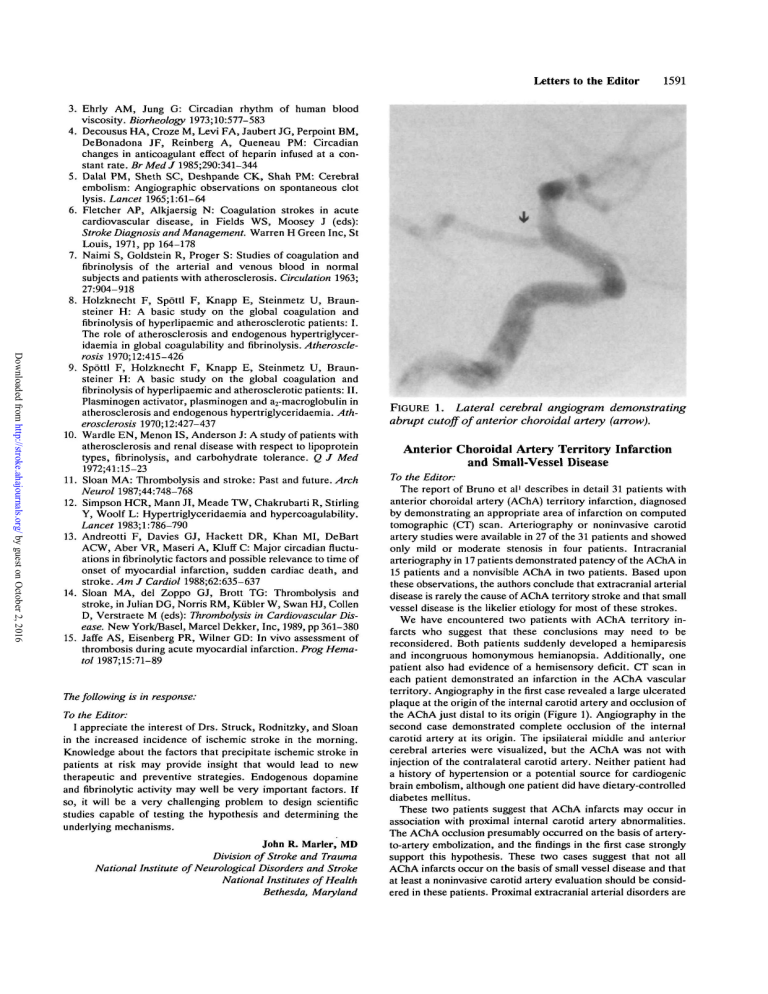
FIGURE 1. Lateral cerebral angiogram demonstrating abrupt cutoff
Letters to the Editor 1591
3. Ehrly AM, Jung G: Circadian rhythm of human blood viscosity. Biorheology 1973;10:577-583
4. Decousus HA, Croze M, Levi FA, Jaubert JG, Perpoint BM,
DeBonadona JF, Reinberg A, Queneau PM: Circadian changes in anticoagulant effect of heparin infused at a constant rate. BrMedJ 1985;290:341-344
5. Dalai PM, Sheth SC, Deshpande CK, Shah PM: Cerebral embolism: Angiographic observations on spontaneous clot lysis. Lancet 1965;l:61-64
6. Fletcher AP, Alkjaersig N: Coagulation strokes in acute cardiovascular disease, in Fields WS, Moosey J (eds):
Stroke Diagnosis and Management. Warren H Green Inc, St
Louis, 1971, pp 164-178
7. Naimi S, Goldstein R, Proger S: Studies of coagulation and fibrinolysis of the arterial and venous blood in normal subjects and patients with atherosclerosis. Circulation 1963;
27:904-918
8. Holzknecht F, Spottl F, Knapp E, Steinmetz U, Braunsteiner H: A basic study on the global coagulation and fibrinolysis of hyperlipaemic and atherosclerotic patients: I.
The role of atherosclerosis and endogenous hypertriglyceridaemia in global coagulability and fibrinolysis. Atheroscle-
rosis 1970;12:415-426
9. Spottl F, Holzknecht F, Knapp E, Steinmetz U, Braunsteiner H: A basic study on the global coagulation and fibrinolysis of hyperlipaemic and atherosclerotic patients: II.
Plasminogen activator, plasminogen and a
2
-macroglobulin in atherosclerosis and endogenous hypertriglyceridaemia. Ath-
erosclerosis 1970;12:427-437
10. Wardle EN, Menon IS, Anderson J: A study of patients with atherosclerosis and renal disease with respect to lipoprotein types, fibrinolysis, and carbohydrate tolerance. Q J Med
1972;41:15-23
11. Sloan MA: Thrombolysis and stroke: Past and future. Arch
Neurol 1987;44:748-768
12. Simpson HCR, Mann JI, Meade TW, Chakrubarti R, Stirling
Y, Woolf L: Hypertriglyceridaemia and hypercoagulability.
