Simulation of Auditory-neural Transduction: Further Studies
advertisement

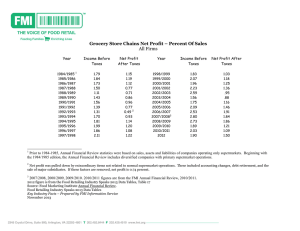
Simulation of auditory-neural transduction: Further studies Ray Meddis DepartmentofHurnanSciences, University of Technology, Loughborough LE I13 TU, England (Received31July 1986;accepted for publication3 November1987) A computationalmodelof mechanicalto neuraltransductionat the hair cell-auditory-nerve synapseis presented.It producesa streamof events(spikes)that are preciselylocatedin time in responseto an arbitrary stimulusand is intendedfor useas an input to automaticspeech recognitionsystemsaswell asa contributionto the theoryof the originof auditory-nervespike activity.The behaviorof the modelis comparedto datafrom animalstudiesin the following tests:(a) rate-intensityfunctionsfor adaptedand unadaptedresponding;(b) two-component short-termadaptation;(c) frequency-limited phaselockingof events;(d) additivityof responding followingstimulus-intensity increases anddecreases; (e) recoveryof spontaneous activityfollowingstimulusoffset;and (f) recoveryof abilityto respondto a secondstimulus followingoffsetof a firststimulus.The behaviorof themodelcompares well with empirical databut discrepancies in tests(d) and (f) pointto the needfor furtherdevelopment. Additionalfunctionsthat havebeensuccessfully simulatedin previoustestsincluderealistic interspike-interval histograms for silenceandintensesinusoidalstimuli,realisticpoststimulus periodhistogramsat variousintensitiesand nonmonotonicfunctionsrelatingincrementaland decrementalresponses to backgroundstimulusintensity.The modelis computationally convenientand well suitedto usein automaticrecognitiondevicesthat usemodelsof the peripheralauditorysystemasinput devices.It is particularlywell suitedto devicesthat require stimulusphaseinformationto be preservedat low frequencies. PACS numbers:43.63.Bq,43.63.Pd,43.63.La INTRODUCTION decidebetweenequallysuccessful accounts. Regrettably,we have not yet reached that stage because no existingmodel Spikeactivity in auditory-nervefibersis a probabilistic has been shown to agree with all of the published results nonlinearfunction of the instantaneousamplitude of the already available. Moreover, as new results are published, it acousticstimulus.In recentyears,a numberof increasingly is difficult to decide whether existing models can account for sophisticated computationalmodelsof this processhave investigation. beenpresented thataimto explaintheparticularnonlineari- themexceptafter a full-scalecomputational ties that occurat the junction betweenthe inner hair cells Armchair evaluationof the issueis normally totally inadeand individualauditory-nervefibers,the point of neurome- quate.The purposeof thisarticleis to reporton a computationalinvestigation of onemodel(Meddis,1986)in theconchanical transduction (Siebert, 1965; Weiss, 1966; Nilsson, 1975; Schroederand Hall, 1974; Oono and Sujaku, 1975; text of three recentresearchresults:(a) the effectof stimulus Eggermont,1973; Geisler et al., 1979; Brachman, 1980; Ross,1982;Schwidand Geisler, 1982;Smithand Brachman, 1982; Westerman, 1985; Westerman and Smith, 1986; stants(Westerman and Smith, 1984); (b) the effectof incre- Cooke, 1986; Meddis, 1986). Thesemodelsare of interestto hearingresearchersfrom a numberof pointsof view. They offer a readilytestable scientific explanationof theobserved phenomena andstimulate the further developmentof theoriesof mechanism. Modelsthatgeneratesimulatedspiketrainsin response to an acousticstimulusare also a necessaryprerequisiteto detailed modeling of physiologicalproee•eu occurring at "higher"levelsof the system,for example,the cochlearnucleusor psychological processes suchas auditoryselective attention(e.g., Evans,1986;Lyon, 1985). In addition,an immediatetechnological applicationof suchspike-generating systems occursin the designof automaticspeechrecognition devices.In hearinglaboratories,spikegeneratorsare alreadyin usefor trainingresearchers and testingapparatus without the needfor live preparations. Ideally, a proliferationof modelsshouldstimulatefruitful empirical studiesby suggestingcrucial experimentsto 1056 J. Acoust.Soc.Am.83 (3), March1988 amplitudeon rapid and short-termadaptationtime conmentsand decrementsof stimulusamplitude(Smith et al., 1985); and (c) the recoveryof rapid and short-term responsecapacity following maskingstimuli (Westerman, 1985). The article will also presentan unintended,emergent propertyof themodelwhichisthesimulation of Roseetal.'s (1967) observationof phase-locked respondingand its restrictionto low-frequencyacousticstimuli. The high-frequencylimit on phaselockingis normallyascribedto lowpassfiltering characteristicsof the hair cell membraneas manifest in the decline of the ac/dc ratio of inner hair cell potentialsas stimulusfrequencyrises(Sellick