Detailed description of four YAC contigs representing 17 Mb of
advertisement

The Plant Journal (1996) 9(5), 755-765
TECHNICAL ADVANCE
Detailed description of four YAC contigs representing
17 Mb of chromosome 4 of Arabidopsis thaliana ecotype
Columbia
Renate Schmidt t, Joanne West, Gerda Cnopst,
Karina Love, Alma Balestrazzi§ and Caroline Dean*
Department of Molecular Genetics, John Innes Centre,
Norwich Research Park, Colney, Norwich NR4 7UH, UK
Summary
The detailed arrangement of 563 YAC clones comprising
four contigs covering -17 Mbp of chromosome 4 is presented. YAC clones were positioned relative to each other
and to markers by taking into account marker and end
fragment hybridization data and the sizes of all YAC
clones. This analysis made it possible to estimate physical
distances between the majority of chromosome 4 markers.
It also identified a relatively large number of YAC clones
containing chimaeric inserts. The YAC contig map of the
Columbia ecotype presents an important resource for
map-based cloning experiments, rapid mapping of DNA
sequences and large-scale genomic sequencing programs.
Introduction
Arabidopsis tha/iana is an important model organism for
the analysis of complex plant processes using molecular
genetic techniques (Meyerowitz and Somerville, 1994).
Many laboratories are currently pursuing map-based cloning strategies to isolate Arabidopsis genes. This experimental approach would greatly benefit from the availability
of a complete physical map of the Arabidopsis genome.
The first attempt to produce a physical map by fingerprinting cosmid clones, paralleling the Caenorhabditis
elegans genome project (Coulson et al., 1986), resulted in
750 contigs with an average size of 120 kb (Hauge et al.,
1991). When yeast artificial chromosome (YAC) libraries
became available (Grill and Somerville, 1991; Ward and
Jen, 1990) an international collaboration was set up
Received 13 November 1995; revised 6 February 1996; accepted 21
February1996.
*For correspondence (fax +44 1603 505725; e-mail arabidopsis@bbsrc.ac.uk).
tpresent address: Max-Delbr0ck-Laboratorium in der Max-PlanckGesellschaft,CarI-von-Linnd-Weg10, 50829Cologne,Germany.
tPresent address: Laboratorium Genetika, Universiteit Gent,
Ledeganckstraat35, 9000Gent,Belgium.
~Present address: Dipartimento di Genetica e Microbiologia, Via
Abbiategrasso207,27100Pavia,Italy.
with the aim of generating a contig map of the whole
Arabidopsis genome based on YAC clones. The initial
experiments used 125 RFLP markers to identify and position
296 YAC clones, representing approximately 30% of the
Arabidopsis genome (Hwang et al., 1991).
Since then many more RFLP markers have become
available and PCR markers have been developed (Bell and
Ecker, 1994; Konieczny and Ausubel, 1993; Reiter et al.,
1992). Currently, more than 100 DNA markers have been
mapped to Arabidopsis chromosome 4. Four Arabidopsis
YAC libraries made from the Columbia ecotype, CIC
(Creusot et al., 1995), EG (Grill and Somerville, 1991), EW
(Ward and Jen, 1990) and yUP (Ecker, 1990) are available
for physical mapping experiments, representing in total at
least 10 genome equivalents. The plant DNA insert sizes
differ between the libraries, with the average being 160 kb
in the EG and EW libraries, 250 kb in the yUP library and
420 kb in the CIC library. The frequency of repetitive
sequences (Creusot et al., 1995; Schmidt eta/., 1994;
Dunn and Ecker, unpublished results) also varies between
the libraries.
We recently published a tiling path of YAC clones
covering more than 90% of the genetic map of chromosome
4. Mapping of the rDNA locus, the repeated sequences
flanking the centromere and 77 genetically mapped
markers on the YAC clones allowed us to integrate the
cytogenetic, genetic and physical maps of chromosome 4
(Schmidt eta/., 1995). To establish the tiling path, 158
probes were used. We have extended this physical mapping and report the results for a total of 263 probes.
Furthermore, we indicate known chimaeric clones and
present all the YAC clones hybridizing to the markers rather
than only the YAC clones which link two or more markers.
