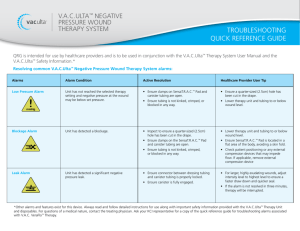
GE Healthcare
Life Sciences
Application note 28-9501-39 AB
Bioreactor systems
A rapid filtration method for
harvesting mammalian cell culture
grown in Cellbag™ bioreactors
Disposable bioreactors have gained widespread
acceptance in cell culture applications because they
provide a flexible resource for multiproduct facilities
and speed the production of biomolecules. We used
ULTA™ Prime GF glass fiber and ULTA Prime CG bioburden
reduction or ULTA Pure HC sterilizing-grade normal flow
filters to harvest Cellbag contents using a simple, turnkey approach that can be easily coupled directly to the
bioreactor chamber at the time of harvest.
Introduction
Disposable bioreactors offer a number of advantages
for scientists including the reduction of preparation time,
elimination of cleaning and sterilization time, and ease of
use (1). WAVE Bioreactor™ is the most popular disposable
bioreactor with over 2000 units in operation worldwide. And
for many applications, WAVE systems have become essential
components in the cGMP production of human therapeutics (2).
Recent advances in filter integration, aseptic connectology
and disposable sensing allow for fully disposable operation
of the bioreactor; however, harvest and clarification
operations remain largely dependent on centrifugation,
cross flow filtration and depth filtration (3)—all techniques
that are yet to be widely adapted to single-use
implementation. The use of these techniques to harvest
disposable bioreactors can result in process bottlenecks (4),
especially in settings where large numbers of relatively
small bioreactors are in use (e.g., clinical and research
laboratories). In these cases, the advantages of ease-of-use,
disposability, and turn-key processing often outweigh the
need for optimized, moleculespecific process development.
Fig 1. Process outline for the harvest of mammalian culture from Cellbag
bioreactors.
Experiments and results
Cell harvest operations that employ normal flow filtration
are generally designed to use serially coupled filters of
decreasing pore size. Most commonly, a depth- or prefilter is coupled to a sterilizing-grade membrane in order to
produce material that can be immediately purified using
chromatography or ultrafiltration operations.
Depth filters are available in a variety of formats and
different material compositions. Historically, mammalian
cell culture operations rely on depth filters composed of
a cellulose matrix blended with inorganic filter aids (e.g.,
diatomaceous earth or perlite); however, these products
are not suitable for single-use applications due to their
inherently high levels of extractables—that must be removed
via a pre-use flush of 50 to 100 L/m2 of water (5)—and the
limited availability of encapsulated formats.
1. Glass fiber has a high void volume and well-defined filter
matrix that provides longevity and efficient cell removal.
2. Glass fiber filters can be preflushed and dried at the point
of manufacture thereby eliminating any need for pre-use
flushing by the end-user.
3. Glass fiber is compatible with common sterilization
techniques such as autoclaving and gamma-irradiation.
Sterilizing-grade membranes are generally coupled to
depth filters in order to facilitate additional removal of cell
debris and particles or fines that may be shed by the depth
filter. In this study, we tested two membrane-based options
for this secondary clarification step—ULTA Prime CG and
ULTA Pure HC. ULTA Pure HC is a sterilizing-grade (0.2 μm)
filter that incorporates an onboard 0.6 μm polyethersulfone
prefilter. ULTA Prime CG is a bioburden reduction filter
(validated for 5 LRV of B. diminuta) that incorporates an
onboard 0.6 μm polyester prefilter. Providing the option of
sterilizing-grade versus bioburden reduction enables the
end-user to choose between lower cost (ULTA Prime CG) and
maximum quality assurance (ULTA Pure HC).
Eight pilot scale experiments were performed on three cell
lines at varying levels of viability and cell density (Fig 2).
All eight cultures were grown in WAVE Cellbag bioreactors
of sizes varying from 5 to 10 liters. All the experiments
were performed with capsule filters under constant flow
conditions. We chose flow rates that allowed complete
processing of the Cellbag contents in one hour.
Cell viability (%)
Meth-A
Hybridoma
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
1 000 000
10 000 000
Total cell density (cells/mL)
Fig 2. Cell culture characteristics. (Two experiments performed at the same
culture conditions).
2 28-9501-39 AB
(error bars represent one standard deviation)
90
80
70
60
50
40
30
20
10
0
ULTA Prime GF
ULTA Prime CG
ULTA Pure HC
Primary filter
Secondary filter
Process step
Fig 3. Capacity of primary and secondary filters.
Based on the experimental results, filter recommendations
were developed for each Cellbag size up to the Cellbag 50 L
(Table 1).