and Russell, 1980;Palmer and Russell, 1986). In the model, it arisesas a consequence of delaysin removingtransmitterfrom the hair cell-nerve fiberjunction. I. THE MODEL The modelhasbeenfully describedelsewhere(Meddis, 1986;modelB) butissummarized in Fig. 1. It canbefully 0001-4966/88/031056-08500.80 @ 1988 Acoustical Societyof America 1056 m Free Transmitter Pool I Reuptake Reprocessing Store dq= y(1-q(t ))+ xw(t)-k(t )q(t ) The model is summarizedby three differentialequationsthat aregivenin Fig. 1. For the purposes of computation, dt is normallysetto 0.00005s exceptwhenexplicitly stated.The threeequationsare evaluated;therefore,20 000 timesper secondandthe quantitiesq(t), c(t), and w(t) are changedafter eachiteration.The modelhassevenparameters,y, x, l, r, g, A, and B, that can be set by the modeler (Table I). dt dc •- = k(t ) q(t)-)c( t)- re(t) II. METHODS OF EVALUATION dw •- = rc(t)-xw(t) FIG. 1. Flowdiagramfor transmittersubstance anddifferentialequations definingthe model.Takenfrom Meddis(1986), modelB, Fig.10. understood in termsof theproduction,movement,anddissi- pationoftransmitter substance in theregionof thehaircellauditory-nerve fibersynapse. An amountq(t) of transmitter In a previousarticle(Meddis, 1986), it wasshownthat the model could realisticallysimulatemammalianadapted spike-rate/intensity functionin the auditorynerve,appropriateintervalandperiodhistograms in response to sinusoidal stimulation,andsuitableintensityrelatedratechanges in response to increases and decreases in stimulusintensity. Subsequent research, to bedescribed below,showed thatthe modelcouldreproduce Smith's(1977)observation thatthe adaptationresponse followingsuddenstimulusincrementis characterizedby the sum of two exponentialdecayfunc- existsinside the cell wall near the junction. A fraction k (t)q (t) dt ofthistransmitterisreleased, between timet, and timet Jr dt acrossthemembraneintothecleft.A permeability factork(t) is a nonlinearfunctionof the instantaneous tions. It alsoindicatedthat the period histogramsdemonphase-locking response that amplitudeof the signalafter mechanicaleffectshavebeen strateda frequency-dependent was analogous to Rose et al.'s (1967) observations. takenintoaccount(althoughmechanicaleffectsareignored Theseobservations werefollowedby a periodof paramin this article), etermanipulationthat aimedto fit themodel'sresponding to k(t) =g[S(t) + A]/[S(t) + A +B], detailedpublishednumericalaccountsof auditoryfiberacfor [s(t) +•t] >0, tivity (WestermanandSmith,1984).Whena usefulconfiguration of parameters hadbeenestablished, the modelwas k(t)=O, for[S(t)+AI<0, (1) furthertestedagainsttwo recentlypublished resultsinvolvwhereA andB are parameters of the modeland$(t) is the ing the "additivityprinciple"and the effectsof masking instantaneous amplitudeof the signal. stimulion the subsequent recoveryof response capability. A fraction lc(t)dt of the amount c(t) of transmitter in The followingaccountisnot a historicalrecordof thesedethe cleft is subjectto chemicaldestructionor lossthrough velopments but a demonstration of thestrengths andweakdiffusion.Another fractionrc(t)dt is takenbackup into the nesses of the modelusingthe final configurationof paramcell.The restremainsin the cleftto stimulatethe postsynapo eters.Thesevaluesaregivenin TableI alongside the values tic membrane.It is assumed, for the sakeof simplicity,that usedin Meddis(1986). Optimizingtheconfiguration of paspikeoccurrence in theauditorynerveislinearly,probabilis- rameters isa problematic affairwithnoguarantee of finding tically relatedto the residueof transmittersubstance in the an idealsetof values.An accountof the process of developcleft.Accordingly,the quantityc(t) is to beidentifiedwith ingthemwill bepostponed untilaftertheexposition of the the "excitation function" of Gaumond et al. (1983), Gau- mondet al. (1982), or Gray's (1967) "recoveredprobability," i.e., the probabilityof spikeemissiondisregardingrefractoryeffects.Resultsbeloware expressed in termsof the excitationfunctionbecauseWesterman(1985) haspresented the results of his observations in these terms and this performance of thecurrentmodel.Unlessotherwise stated, the stimuli used to evaluate the model are 1-kHz sinusoidal TABLE I. Valuesofparameters usedin thisanda previous evaluation ofthe model (Meddis, 1986). methodavoidsthe needto overlaythe model'sperformance with additional,possiblycontroversial, assumptions concerningtherecoveryfunctionof auditory-nerve activity. Transmitter taken back into the cleft is not immediately Meddis, 1986 A 8 5 B 320 300 g I 660 availablefor releaseagainbut is delayedin a reprocessing y store.A fraction xw(t)dt of the amount of transmitter w(t) in thisstoreis continuously transferredto the freetransmitter pool.The transmitteroriginatesin a manufacturing base or "factory"that replenishes the free transmitterpool at a ratey [ tn -- q(t) ], wheretn is the (approximate)maximum l r x dt 16.67 500 12 500 I 000 0.00005 10013 11.11 I 250 16 667 250 0.00005 Time constants (ms) T• 60 T, 2 quantified versionof the model,rn is setto unityand all T, 0.08 transmitter amounts are construed as fractions of the total Tx 1 amount of transmitter to be found in the pool. In the un- New values 198 0.40 0.152 15.08 possibleamount. 