Lancet 1983;l:786-790
13. Andreotti F, Davies GJ, Hackett DR, Khan MI, DeBart
ACW, Aber VR, Maseri A, Kluff C: Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death, and stroke. Am J Cardiol 1988;62:635-637
14. Sloan MA, del Zoppo GJ, Brott TG: Thrombolysis and stroke, in Julian DG, Norris RM, Kiibler W, Swan HJ, Collen
D, Verstraete M (eds): Thrombolysis in Cardiovascular Dis-
ease. New York/Basel, Marcel Dekker, Inc, 1989, pp 361-380
15. Jaffe AS, Eisenberg PR, Wilner GD: In vivo assessment of thrombosis during acute myocardial infarction. Prog Hema-
tol 1987;15:71-89
The following is in response:
To the Editor:
I appreciate the interest of Drs. Struck, Rodnitzky, and Sloan in the increased incidence of ischemic stroke in the morning.
Knowledge about the factors that precipitate ischemic stroke in patients at risk may provide insight that would lead to new therapeutic and preventive strategies. Endogenous dopamine and fibrinolytic activity may well be very important factors. If so, it will be a very challenging problem to design scientific studies capable of testing the hypothesis and determining the underlying mechanisms.
John R. Marler, MD
Division of Stroke and Trauma
National Institute of Neurological Disorders and Stroke
National Institutes of Health
Bethesda, Maryland
FIGURE 1. Lateral cerebral angiogram demonstrating abrupt cutoff of anterior choroidal artery (arrow).
Anterior Choroidal Artery Territory Infarction and Small-Vessel Disease
To the Editor:
The report of Bruno et al
1
describes in detail 31 patients with anterior choroidal artery (AChA) territory infarction, diagnosed by demonstrating an appropriate area of infarction on computed tomographic (CT) scan. Arteriography or noninvasive carotid artery studies were available in 27 of the 31 patients and showed only mild or moderate stenosis in four patients. Intracranial arteriography in 17 patients demonstrated patency of the AChA in
15 patients and a nonvisible AChA in two patients. Based upon these observations, the authors conclude that extracranial arterial disease is rarely the cause of AChA territory stroke and that small vessel disease is the likelier etiology for most of these strokes.
We have encountered two patients with AChA territory infarcts who suggest that these conclusions may need to be reconsidered. Both patients suddenly developed a hemiparesis and incongruous homonymous hemianopsia. Additionally, one patient also had evidence of a hemisensory deficit. CT scan in each patient demonstrated an infarction in the AChA vascular territory. Angiography in the first case revealed a large ulcerated plaque at the origin of the internal carotid artery and occlusion of the AChA just distal to its origin (Figure 1). Angiography in the second case demonstrated complete occlusion of the internal carotid artery at its origin. The ipsiiaterai middle and anterior cerebral arteries were visualized, but the AChA was not with injection of the contralateral carotid artery. Neither patient had a history of hypertension or a potential source for cardiogenic brain embolism, although one patient did have dietary-controlled diabetes mellitus.
These two patients suggest that AChA infarcts may occur in association with proximal internal carotid artery abnormalities.
The AChA occlusion presumably occurred on the basis of arteryto-artery embolization, and the findings in the first case strongly support this hypothesis. These two cases suggest that not all
AChA infarcts occur on the basis of small vessel disease and that at least a noninvasive carotid artery evaluation should be considered in these patients. Proximal extracranial arterial disorders are
1592 Stroke Vol 20, No 11, November 1989 being increasingly identified in patients with lenticulostriate artery lacunar syndromes, and it is not surprising that a percentage of
AChA territory infarct patients will also harbor such lesions.
2
Marc Fisher, MD
James F. Lingley, MD
Andrew Blumenfeld, MD
Kevin Felice, DO
Departments of Neurology and Radiology
The Medical Center of Central Massachusetts-Memorial
Worcester, Massachusetts
References
1. Bruno A, Graff-Radford NR, Biller J, Adams HF: Anterior choroidal territory infarction: A small vessel disease. Stroke
1989;20:616-619
2. Tegeler CH, Shi F, Hart RG, Sherman DG, Morgan T:
Carotid stenosis in lacunar stroke (abstract). Stroke 1989;
20:141
The following is in reply:
To the Editor:
We read with interest about the experience of Dr. Fisher and his colleagues regarding anterior choroidal artery (AChA) territory infarction. We reported carotid artery studies on all 31 of our patients with AChA territory infarction
1
and not 27 as stated by Dr. Fisher in his letter. The first case of AChA territory infarction presented by Dr. Fisher suggests an embolic AChA occlusion. The second case is associated with ipsilateral internal carotid artery occlusion and nonvisibility of the AChA. Nonvisibility of the AChA on arteriography should not be interpreted as an abnormality since it occurs in about 5% of normal studies.
2
Small size of the AChA and obscuration by middle cerebral artery branches are contributing factors. The abstract referenced by Dr. Fisher in his letter
3
does not refer to the AChA and, therefore, is not pertinent to our article. We reported patients with brain infarction in a specific vascular territory and not lacunar infarction in general.
Based on our experience with 31 patients with AChA territory infarction
1
and 55 additional patients in other reports,
4
-
7
the two cases presented by Dr. Fisher are interesting and unusual, but they do not invalidate our conclusion that AChA territory infarction is usually caused by small vessel disease.