The emphasis of the work presented here is on the relative
positioning of YAC clones and markers, allowing the physical distances between markers to be estimated and greatly
increasing the usefulness of the YAC contigs in map-based
cloning experiments.
Results
Southern blot analysis of YAC clones
The YAC tiling path for Arabidopsis chromosome 4
(Schmidt et al., 1995; World Wide Web at URL: http://
755
756
Renate Schmidt et al.
YAC contigs on Arabidopsis chromosome 4 757
758
Renate Schmidt et al.
nasc.nott.ac.uk/JIC-contigs/JIC-contigs.html) shows the
order of 158 probes along the chromosome and all YAC
clones which link two or more probes. However, it neither
displays the different physical sizes of the YAC clones
nor the distances between particular markers. In order to
achieve a representation which also fulfils these criteria
additional data had to be determined for the YAC clones.
First, the YAC insert sizes for all YAC clones which have
been mapped on to chromosome 4 were determined using
pulsed field gel electrophoresis (PFGE). The sizes of the
YAC inserts in the CIC library were already available
(Creusot et al., 1995).
Second, Southern blot analyses were used to position
YAC clones relative to each other and to probes. The
Southern blots contained all YAC clones identified as
carrying chromosome 4 DNA, digested with EcoRI/BamHl.
This restriction digest ensured that for the EG, yUP and
the CIC clones the insert DNA was removed completely
from the vector sequences. For EW YAC clones the vector
sequences could not be removed entirely, as these clones
were constructed using sheared DNA and the cloning site
was destroyed in the cloning process. The Southern blot
analysis showed that a particular single-copy marker
hybridized to common restriction fragments in all the YAC
clones it had hybridized to in the colony hybridization
experiments, demonstrating overlap between the different
YAC inserts. When all the EcoRI/BamHI restriction fragments of a particular marker were found in a YAC clone it
was concluded that the marker was fully contained within
that clone. Some of the YAC clones only contained a subset
of the restriction fragments of the marker, especially when
the relatively large cosmid or Lambda DNA markers were
used as probes. This indicated that these particular YAC
clones did not span the marker completely but ended
within it. Sequences adjacent to the YAC vector sequences
in EW YAC clones were found on a junction EcoRI/BamHI
restriction fragment, which was a different size to that in
the probe.
The knowledge whether a particular marker is fully
contained within a YAC clone can give important information about the order of markers which map physically very
close to each other. For example, markers AGL19 and
pCIT-d23 could only be unambiguously placed relative to
markers B10206 and m210 in contig III due to the information that YAC clone CIC9G5 is not fully contained within
pCIT-d23 (compare Figure 1).
The Southern blot analysis also detected aberrations in
YAC clones. In a few cases YAC clones (e,g. CIC5D2/E2,
CIC9H6) were found not to contain an entire marker despite
the fact that they spanned the two flanking markers. We
interpret this as an indication of small deletions. Also, in
some cases one of the hybridizing EcoRI/BamHI restriction
fragments varied in size in different YAC clones. This is
most likely caused by chimaeric inserts in the YAC clones.
Examples of this kind were found in YAC clones EG5D4
and yUP6B4, which contained aberrant sized restriction
fragments hybridizing to markers m210 and mi128, respectively (see Table 2).
A considerable proportion of the DNA sequences used
to probe the YAC libraries were cosmid or Lambda clones.
Since some of the YAC clones could potentially contain
only a very small part of the marker it could not be ruled
out that some YAC clones corresponding to a particular
marker were not detected in the colony hybridization
experiments. To ensure that all linking clones between
markers had been identified, a Southern blot analysis of
all clones mapping to a genomic region was carried out
using all markers in this region as probes. This test was
very important to avoid the non-detection of particular
clones due to experimental shortcomings which could have
resulted in the false ordering of markers in particular
genomic regions.