1057 J. Acoust. Sec. Am., Vol. 83, No. 3, March 1988 Ray Meddis: Model of auditory-neural transduction 1057 stimuli with either an instantaneous rise time or a rise time of 2.5 ms. The outputof the model,its excitationfunction,is based on the cleft contents c(t). The cleft contents are al- waysaveragedoveronewholecycleof a 1-kHz signalthen multipliedby a factor 69 080 in order to estimatethe approximatefiring rate in events(spikes) per secondfor that cycle.This valuewasbasedon fittingthe functionsby eyein Fig. 2 in orderto arrive at a compromise,goodfit between modelresultsand empiricaldata. A. Rate intensity Usingthe parametersin Table I, column2, a new setof rate-intensity curveswasproducedandisgivenin Fig. 2. The resultsare comparedwith Westermanand Smith's (1984) resultsfor a singlefiber (E8F2) with a centerfrequencyof 1170Hz. Figure2 distinguishes two rate-intensity functions. The steady-statefunction representsthe firing rate after adaptationto the stimulustoneandis sampled300 msafter the toneonset.The onsetfunctionrepresents the firingrate in the 1-msperiodwiththehighestrateof firingimmediately followingtoneonset. WestermanandSmithgive0 dB as"AV threshold."In the absenceof a more precisedefinition,we have defined0 dB for the modelasthe pointat whichtheonsetandsteadystatefunctionsdiverge. B. Rapid and short term adaptation Westermanand Smith (1984) alsostudiedthe adaptation functionsof fibersto brief tonebursts.Figure 3 showsa recoveredPST histogramfor the samefiber (E8F2) in response to toneburstsat 63 dB abovethreshold.Resultsof the modelin response to the samestimuliare superimposed on their data. Westermanand Smith characterizedthe adaptation functionasthe sumof two exponentialdecayfunctionsplus a constant.The morerapiddeclinehad a time constantless than 10 ms and the slower (short-term) declinehad a very muchslowertime constantin the regionof 70 ms. Figure4 shows their estimate of the two time constants for the same fiber as a functionof the intensityof the tone pulse.The short-term time constant remains steady acrossa 40-dB range. The rapid time constant,however,showsa steady declinefrom approximately8 to 1.5 ms. The resultsof the model (dottedline) aresuperimposed on the empiricalvaluesin Fig. 4. The methodof fittingthe exponentials to themodelresultsis givenin the Appendix. Becausethe model results take the form of smooth curves, the process of curvedfittingis relativelystraightforward. Time constants were the same for both instantaneous and 2.5-ms rise times. C. Phase locking From an early stageit was clear that the ability of the model's excitation function to reflect the fine structure of the stimuluswas limited by the rate at which the transmitter could be cleared from the cleft. In the model this is affected 1200 by two routes:(a) dissipationand chemicaldestructionin the cleftand (b) reuptakeinto thecell.Whentheseare slow relativeto the stimulusfrequency,phaselockingwill beless evident.The process will alsobeaffectedby theabilityof the haircellpermeability functionto respondquicklyenoughto Instantaneous rise-time lOOO 800 lO00 2.5 ms 600 rise-time 400 200 Steady state 0 10 20 30 40 500 Relative Intensity (dB) Time (ms) FIG. 2. Comparisonof rate/intensityfunctionsbetweenmodel behavior (dotted) andWesterman's ( 1985,p. 74) gerbildata (solidline). In thecase of the model,I ms (onsetfunction) refersto the excitationfunctionduring the first (or highest)millisecondaftertoneonset.The steady-state function is based on the excitation 1058 function 300 ms after tone onset. J. Acoust.Sec. Am., Vol. 83, No. 3, March 1988 FIG. 3. Poststimulustime excitation function for the model (dotted line) comparedto Westerman's( 1985,p. 72) derivedexcitationfunction(solid line) for thesamefiberusedin Fig. 2. The stimulusfor themodelwasa 43dB, 300-ms,1-kHz tone againsta backgroundof silence. Ray Moddis:Modelof auditory-neuraltransduction 1058 •0o kHz wereusedasstimulianddt reducedto 0.01ms.Figure5 givesthesynchronization coefficient for thecomputersimulationasa functionof frequency. Johnson (1980) alsoshowedthat,for a givenfrequency/, synchronization increasedwith stimulusamplitudeover a limitedrange.This rangewasnot the sameasthe dynamic rangeof the adaptedfiringrate of the fiberbut commenced itsupswingwellbeforethe firingraterisesabovethe spontaneouslevel.The modelsuccessfully mimicsthis effect.Figure 6 showsbothsynchronization andratemeasuresderived