Askiel Bruno, MD
Neurology Service
Veterans Affairs Medical Center
Albuquerque, New Mexico
Neill R. Graff-Radford, MBBCh, MRCP
Mayo Clinic Jacksonville
Jacksonville, Florida
Jose Biller, MD
Harold P. Adams Jr., MD
Division of Cerebrovascular Diseases
Department of Neurology
University of Iowa
Iowa City, Iowa
References
1. Bruno A, Graff-Radford NR, Biller J, Adams HP Jr: Anterior choroidal artery territory infarction: A small vessel disease. Stroke 1989;20:616-619
2. Huber P: Cerebral Angiography. Stuttgart, George Thieme
Verlag, 1982, p 76
3. Tegeler CH, Shi F, Hart RG, Sherman DG, Morgan T:
Carotid stenosis in lacunar stroke (abstract). Stroke 1989;
20:141
4. Decroix JP, Graveleau PH, Masson M, Cambier J: Infarction in the territory of the anterior choroidal artery: A clinical and computed tomographic study of 16 cases. Brain
1986;109:1071-1085
5. Sterbini GLP, Agatiello LM, Stocchi A, Solivetti FM: CT of ischemic infarctions in the territory of the anterior choroidal artery: A review of 28 cases. AJNR 1987;8:229-232
6. Helgason C, Caplan LR, Goodwin J, Hedges T III: Anterior choroidal artery-territory infarction: Report of cases and review. Arch Neurol 1986;43:681-686
7. Helgason C, Wilbur A, Weiss A, Redmond KJ, Kingsbury
NA: Acute pseudobulbar mutism due to discrete bilateral capsular infarction in the territory of the anterior choroidal artery. Brain 1988; 111:507-524
Effect of Insulin on Acute Experimental
Cerebral Ischemia in Gerbils
To the Editor:
We would like to comment on the article by Fukuoka and coworkers
1
in the March 1989 issue of Stroke regarding the effect of insulin after transient common carotid artery occlusion in
Mongolian gerbils. The results showed that nonhypoglycemic animals treated with insulin had the most favorable outcome. In this study, glycemia after ischemia was not monitored, and the effects of plasma glucose concentration in the first hours of the experiment could have influenced the final results.
We compared the effects of serum glucose concentration on the neurologic outcome
2
and mortality in a group of Mongolian gerbils subjected to unilateral common carotid artery occlusion.
Group A was treated with 3.5 mg/kg of glucose intraperitoneally immediately after carotid occlusion and 1.75 mg/kg at subsequent intervals of 30, 60, and 180 minutes. Control Group B was treated with 0.9% saline injections and Control Group C was treated with 8.0% saline injections. After the first 210 minutes of the experiment, Group A animals demonstrated more severe neurological disability (/><0.01). Group A showed 100% mortality by the end of 24 hours, which was significantly different from
Groups B and C in which no animals died (/?<0.00001). In a similar experiment
3
with gerbils made hyperglycemic by administration of 3.5 mg/kg of glucose i.p. immediately after unilateral common carotid artery occlusion, we compared cerebral neuropathologic changes 24 hours later with a control treated with
0.9% saline injections. In the hyperglycemic gerbils, we found a greater degree of neuronal ischemic changes (p<0.05) and, unlike the normoglycemic gerbils, most brains exceeded the stage of selective neuronal necrosis (/?<0.01).
We conclude that experiments dealing with the protective effects of insulin on cerebral ischemia should take into account the plasma glucose concentration during and immediately after ischemia.
J. Vazquez-Cruz, MD
J.L. Marti-Vilalta, MD
Sabadell, Spain
References
1. Fukuoka S, Yeh H-S, Mandybur TI, Tew JM Jr: Effect of insulin on acute experimental cerebral ischemia in gerbils.
Stroke 1989;20:396-399
2. McGraw CP: Experimental cerebral infarction. Effects of pentobarbital in mongolian gerbils. Arch Neurol 1977;
34:334-336
3. Vazquez-Cruz J: Neurologic and neuropathologic effects of acute hyperglycemia after regional cerebral ischemia in mongolian gerbil (thesis). Barcelona, Spain, Universitat
Autonoma Barcelona, 1989
Anterior choroidal artery territory infarction and small-vessel disease.
M Fisher, J F Lingley, A Blumenfeld and K Felice
Stroke. 1989;20:1591-1592 doi: 10.1161/01.STR.20.11.1591
Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1989 American Heart Association, Inc. All rights reserved.
Print ISSN: 0039-2499. Online ISSN: 1524-4628
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://stroke.ahajournals.org/content/20/11/1591.citation
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office.
Once the online version of the published article for which permission is being requested is located, click Request
Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer
Reprints: Information about reprints can be found online at: document. http://www.lww.com/reprints
Subscriptions: Information about subscribing to Stroke is online at: http://stroke.ahajournals.org//subscriptions/






![Advanced Practice Clinicians Section 2014 []](http://s2.studylib.net/store/data/005266347_1-e47bbdee2346242f6ff85363ec775d6a-300x300.png)