The Southern blot analysis also allowed the unambiguous integration of multilocus markers into the map. For
markers mapping to multiple loci in the Arabidopsis
genome (e.g. UM177, UM415, BIO206, g4564) distinct
Southern hybridization patterns for the different loci could
be established. A locus was integrated into the YAC contig
map if some of the YAC clones representing that locus had
previously been anchored on to the chromosome 4 map
by a single-copy marker. For example, marker BIO206
showed hybridization to two different sets of restriction
fragments, one represented by clones EW8C7, yUP15D11,
yUP 16F9, CIC4A7 and CICll B4 and the other one by EW5C9,
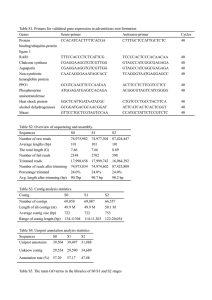
Figure 1. YAC contigs coveringchromosome4.
The arrangementof the YAC clones is consistentwith hybridizationto markers (shown at the top of each contig) and a limited numberof chromosome
walking experiments.The linescrossingthe YACclonesrepresentthe approximatelocationof the markers/endfragmentswithin the clones.The approximate
size of a markeris given by the thicknessof the line. For markersmappingto the samegenomiclocation,the lines are shown in differentshadesof grey
and bars indicatethe extentof each marker.The sizesof all the YAC clonesare drawn to scale, if a clonehas beenshownto containseveralYACs,the size
of this clone is indicatedby multiple boxes, however, it has not been determined if all the YACsof a particularclone hybridizeto a given markerand/or
repetitive sequence.For known chimaericclones the non-contiguoussequencesare indicatedas dark grey boxes. For those clones, for which marker
hybridizationdata and the physicalsizeof the clone are inconsistent,the putativechimaericpart of the cloneis shown by light grey shading.The location
and the extentof the chimaericsequencesin a cloneare consistentwith the markerand end fragmenthybridizationdata,but can vary from thoseshown in
the figure. All YAC end fragmentsthat havebeenanalysedare indicatedin the figure, left-endfragmentsas ellipsesand right-endfragmentsas triangles.
Only end fragmentswhich havebeenfully integratedinto the YACcontigs are representedin the sameway as markers.Chimaericend fragmentsare shown
in black.For markersmappingto multipleloci, only the YACclonescorrespondingto the particularchromosome4 locusare representedin the figure.Where
marker order is ambiguous,markersare eithershown at identicalpositionsor an arrow indicatesthat the order of markerscould be reversed.
YAC contigs on Arabidopsis chromosome 4 759
CIC3G3, CIC3H5, CIC9G5, EG10C4, CIC8B4, EW15B12,
EW22E4 and yUP4D12. All clones corresponding to the
first pattern had previously been shown to hybridize to LD
and/or GA1, hence this BIO206 locus maps between LD
and GA1 (Contig I, Figure 1). A number of clones which
revealed the second hybridization pattern had been found
to hybridize to m518 and/or AGL19, thus placing the second
BIO206 locus between these markers in Contig Ill (Figure 1).
Since clones EW5C9, EG10C4, EW15B12, EW22E4 and
yUP14D12 showed common restriction fragments when
compared with CIC3G3, CIC3H5 and CIC9G5, these clones
could also be incorporated into the contig, although they
did not hybridize to any of the markers flanking BIO206.
Previously 132 markers and a repetitive sequence had
been used to establish YAC contigs for chromosome 4
(Schmidt et al., 1995). Here, we have incorporated an
additional eight markers into the map (AtPLCI: Yamamoto
et al., 1995; B31: B. Osborne and B. Baker (Plant Gene
Expression Center, Albany); I/dSPM312: M. Aarts and A.
Pereira (CPRO, Wageningen); JAG9: J. Glover, A.
Chaudhury and E. Dennis (CSlRO, Canberra); PDS: Wetzel
et al., 1994; phyD, phyE: Clack et al., 1994; r808-b: S.
Naito (Hokkaido University, Sapporo)). Some of these new
markers identified YAC clones which had not been mapped
to chromosome 4 before. Furthermore, it is interesting to
note that the incorporation of these eight more markers
into the contig map reduced the number of total contigs
on chromosome 4--solely generated by marker hybridizations - - from 14 (Schmidt et al., 1995) to 12 (Contig la:
BIO217-mi122; Contig Ib: g3843-mi87; Contig I1: HY4nga8; Contig Ilia: RPS18C-pCITf3; Contig IIIb: H2761-mi198;
Contig IIIc: PRL1-PHYE; Contig IIId: CH42-g17340; Contig
IVa: PRHA; Contig IVb: g8300; Contig IVc: AtKC1/mi431AP2; Contig IVd: ELI3-ve031; Contig IVe: g3713-DHS1/
mi369). Five of these contigs were established by one or
two markers (contigs II, Ilia, IVa, IVb), while contigs Ib, Illb
and IIId spanned over 20 markers each.