from the model'sperformance asa functionof stimulusamplitude. Short term lO D. Additivity test The increasein firingrate followinga stimulusamplitude incrementhasbeenshownto be independent of the stateof adaptationof the fiber (Smith and Zwislocki, 1975; Rapid 0 10 20 Relative Intensity 30 Smith, 1977; Smith etal., 1985). This effectis true for onset ( 1-ms window) and short-term ( 10-ms window) measures 40 of rateincrease. The effectis alsovalid,followingstimulus (dB) FIG. 4. Time constants for two additiveexponential components fittedto the excitationfunctionfollowingbrief tonebursts.The toneburstsfor the model(dottedline) werel-kHz sinusolds (2.5-msrisetime). Theintensity ofthetoneburstsisrelativeto threshold. Theempiricalvalues(solidline) aretakenfromWesterman andSmith( 1984,Fig. 8). amplitudedecrement,for short-termmeasuresof rate decreasebut notfor onsetratemeasures. Followingthemethod of Smithet al. (1985), a 1-kHz pedestaltone, 13 dB above thresholdwas presentedto the model followedby a 6-dB increment or decrementat 0, 10, 20, and 30 ms after the onsetof thepedestal.The increment/decrement in rateisthe differencebetweenthe response to the pedestalplusincre- theinstantaneous amplitude ofthesignal. Thislatterprocess isnotsimulated heresothattheformerprocess canbestud- ment and the pedestalalone. ied in isolation. Figure5 showsRoseet al.'s ( 1967) synchronization coefficient expressed asa functionof stimulusfrequency. This coefficient isbasedonperiodhistograms andrepresents the "mostpopulous" halfof thehistogram asa percentage of its total area.A valueof 50% indicatesno phaselocking.A well-replicated findingis that synchronization measures declinein strengthbetween1 and 5 kHz. 90 Synchronizalion 8O To testthe model, sinusoidalstimuli of 1, 2, 3, 4, and 5 lOO Synchronization Coefficient % 6O 70 60 5O 5O -70 I 2 3 Frequency 4 (kHz) FIG. 5. Synchronization coefficientas a functionof stimulusfrequency. Modelbehavior(dottedline) wascomputedusingdt = 0.01ms.Empirical data (solid line) are taken from Rose et al. (1967). 1059 J. Acoust.Sec. Am., Vol. 83, No. 3, March 1988 0 60 5 Amplitude d I• re threshold FIG. 6. Synchronizationcoefficientasa functionof stimulusamplitudefor a l-kHz tone (solid line). Steady-statefiring rate of the modelasa function of amplitudefor the samestimulus(dotted line). Ray Meddis:Model of auditory-neuraltransduction 1059 250 200 (a)+6clB e 150 (c)-6clB ß•,, FIG. 7. Effectsof prior adaptationon response to 6-dBincrements anddecrements of a 1-kHz pedestaltonepresented at 13dB abovethreshold.Here, (a) and (c) are rap- 100 (b)+6rib m --...'-.ß. Onset (1ms) id effects based on the first millisecond after stimuluschange, and(b) and(d) areshortterm effects derived from the first 10 ms 5O afterstimuluschange.Empiricalstudiesobtain horizontal lines for (a), (b), and (d). Shortterm,(10 ms) Delay (ms) The resultsdo not agree with their results (Fig. 7). Their studyshowedhorizontalfunctionsfor 6-dBincrement CF = 842 Hz). Clearly, the ability to producea brief response recoversmorequicklythanthe abilityto sustainthat (I- and 10-ms window) and for 6-dB decrement (10-ms window). The short-term decrement function (1-ms win- response. dow) wasshownto reducewith increasingdelay.The model doesshowthe requiredresponses followingstimulusdecrementsbut isclearlydiscrepantduringthefirst 10msof delay for stimulus increments. E. Recovery of function Followingan intensemaskingtone,spontaneous firing of the fiberis brieflysuppressed beforeslowlyrecovering to normalspontaneous levels.Westerman(1985) givesmean recoverytime constantsof 40 ms (standarddeviationof 25 ms) basedon 12 fibers.The model,usingthe specifiedfinal parameters,hasa recoverytime constantof 46 ms. In the first millisecondfollowingstimulusoffset (50 dB), theexcitationfunctionof thecomputermodelfallsto a valueequivalent toa rateof9 spikes/s. Thiscontrasts witha periodof totalsuppression thatiscommonly observed. The modelis,therefore,unableto explaina totalsuppression of spikeactivity.Alternatively, wemaybeseeking anexplanationof thedeadperiodin thewrongplace.The totalpoststimulus suppression mayreflectpostsynaptic fatiguethatis not represented in the modelat all. The dashedlinesin Fig. 8 showthe resultsof applying this experimentalparadigmto the model.There are some cleardiscrepancies betweenthe model'sbehaviorand the empiricaldata.However,thedashed linesareapproximately straightfor muchof theirlengthand,therefore,indicatean exponentialimprovementin the capacityto respond.Between0 and 20 ms,thereis an upturnin the modelresults tooo lOO lO Westerman (1985) also measuredrecoveryof the ca- pacityto respondto a secondstimulusby presenting 30-ms testtonesat varyingintervalsafterthe cessation of a 300-ms durationmaskingtone.Bothtoneswere43 dB abovethreshold. The responsewas measuredas a decrementwhen