194

SINGLE-CELL ANALYSIS BY DROPLET RADIOFLUIDICS
S. Türkcan
1
, J. Nguyen
2
, M.
Vilalta
3
, G. Pratx
1
, and P. Abbyad
2*
1
Stanford University School of Medicine, USA
2
Santa Clara University, USA and
3
Stanford University, USA
ABSTRACT
We present a new microfluidic technique, dubbed droplet radiofluidics, that can measure radiotracer uptake for single cells encapsulated into an array of microdroplets. This method was used to measure the cell-to-cell heterogeneity of [18F]fluorodeoxyglucose (FDG) uptake in single cancer cells subjected to different incubation conditions and expressing different types of glucose transporters.
KEYWORDS: droplet, single cell, radiolabel
INTRODUCTION
Radiolabels can detect small biomolecules with high sensitivity without interfering with biochemical activity. For instance, the radiolabeled glucose analogue, [18F]fluorodeoxyglucose (FDG), is routinely used in PET scans for cancer diagnosis, staging and monitoring. Despite its widespread usage, many questions and contradictory observations arise about FDG uptake in cancer cells, even within the same cell line [1]. Radioluminescence microscopy, first reported in 2012 by Pratx et al., allowed for the first time the imaging of radiotracers on single cells [2]. Droplet radiofluidics, presented here, confines cells in droplets to extend the throughput and versatility of the method.
EXPERIMENTAL
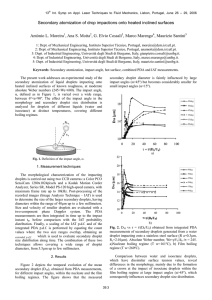
The general principal of droplet radiofluidics is shown in Fig. 1. Flow focusers are used to produce droplets and encapsulated cells. Using a technique called “rails and anchors” [3], microfabricated wells are used to anchor flattened droplets into an array by a decrease in confinement. Intracellular radiotracers are then imaged on a conventional microscope by capturing individual flashes of visible light that are produced as individual positrons, emitted during radioactive decay, traverse a scintillator plate below the cells. The advantages of the droplet approach are multiple. First, droplets are easily positioned in a predetermined pattern for optimal imaging throughput. Second, droplet encapsulation ensures that effluxed radionuclide is trapped in the droplet. Last, multiplexed measurements can be performed using fluorescent labels.
Figure 1: a) Cross-sectional diagram of the droplet radiofluidics platform. Droplets encapsulating cells are anchored in locations of higher channel height. Radioactive decay of intracellular FDG leads to a beta particle producing an ionization track and the emission of visible light in the CdWO4 scintillator, which is collected by an objective. b) Microfluidic channel design contains two flow focusers to make droplets. The wider channel contains an anchor array. Inset: Picture of device.
978-0-9798064-8-3/µTAS 2015/$20©15CBMS-0001 19 th
International Conference on Miniaturized
Systems for Chemistry and Life Sciences
October 25-29, 2015, Gyeongju, KOREA
RESULTS AND DISCUSSION
To validate the technique, two types of droplets, one containing radioactive FDG and the other fluorescein were flowed into the anchor array. Figure 2a shows a fluorescence image where only fluorescein containing droplets are bright. In contrast, when the excitation light is turned off, the only source of light is produced by radioluminescence. The locations of individual decay events are reconstructed to produce an image (Figure 2b). Comparison of Fig. 2a and b confirms that droplets light up either the fluorescence or radioluminescence image, but not in both. The number of FDG molecules observed for each droplet is summarized in Fig 2c.
Figure 2: Comparison of droplets containing a green fluorophore and a radioactive glucose analog, FDG. a) Fluorescence image of the droplet array superimposed on a bright-field image. The green droplets contain fluorescein while clear droplets contain FDG b) Radioluminescence image with heat map intensity ranging from blue to red with radiolabel count. c) Number of observed FDG molecules for single droplets of control and FDG droplets. The average value is indicated by a red line. The standard deviation and 95% confidence interval are indicated by a blue and red bar, respectively.
The method is used to measure cell-to-cell heterogeneity in FDG uptake in single colon and breast cancer cells. Multiplexed measurements were demonstrated by measuring FDG uptake of cells subjected to short (15 minutes) and long (60 minutes) incubation with FDG (Figure 3). An increase in radioactivity is observed for longer FDG incubation (Figure 3c).
Figure 3: Breast cancer cells with short (15 minutes) and long (60 minutes) incubations with FDG. a) Fluorescence with green droplets for long incubation and blue for short incubation. Cells marked in red. b) Radioluminescence with intensity primarily coincides with the position of long incubation cells. c) Number of observed FDG molecules for cells for long and short incubation.
We also measured differential FDG uptake in an isogenic cancer model, composed of two cell lines in which the glucose transporter 1 was either wild-type or knocked-out (Figure 4). At the single-cell level, the knockout reduces, but does not entirely block, FDG uptake (Figure 4c).
195
Figure 4: Colon cancer cells with wild-type (RKO WT) and glucose transporter 1 knockout (RKO KO). a) Fluorescence of the droplet array with clear droplets containing WT cells while green droplets contain KO cells. Cells marked in red. b) Radioluminescence intensity primarily coincides with the position of WT cells. c) Number of observed FDG molecules for single cells for the WT and KO.
CONCLUSION
Droplet radiofluidics allows the single-cell measurement of small molecules that cannot be easily labeled with a fluorescent dye, such as ions, amino acids, metabolites, and drugs. This method opens many new avenues of research in basic cell biology and human disease by capturing the full range of stochastic variations in highly heterogeneous cell populations in a repeatable and high-throughput manner.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Stanford small-animal imaging facility (SCI3); the Olympus
Corporation for providing the LV200; Stanford Cyclotron Radiochemistry Facility Staffs (Bin Shen,
Frederick T. Chin, Murugesan Subbarayan, George Montoya and Shawn Scatliffe) for producing/packing
18F-FDG. We would also like to thank International Electronic Components Inc. for their generous donation of dry photoresist films and Rob Campell for the notBoxPlot matlab function. This work was supported in part by a grant from the National Institutes of Health (R01CA186275) and by a Prostate Cancer
Research Program fellowship (W81XWH-14-1-0288). The radiotracer production was supported in part by a Stanford University, Department of Radiology Startup Funding (FTC), and a NCI ICMIC P50
CA114747 grant.
REFERENCES
[1] N.J. Avril, “GLUT1 Expression in Tissue and 18F-FDG Uptake,” J. Nucl. Med.
, 45, 930–932, 2004.
[2] G. Pratx, K. Chen, C. Sun, L. Martin, C.M. Carpenter, P.D. Olcott, and L. Xing, “Radioluminescence microscopy: measuring the heterogeneous uptake of radiotracers in single living cells,” PLoS
One , 7, e46285 2012.
[3] P. Abbyad, R. Dangla, A. Alexandrou, and C.N. Baroud, “Rails and anchors: guiding and trapping droplet microreactors in two dimensions,” Lab Chip , 11, 813–821, 2011.
CONTACT
* Paul Abbyad ; phone: +1-408-554-6948; pabbyad@scu.edu
196