The vast majority of the markers (137 out of 140) were
used to screen all four Columbia YAC libraries. These 137
markers, representing 140 chromosome 4 loci, identified
between two and 19 YAC clones per locus, with an average
of 8.5 YAC clones. None of the four YAC libraries used
yielded YAC clones for every marker tested, demonstrating
the need to use multiple libraries. For example, markers
PHYE, PRL1, PHYD, g3713, g3265, DHSl and mi369 did not
detect YAC clones in the CIC library.
YAC end fragment analysis
Chromosome walking experiments were employed to join
the remaining 12 YAC contigs reducing the number of YAC
contigs to four (Schmidt et aL, 1995). In addition to the
eight end fragments presented on the tiling path 116 end
fragments have been produced in the course of generating
the YAC contig map for chromosome 4, all of which are
indicated on Figure 1. End fragments which either joined
adjacent contigs, established by the marker hybridzations,
or which provided an additional link in an area with sparse
YAC coverage have been analysed using Southern blots
of all YAC clones mapping to a particular area to ensure
that all linking YAC clones have been identified. These end
fragments are represented in Figure 1 in the same way as
the markers. The majority of end fragments have only
been tested on a subset of YAC clones mapping to a
particular genomic region preventing an unambiguous
positioning of the end fragment relative to all YAC clones
in this area. However, even the partial information is useful
in generating the YAC contig map and more importantly
the end fragment analysis reveals chimaerism of YAC
clones (see below).
The cosmid clones which have been isolated using
YACs or end fragments (g14587, CC5P13, CC6N7, CC7J19,
CC10M20, CC12J20, CC15D15, CC15017, CC16N19, CC2012,
CC27Pll, CC28C17, CC34A17, CC44C20, CC44H2, CC50H10,
CC50K21; Schmidt et al., 1995) were particularly useful for
contig generation. They assessed a bigger genomic region
than most end fragments in the Southern blot analysis - end fragments produced by inverse polymerase chain
reaction (IPCR) were often smaller than 300 bp - - hence
the positioning of YAC clones relative to each other and to
the cosmids was more accurately assessed.
Alignment of YAC clones relative to markers and end
fragments in the YAC contigs
Southern blot analysis of YAC clones using markers or
end fragments as probes in combination with the size
information allowed us to position the YAC clones relative
to each other and to the markers in the YAC contigs. The
arrangement of the YAC clones was drawn out using the
following rules:
(i) Where a YAC clone spans two markers the physical
distance between those markers is the size of the YAC
insert or smaller;
(ii) where a YAC clone lies between two markers but does
not contain any of the restriction fragments of these
markers the distance between these markers is bigger
than the size of the YAC insert;
(iii) the order of markers given is consistent with all Southern hybridization data, especially if markers end within
a particular marker;
(iv) unless additional data prove otherwise, the clones are
positioned relative to the markers under the assumption that none of the clones is chimaeric. If this was
not feasible, an arrangement was chosen which
required the fewest chimaeric clones possible and
putatively chimaeric clones are indicated.
760
Renate Schmidt et al.
Figure 1 shows an arrangement of all 563 YAC clones
mapping to chromosome 4 which is consistent with the
results of all marker and end fragment hybridizations and
the sizes of all YAC inserts. This representation of the YAC
clones provides important information on regions with
sparse YAC coverage. Although multiple YAC coverage has
been achieved for the majority of the chromosome we
have reported that some of the links are spanned by a
single YAC clone (Schmidt et al., 1995). For example,
CIC12G2 is the only clone which links contigs IVc and IVd.
However, Figure 1 shows that multiple clones extend into
the interval between CC12J20 and CC5P13 (e.g. yUP10Bg,
yUP10B10, yUP15H2 and yUP10H11). End fragments from
such clones could prove useful to establish additional links.