com- paredto theresponse in theabsence of a preceding masking tone. The onset rate is based on the first millisecond after the o 1 DO 200 Time after masker testtoneonset.The short-termresponse isbasedon the period 20-30 ms after the onset of the test tone. Westerman's resultsaregivenassolidlinesin Fig. 8. The useof a logarithmic scalefor theresponse decrementmeasuremeansthat the two straight line functionsobtainedrepresentexponential recoveryin both cases.Westermanfound two recoverytime constants,49 ms for the onsetresponseand 68 ms for the short-term responsefor this particular fiber (E27F13, 1060 J. Acoust. Sec. Am., Vol. 83, No. 3, March 1988 FIG. 8. Recoveryof responsefollowingadaptation.The decrementis the differencebetweenthe responseof an unadaptedfiber to a 43-dB (re: threshold) 1-kHz tone and the responseof a fiber soonafter the offsetof a 300-ms,43-dB maskingtoneof the samefrequency.The onsetdecrements are basedon the maximal 1-msfiring rate after test tone onset.The shortterm decrements are based on the rate between 10 and 30 ms after test tone onset.Solidlinesaretakenfrom Westerman( 1985,p. 52, Fig. 21). Dotted linesrepresentthe response of the model. Ray Meddis: Model of auditory-neural transduction 1060 suggesting a departurefroma simpleexponential improvement.This is not necessarily inconsistent with Westerman's data pointseventhoughhe choseto fit a singlestraightline timeconstants; y, thereplenishment factor,affectsspontaneousandadaptedfiringratesaswellastheshort-termadapta- throughout.I haveredrawnhis "best-fitlines" to illustrate the cleft into the cell, affectsphaselockingand the shortterm adaptationtime constant;x, the rate of transmitterreprocessing, affectsonly the adaptationtime constants; l, the rate of lossof transmitterfrom the cleft--and, hence,from the wholesystem--influences all firingratesandthe shortterm adaptationtime constant. this possibility.Perhapsa more detailedanalysisof additional data will resolve this issue. Thereare two importantdiscrepancies that deserveattention. First, Westermanfound differenttime constantsfor the recoveryof onsetandshort-termresponding. For seven tion time constant;r, the rate of return of transmitter from fibers studied in detail, all had faster time constants for the recovery of the onset response.The model, by contrast, IV. DISCUSSION showsequivalenttimeconstants forbothrecoveryprocesses. For Fig. 8, Westermangivestime constantsof 49 and 68 ms Two possibleusesof hair cell modelswere identifiedin the Introduction. First, theyarea readilytestablestructural for onset and short-term functions. The time constant for account of what is actuallyhappening at thehaircell-audibothfunctions usingthecomputermodelwasapproximately tory-nerve synapse. Second, they can be usedasa generator thesameashisshort-termrecoveryfunction.For a sampleof of trainsof spikesto act as input to other modelsof, for sevenfibers,Westermangivesmeanrecoveryfunctionsof 48 example, cochlear nucleus functioning, binauralhearing,sems (s.d. = 25 ms) for onsetand 169 ms (s.d. = 79 ms) for lective attention, speech recognition, etc. Differentcriteria short term. of usefulness applyin thesevariouscases. Certainly,weaker criteriamustapplyto the modelasan inputdeviceto other III. OPTIMIZING PARAMETERS modelsbecausethe development of theoriesconcerning The preceding exposition isbasedonsimulations using "higher" processes cannotwait until all the problemsof anunchanging parameter set.Similarly,therescaling ofcleft characterizingthe "lower" processes have been solved. contentsto indicatepotentialfiringrateusedthesamescale Compromiseis unavoidable. factorthroughout.While it is encouraging that the model The currentmodelis clearlyverysuitablefor useas a wasableto fit the empiricaldata aswell asit did, we haveno spikegenerator because the cleftcontents, whenmultiplied guaranteethat its performancecould not have been imby a suitableconstant,can be viewed as a statementof the provedwith a bettersetof parameters. The methodof paprobabilitythat a spikewill occurat that time. A random rameter optimization used here was the laborious "hillnumbergeneratorcan,therefore,beusedto decidewhethera climbing"approachof changingoneparameterat a timeand spikedoesoccurat thattime.The modelwill acceptanarbinotingthe effect.If the effectwasbeneficial, thissetof patrary stimulussampledat any rate that wouldnormallybe rameterswasusedasa new startingpoint;otherwise,it was acceptablein acousticanalysis.In response,it producesa necessaryto revert to the previousset and make a different streamof spikespreciselylocatedin time. Rate measures can change. bederivedfromthisoutput,asrequired.Moreimportantly, The benefitof eachnewparameterchangewasassessed knowingthe precisetimingof eacheventis a specialvirtue in termsin a numberof dependent measures thatcompared modelperformance