Table 1. Recommended filters for Cellbag harvest
Filter sizing and selection
CHO
Primary (i.e., ULTA Prime GF) and secondary (ULTA Pure HC
or ULTA Prime CG) filters were run in series with pressure
transmitters located upstream of each filter. Filtrate volume
and pressure data was collected as a function of time. Each
experiment was continued until a total pressure drop of
1 bar was observed or until the feed vessel was exhausted.
Capacity calculations were performed for both filters (Fig 3).
Capacity (L/m2)
In this study, we focused on glass-fiber based depth filters
over traditional depth-filter technologies for the following
reasons:
Cellbag
size
Culture
volume (L)
ULTA Prime GF
5.0 μm
ULTA Prime CG or
ULTA Pure HC
2L
up to 1
2 in
2 in
10 L
1 to 5
5 in
6 in
20 L
5 to 10
10 in
5 in
50 L
10 to 25
20 in
10 in
100 L
25-50
2 × 20 in
30 in
200 L
50-100
3 × 30 in
2 × 30 in
Conclusions
Ordering information
ULTA Prime GF filters coupled to a sterilizing-grade ULTA
Pure HC or bioburden reduction ULTA Prime CG filter is
a highly efficient method for harvesting WAVE Cellbag
bioreactors. This combination provides a rapid solution
that can be implemented with very little preparation. Glass
fiber and polyethersulfone membranes are inherently low
in extractables and are validated for low levels of endotoxin
and other contaminants. The methods presented in this work
provide a reliable solution designed to work in the majority of
mammalian cell culture applications with no upfront process
development efforts.
Product
References
1.
2.
Fries, S. et al. Evaluation of disposable bioreactors—Rapid Production of
Recombinant Proteins. By several Animal cell Lines. BioProcess International Vol. 3,
Supplement 6 (2005).
Disposable bioreactors become standard fare. Genetic Engineering & Biotechnology
News 25, No. 14 (2005).
3.
Shukla, A. et al. Harvest and recovery of monoclonal antibodies from large-scale
mammalian cell culture. BioPharm International. 21, No. 5 (2008).
4.
Tathagata, R. et al. Cell culture clarification by depth filtration. Biospectrum Asia.
http://www.biospectrumasia.com/content/100908IND7060.asp (2008).
5.
Application note: Using Millistak+® HC Filters for mammalian cell culture
clarification. Millipore Corporation, AN1100EN00 (2003).
Code number
ULTA Prime GF, 2 in
28-9084-27
ULTA Prime GF, 5 in
28-4136-61
ULTA Prime GF, 10 in
28-4136-64
ULTA Prime GF, 20 in
28-4136-68
ULTA Prime GF, 2 × 20 in
28-4136-68
ULTA Prime GF, 3 × 30 in
28-4136-72
ULTA Prime CG, 2 in
28-9085-17
ULTA Prime CG, 6 in
28-9085-23
ULTA Prime CG, 5 in
28-4137-08
ULTA Prime CG, 10 in
28-4137-12
ULTA Prime CG, 30 in
28-4137-18
ULTA Prime CG, 2 × 30 in
28-4137-18
ULTA Pure HC, 2 in
28-4002-31
ULTA Pure HC, 6 in
28-4002-37
ULTA Pure HC, 5 in
28-4004-27
ULTA Pure HC, 10 in
28-4004-28
ULTA Pure HC, 30 in
28-4004-30
ULTA Pure HC, 2 × 30 in
28-4004-30
28-9501-39 AB 3
For local office contact information, visit
www.gelifesciences.com/contact
www.gelifesciences.com/readytoprocess
GE Healthcare Bio-Sciences AB
Björkgatan 30
751 84 Uppsala
Sweden
GE, imagination at work and GE monogram are trademarks of General
Electric Company.
Cellbag, WAVE Bioreactor, and ULTA are trademarks of GE Healthcare
companies.
Millistak is a trademark of Millipore.
© 2009–2012 General Electric Company — All rights reserved.
First published Jan. 2009.
All goods and services are sold subject to the terms and conditions of
sale of the company within GE Healthcare which supplies them. A copy
of these terms and conditions is available on request. Contact your
local GE Healthcare representative for the most current information.
GE Healthcare UK Limited
Amersham Place
Little Chalfont
Buckinghamshire, HP7 9NA
UK
GE Healthcare Europe, GmbH
Munzinger Strasse 5
D-79111 Freiburg
Germany
GE Healthcare Bio-Sciences Corp.
800 Centennial Avenue, P.O. Box 1327
Piscataway, NJ 08855-1327
USA
imagination at work
GE Healthcare Japan Corporation
Sanken Bldg., 3-25-1, Hyakunincho
Shinjuku-ku, Tokyo 169-0073
Japan
28-9501-39 AB 09/2012