Redundant YAC coverage for the majority of genomic
intervals and a high density of markers in most areas of
chromosome 4 ensured that the markers could be placed
within the YAC contigs with high accuracy. This allowed
the determination of physical distances between adjacent
markers and the extent of the YAC contigs. From the low
degree of flexibility in the positioning of the YAC clones,
we estimate that the error on the majority of distances is
less than 10%. A few genomic intervals, however, are only
spanned by one or several large insert YAC clones. The
lack of small YAC clones linking the markers flanking these
intervals prevents an accurate estimate of the physical
distances in these cases (e.g. intervals: HY4-ngaS, RJSmi422, mi 123-02213-2, PRHA-g8300). The four YAC contigs
shown in Figure 1 cover approximately 17 Mb.
Chimaeric YAC clones
The YAC clones forming the chromosome 4 map were
analysed for chimaerism using various criteria. For all YAC
libraries, clones carrying chloroplast DNA, rDNA sequences
and the 180 bp repeated DNA sequence have been identified (Creusot et aL, 1995; Schmidt et aL, 1994; Dunn and
Ecker, personal communication). These coordinates have
been compared with the YAC clones mapping to chromosome 4. Southern blot analysis using the repetitive
sequences as probes verified that 74 YAC clones, representing 68 independent clones, carried chromosome
4-specific sequences in addition to unlinked repetitive
sequences (Table 1). In some cases it was shown that end
fragments of the clones corresponded to the repeated
DNA sequences (EG2D2LE, yUP14B12RE: rDNA-sequences;
EG1B10RE, EG15C10LE, yUP8A5LE: chloroplast DNA
sequences). Thirteen YAC clones hybridized to two or three
different locations on chromosome 4, as shown by the
marker hybridization results and thus must be chimaeric
(Table 2). End fragment analysis revealed that at least 22
out of 106 YAC clones tested were carrying non-contiguous
sequences (Table 3 and see above). Interestingly, several
YAC clones are comprised of multiple unlinked sequences
(e.g. EG5D4, yUP6B4, EG15C10, EG17C8/C9).
Putative chimaeric clones were also detected when the
YAC clones were aligned with the markers in order to form
the contigs. For some clones the size ascertained in the
PFGE analysis was not consistent with the marker hybridization data and the distance of the markers, as established
by other YAC clones in the area; these clones could only
be integrated into the contigs based on the assumption
that the clones were chimaeric. In some cases it was
shown that the clones were indeed chimaeric, as they also
hybridised to unlinked markers (e.g. yUP20H1, yUP19E11;
Schmidt et al., unpublished results).
Discussion
The YAC contig map for chromosome 4 has been produced
using genetically mapped markers and YAC end fragments
as probes, guaranteeing that the YAC contig map is of direct
use for map-based cloning experiments. Four different YAC
libraries were used to generate the YAC contigs. On average
8.5 YAC cJones were isolated per marker, resulting in a
highly redundant YAC coverage for most areas of the
chromosome. Use of YAC libraries with different average
insert sizes has a number of advantages. The big insert
YACs were advantageous for linking genomic regions with
sparse marker cover, while in areas of high marker density
small YAC clones were extremely useful for determining
marker order. The integration of small YAC clones into the
physical map will be an important resource in the finemapping and subcloning stages of map-based cloning
experiments since they cover smaller genomic regions.
YAC clones carrying single-copy sequences derived from
chromosome 4 and unlinked repetitive sequences were
found in three of the four YAC libraries. Twenty-eight
percent and 14% of the EG and yUP YAC clones, respectively, belonged to this class of chimaeric clones, while only
2% of the CIC YAC clones showed this problem. The
limited analysis for chimaeric clones on chromosome 4 as
described in this paper revealed that at least 2-3% of the
EW and CIC YAC clones are chimaeric, while for the yUP
(>21%) and the EG library (>35%) the percentage of
chimaeric clones is much higher. Despite the presence of
chimaeric clones the map is reliable, since most intervals
are covered by a minimum of two YAC clones.