withtargetvaluesderivedfromempiricaldata.The measures andthetargetsgivenin bracketsare asfollows:( 1) ratioofspontaneous to 100-dBadaptedfiring rate (0.2); (2) dynamicrange(30 dB); (3) rapid adaptationtimeconstant nearthreshold( 8 ms); (4) rapidadaptationtimeconstants at 40 dB (2 ms); (5) short-termadaptation time constantnear threshold(75 ms); (6) short-term adaptationtimeconstantat 100dB( 75 ms); ( 7) phaselocking (valuesgivenby Roseet al., 1967). It wasnotpossible to matchall of thetargetsexactlyand theparameter setusedrepresents ajudicious compromise. It is quitelikelythatthis'setcouldbeimprovedupon. It is not a simplematter to identifyindividualparametersof the modelwith the dependentmeasuresused.If it were,thenthediscovery of anoptimumparametersetwould havebeenverymucheasier.Changinganyoneparameterin isolationtypicallyaffectsall measures. However,individual for thoseanalysis systems thatdependuponthetimeintervalsbetweenspikes(e.g.,Moore,1982)or, moregenerally, which involveany kind of phasesensitivity(Patterson, 1987).The simplicityof themodelallowsfor rapidnumericalevaluation.A recentimplementation onan 8-bit6502 ( 1MHz) processor runsat 10timesrealtimewhenusinga 20kHz samplingrate and we expectto producea real-time implementation usinga morepowerfulprocessor in thenear future.Moreover,themodelappearsto mimicall of themajor propertiesof auditory-nerve response. Suchdefectsas havebeenrevealedso far are unlikelyto affectadversely research progress for systems usingthismodelasan input device. The failingsof the model,however,are muchmorecritical whenevaluatingits potentialas a structuralaccountof eventstaking placeat the point wherethe auditorynerve meetsthe hair cell. It is not possibleto makea directcomparisonwith other publishedaccountsbecausethis is the parameterstypicallyaffectsomemeasuresmorethan others: firsttime that thisparticularsetof experimentalparadigms .4, whichoccursin the permeabilityequation,affectsthe hasbeensimulated asa complete set.However,therangeof spontaneous firing rate and the responsethreshold;B affects phenomenasuccessfully simulatedsuggests that the model everythingexcept the adaptationtime constants;g indefectsare relativelyminor. Theseinvolvetwo anomalies. fluences the rate of outflow of transmitter from the cell into First, the onsetresponse showsan unrealisticsensitivityto thecleftandthusaffects all firingratesandrapidadaptation levelof adaptationduringthe first 10 ms of the adaptation 1061 J. Acoust.Soc.Am.,Vol.83, No.3, March1988 RayMeddis:Modelof auditory-neural transduction 1061 process. Second,recoveryof theabilityto respondto a new stimulusfollowingan intensemaskingstimulusshowsimportantdiscrepancies with empiricalresults. The failure to replicatethe additivity effectfor onset responses is an importantproblem.A numberof existing models (Sehwid and Geisler, 1982; Smith and Brachman, 1982;Cooke,1986;WestermanandSmith, 1986) haveexpli- citly directedtheirmodelingeffortstowardsexplainingadditivityby suggesting that stimulusintensitymodulatesthe amountof transmittereligiblefor release,i.e., the stimulus controls the volume of the free-transmitter reservoir. Wes- terman and Smith's (1986) most recentaccountproposes that both the volumeand the membranepermeabilityare stimulusdependent. If thesemodelsin anexplicitsimulation canbeshownto reproducethe additivityeffectsin the paradigmillustratedabove,a casecouldbemadefor introducing multiplereleasesitesor variablevolumereservoirsinto the modelcurrentlyunderdiscussion. The attemptto simulateWesterman's(1985) function for therecoveryof the abilityto respondto a stimulusafter previousintensestimulationalsorevealeddiscrepancies. In addition,Westerman(1985) findstwo differentexponential recoveryfunctions,one for effectsmeasuredimmediately after the onset of the test stimulus and one for the increase measuredduringa period10-30 msafterwards.For thebulk of the recoveryperiodthe modelgenerates only onerate of recoverythat is the samefor both measures.Westerman and Smith(1986) haveshownthat thiseffectcanbemodeledby introducingan additionaltransmitterreservoirbetweenthe globalstore fraetory) and the free-transmitter pool. Ross (1982) usedtwo additional reservoirsin cascade.Some such amendmentto the modelmay be requiredif it is not found possibleto solvethe problemby parametermanipulation. One problemwith the currentpositionis that Westerman givesmeantime constantsof 48 and 169 ms for rapid and short-termeffects,respectively.However,the standard deviationsfor thesetime constantsare veryhigh indeed(25 and 79 ms). Somerapid recoverytime constantsfor certain animals must be slower than some short-term time constants for other animals. Moreover, some short-term time ari- srants,in excessof 350 ms, appearto have beenestimated overonly200-mstimeperiods.Insofarastheremayberoom for