. C. Flemings and Y. Shichara, Mat. Sci. and Eng., 1984, vol. 65, pp. 157-170. 61. K. Mori, K. N. Ishihara, and P. H. Shingu, Mat. Sci. and Eng., 1986, vol. 78, pp. 157-162. 62. H. J. Fecht and J. H. Perepezko: Met. Trans., 1989, vol. 20A, pp. 785-803. 63. H. A. H. Steen and G. Becker: Brazing and Soldering, 1986, Autumn (NO.ll),pp. 4-11. Chapter I : Physical Metallurgy of Solder Systems G. A. WolE Intermetallic Compounds, J. H. Westbrook, Ed., John Wiley and Sons, New York, 1967. M. A. Carroll and M. E. Warwick: Mat. Sci. and Tech., 1987, vol. 3, pp. 1040-1045. G. Petzow a n d G. Effenberg: Ternary Alloys Volume 2, Silver, 1988, VCH Verlagsegesellschaft. W. Hofmann: Lead and Lead Alloys, 1970, Springer-Verlag, p. 115. L. G. Earle: J. Inst. Metals., 1946, vol. 72, pp. 403-413. S. K. Tarby and M. R. Notis: Metall. Trans. B, 1986, vol. 17B, pp. 829-832. C. J. Thwaites: Brazing and Soldering, 1986, no. 10, pp. 38-42. J. Hoyt: Brazing and Soldering, 1987, no. 13, pp. 10-19. J. S. Hwang and R. M. Vargas: Soldering and Surface Mount Technology, 1990, no. 3, pp38-45. A. Prince, G. V. Raynor and D. S. Evans: Phase Diagrams of Ternary Gold Alloys, 1990, Institute of Metals, London. K. 0kamura: Bull. Alloy Phase Diagrams, 1988, vol. 9, pp. 274-281. W. Guertler, M. Guertler and E. Anastasiadias: WADC Tech. Report 58615, 1969, WPAFB, Jerusalem, p. 683. P. A. Savintsev, e t al.: Izu. Akad. Nauk. S S S R Metal, 1984, vol. 5, pp. 166-169. J. F. Major and J. W. Rutter: Mat. Sci. and Tech., 1986, sol. 2, pp. 534-538. Y. A. Chang, J. P. Neu;man, A. Mikula and D. Goldberg: Phase Diagrams and Thermodynamic Properties of Ternary Copper-Metal Systems, 1979, INCRA Monographs Series, vol. VI. V. C. Marcotte and K. Schroeder: Proc. 13th No. Amer. Thermal Analysis Soc., 1984, vol. 13, pp. 294-298. A. N. Campbell, R. M. Screaton, T. P. Schaefer and C. M. Hovey: Can. J. Chem., 1955, vol. 33, p. 511. D. S. Evans and A. Prince: Met. Sci., 1980, vol. 14, pp. 34-37. B. W. Meagher and W. G. Bader: Int'l. Metallographic Society, 1987, Monterey, CA. K. Osamura: Bull. Alloy Phase Diagrams, 1985, vol. 6, pp. 372-379.