reevaluatingWesterman'spioneeringfindings,it may be wiseto delayradicalrevisionof the computermodel. Havingdweltat lengthon the difficultieswith the model, it isusefulto rehearsethemanyphenomena that themodel has successfully simulated.This summaryis alsodrawn from a previousreport (Meddis, 1986) that usedthe same decrements in stimulation intensityasa functionof adaptation level for both onset and short-term measures and for short-termmeasuresafter stimulusincrement;and (8) real- istic rate of recoveryof spontaneous firing ratesfollowing intense stimulation. An interestingfeatureof the modelis its relianceon transmittermovementdelaysto generatethe familiarpropertywherebyphaselockingislimitedbystimulus frequency. Little attentionhasbeengivento thispossibility whichexists whetheror not we acceptthe ideaof transmitterreuptake intothehaircell.Evenona puredissipation anddestruction principle,theremustbe somedelayin clearingtransmitter from the cleft. Recent work on the close association between the receptorpotentialsof innerhair cellsandphase-locking indicesis troubledby two difficulties: First, directextrapolation from receptorpotentialseemsto underestimate phaselockingability,and,second,that the greatvariationamong species in phase-locking abilitymayimposetoogreata strain on the theory (Palmer and Russell,1986). While the model's successful use of transmitter movement as a basis for generatingthe phenomenon doesnot grantit the statusof a true explanation,it doesrequirethat thispossibilitybetaken into account in future discussion of the matter. The reuptakeprinciplewasoriginallyadoptedfor reasonsof computationalexpediency.However,a recentstudy (Siegeland Brownell, 1986) has shownthat this process may indeed be at work. They offer evidenceof membrane recyclingat the inner hair cell synapse.Someof the membranerecoveredfrom the cleft (presumablyby a processof invagination)appearsto be usedin the formationof new synapticvesicles.This providescircumstantialevidence,at least,that a fastrouteexistsfor the reuptakeof largemoleculesfrom the cleftinto the presynapticregion. While the modelhasmanyinterestingand satisfactory features,it isacceptedthat it maybenefitfrommodifications basedon other publishedmodels.Unfortunately,no direct comparisonhasyet beenmadethat would allow a summary of therespective strengths of the differentmodels.It is proposedthat thesetof testsdescribed in thisarticlecouldprovidea minimumsubsetfor comparativetestingof all current modelsand a project to do this is currently planned.For sucha comparisonto be fully effective,someobjectiveand preferablyautomaticmethodfor optimizingthe parameters in eachmodelisclearlycalledfor--especiallyfor thosemodelsthat do not allow for analyticsolutions.This problemis underactivediscussion in many areasof scientificendeavor (Kirkpatrick et al., 1983) and someof the proposedsolutionswill be activelyexploredin this context. modelwithonlyparameterchanges. Phenomena successfully simulatedare as follows:(1) steady-state rate/intensity APPENDIX functions;(2) poststimulusperiod histogramsat various The adaptationcurve of the excitationfunction followlevelsof stimulusintensity;(3) interspike-intervalhistoinga stimulusincrementwasdescribed in termsof theequagramsfor silenceand 70-dB, 1-kHz sinusoid;(4) nonmono- tion tonic functionsrelating incrementaland deerementalreY, = a + be- ,/r, + ce- ,/r., (A1) sponsesto stimulation amplitude changesas a function of backgroundstimulationintensity;(5) adaptationfunctions that can be describedas the sumof two exponentialdecay functions;(6) realisticsynchronization coefficients decayingasa functionof frequency;(7) realisticeffectscausedby 1062 J. Acoust.Soc. Am., Vol. 83, No. 3, March 1988 where¾,istheexcitationfunctionat timet, e istheexponential constant,and a, b, c, T•, and T2 are valuesto be discovered by the method. To begin, we need to find a minimum valueof Y ( Ymi,), SOthat Ycan be rescaledthus, Ray Meddis:Modelof auditory-neuraltransduction 1062 Y = Y-- Y•,i,- (A2) Sinceweknowthatadaptation, for ourpurposes, isvirtually completeaftera quarterof a second,we canusea valuenot much smaller than the excitation function after 300 ms of adaptationhaveelapsedfor Y.,i,. Here, Y•,,, is alsotakenas our estimateof the parametera. We assumethat the firsttimeconstant(T•) is unlikely to be greaterthan 10ms.As a result,we do not expectthis firstprocessto make muchcontributionto the functionafter 40 ms. We, therefore,computeT2 on the basisof values Soc. Am. 68, 1115-1122. Lyon,R. F. (1985). "Processing Speechwith the Multi-SerialSignalProoessor,"IEEE ICASSP-85, 981-985. Meddis, R. (1986). "Simulation of Mechanical to Neural Transduction in the AuditoryReceptor,"J. Aeoust.Soc.Am. 79, 702-711. Moore,B.C. J. ( 1982). An IntroductiontothePsychology of Hearing( Academic, London). Nilsson,H. G. (1975}. "Modelof Discharge Patternsof Unitsin theCochlear Nucleusin Response to SteadyStateand Time-VaryingSounds," between,say,40 and 80 ms: T2 = (80 - 40)/[ln(Y4o) -- In(yea)] Biol. Cybernet.20, 113-119. Oono, Y., and Sujaku, Y. (1975). "A Model for Automatic Gain Control and c = exp[ln(y4o)+ 40/T2]. If we now removethe asymptoteand the effectsof the slow adaptationfrom the originaldata, y• = Y, -- a -- cewecanfindtheparameters of therapidadaptationfunction usingthetwo datapointsat 1 and2 ms: T• = (2- 1)/[ln(y• ) --ln(yl) ], b=exp[ln(y[) + 1/T•], a= Ymi,. Observedin the Firingsof Primary Auditory Neurons,"Trans. Inst. Electron.Comm.Eng.Jpn.58, 352-358. Palmer,A. R., and Russell,I. J. (1986). "Phase-locking in the Cochlear Nerve of the GuineaPig and Its Relationto the ReceptorPotentialof Inner Hair Cells," HearingRes. 24, 1-15. Patterson, R. D. (1987). "A Pulse Ribbon Model of Monaural Phase Per- ception,"J. Acoust.Soc.Am. 82, 1560-1586. Rose,J. E., Brugge,J. F., Anderson,D. J., and Hind, J. E. (1967). "PhaselockedResponse to Low-Frequency Tonesin SingleAuditoryNerveFi- bersof theSquirrelMonkey,"J. Nenrophysiol. 30, 767-793. Ross,S. (1982}. "A Model of the Hair Cell-PrimaryFiber Complex,"J. Acoust. Soc. Am. 71, 926-941. Schroeder,M. R., and Hall, J. L. (1974). "Model for Mechanicalto Neural Transductionin the Auditory Receptor," J. Acoust. Soc. Am. 85, Thismethodisadequatewhenthefunctionsaresmooth and the basic model holds. If random variation Gray, P. R. (1967). "ConditionalProbabilityAnalysesof the SpikeActivity of SingleNeurons,"Biophys.J. 7, 759-777. Kirkpatrick,S.,Gelatt,C. D., Jr., andVicchi,M.P. (1983). "Optimization by SimulatedAnnealing,"Science220,671-680. Johnson,D. H. (1980). "The RelationshipbetweenSpikeRateandSynchronyin Responses of Auditory-NerveFibersto SingleTones,"J. Aeoust. affects the data, then the time constantsT• and T2 mustbe estimated overa rangeofvaluesusingleast-squares methods.An alternativemethodand references to the literatureare givenby Westerman( 1985,AppendixB). Brachman,M. L. (1980). "Dynamic ResponseCharacteristics of Single AuditoryNerve Fibers,"SpecialReportISR-S-19,Institutefor Sensory Research,SyracuseUniversity,Syracuse,New York 13210. Cooke,M.P. (1986). "A ComputerModelof Peripheral AuditoryProcessing," SpeechCommun.S, 261-281. Eggermont, J. J. (1973). "AnalogueModellingof CochlearAdaptation," Kybernetic14, 117-126. Evans,E. F. (1986). "CochlearNerveFibreTemporalDischargePatterns, CochlearFrequencySelectivityand the Dominant Regionfor Pitch," in AuditoryFrequency Selectivity, editedbyB.C. J. MooreandR. D. Patterson (Plenum, New York). Gaumond,R. P., Kim, D. O., and Molnar, C. E. (1983). "Responseof CochlearNerveFibersto BriefStimuli:Role of DischargeHistoryEf- 1055-1060. Schwid,H. A., andGeisler,C. D. (1982). "Multiple ReservoirModelof Nenrotransmitter Releaseby a Cochlear Inner Hair Cell," J. Acoust. Soc. Am. 72, 1435-1440. Sellick,P.M., and Russell,I. J. (1980). "The Responses of Inner Hair Cells to BasilarMembraneVelocityduringLow FrequencyAuditoryStimulation in the GuineaPig Cochlea,"Hear. Res.2, 439 Siebert,M. W. (1965). "SomeImplicationsof the Stochastic Behaviourof PrimaryAuditoryNeurons,"Kybernetic2, 206-215. Siegel,J. H., andBrownell,W. E. (1986). "SynapticandGolgiMembrane Recyclingin CochlearHair Cells,"J. Neurocytol.15, 311-328. Smith, R. L. (1977). "ShortTerm Adaptationin SingleAuditory Nerve Fibers:SomePoststimulatoryEffects,"J. Neurophysiol.40, 1098-1112. Smith, R. L., and Brachman,M. L. (1982). "Adaptation in Auditory Nerve Fibers:A RevisedModel," Biol. Cybernet.44, 107-120. Smith, R. L., Brachman,M. L., and Frisina,R. D. (1985). "Sensitivityof Auditory Nerve Fibersto Changesin Intensity:A Dichotomybetween Decrements and Increments," J. Acoust. Soc. Am. 78, 1310-1316. Smith,R. L., and Zwislocki,J. J. (1975). "Shortterm Adaptationand IncrementalResponses in SingleAuditory-NerveFibers,"Biol. Cybernet. 17, 169-182. Weiss,T. F. (1966). "A Modelof thePeripheralAuditorySystem,"Kybernetic 3, 153-157. Recovery Dependence ofCatCochlearNerveSpikeDischarge Probabili- Westerman,L. A. (1985). "Adaptationand Recoveryof Auditory-Nerve Responses," Ph.D. thesis,Syracuse University,Syracuse, NY. Westerman,L. A., andSmith,R. L. (1984). "RapidandShortTerm Adaptationin Auditory-NerveResponses," Hear. Res.15, 249-260. ty,"J. Neurophysiol. 48,856-873. Westerman, L. A., and Smith, R. L. (1986). "A Diffusion Model of the fects,"J. Acoust. Soe.Am. 74, 1392-1398. Ganmond, R. P., Molnar, C. E., and Kim, D. O. (1982). "Stimulus and Geisler, C. D., Sanh Le, and Schwid, J. (1979). "Further Studieson the Schroeder-Hall Hair-Cell Model," J. Acoust. Soc.Am. 6S, 985-990. 1063 J. Acoust.Soc. Am.. Vol. 83, No. 3, March 1988 TransientResponse of the CochlearInner Hair Cell Synapse,"Manuscript. Ray Meddis:Modelof auditory-neuraltransduction 1063