The size of the four YAC contigs comprising more than
90% of chromosome 4 is approximately 17 Mb, while the
nucleolar organizing region carrying the tandemly repeated
rDNA units covers approximately 3.5 Mb (Copenhaver and
Pikaard, personal communication). The gaps between YAC
contigs I, II and III amount to 6.6 cM while the gap between
YAC contigs III and IV is 2.3 cM (Schmidt et al., 1995).
Based on the average kb/cM ratio and taking into account
that the YAC contigs extend beyond the markers which
YAC contigs on A r a b i d o p s i s c h r o m o s o m e 4
Table 1. Chimaeric YAC clones carrying single-copy nuclear sequences as well as repetitive DNA
YAC co-ordinate
Hybridization to repetitive
DNA sequences
Location on chromosome 4
CIC5C2
CIC8B4
EG1B10
EG1B11
EG2A8
EG2D2
EG2D11
EG2G4/H4/H6
EG3A11
EG3F12
EG4G9
EG5C1
EG5F2
EG5G2
EG7B11
EG7C4
EG7G2/H2
EG7G6
EG8E6
EG8F7
EG8G8
EG10C12
EG10F2
EG10G6/H6
EG11G8
EG11H6
EG13C7
EG13G5
EG14G5
EG15C10
EG15E1
EG15H3
EG17A11
EG17C8/C9
EG17G3/H3
EG18A2
EG18A12
EG18B4
EG18G1
EG19D12
EG19E10
EG19E11
EG19E8
EG19H3
EG23A3
yUP2B9
yUP3G10
yUP5G9
yUP6D1
yUP6F11
yUP7A6
yUP7B10
yUP7H4
yUP8A5
yUP8E11
yUP9B4
yUP9E4
yUP10D3
yUP14B12
yUP15B7
yUP16G2
yUP17B8
yUP18G9
yUP20A2
yUP20C1
yUP20H3
yUP21D11
yUP24B7
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA / rDNA
Chloroplast DNA
rDNA
rDNA
Chloro )last DNA
Chloro )last DNA
Chloro )last DNA / rDNA
Chloro )last DNA
Chloro )last DNA
Chloro )last DNA / rDNA
Chloro )last DNA
Chloro )last DNA
Chloro )last DNA
Chloro )last DNA / rDNA
rDNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA / rDNA
Chloroplast DNA
rDNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA / rDNA
rDNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
Hindlll repeat sequence
rDNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
rDNA
Chloroplast DNA
Chloroplast DNA / rDNA
rDNA
rDNA
Chloroplast DNA
Chloroplast DNA / rDNA
Chloroplast DNA / rDNA
rDNA
rDNA
rDNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
Chloroplast DNA
rDNA
rDNA
Chloroplast DNA
rDNA
rDNA
Chloroplast DNA
rDNA
Chloroplast DNA
rDNA
Chloroplast DNA
rDNA
rDNA
Chloroplast DNA
Contig II1:UM415-KG32
Contig II1:BIO206-m210
Contig II1:COP9-g10086
Contig II1:H2761
Contig II1:mi232 / I/dSpm64-RLK5
Contig II1: EG15C8RE
Contig IV: AtKC1 / mi431
Contig IV: yUP24C6LE-g3088
Contig II1:CC28C17
Contig I: m506
Contig Ill: CCl14 / g4564
Contig IV: g15064
Contig IV: g15064
Contig II1:CC28C17
Contig IV: yUP7A3LE-I/dSpm76
Contig IV: r808-b
Contig IV: PRHA
Contig II1:B31
Contig II1:ve030-KG32
Contig II1:ve030-KG32
Contig IV: g8300
Contig II1:m557-g3883
Contig Ill: AG--g19838
Contig I: Ms2 / I/dSPM312-ve023 / GT148
Contig II1: m557-261aContig I: BIO219
Contig I: BIO217-CIC10C8RE
Contig II1:KG32
Contig II1:g4513-g17340
Contig II1:m326/455 / ve024
Contig II1:CC2012-g4539
Contig I: CC50K21
Contig II1:SEP2B-CC128
Contig IV: pCITdl04; UM415/555
Contig I: g3843
Contig II1:ve030-KG32
Contig II1: EW14E4LE
Contig II1:m557-CC10
Contig IV: AtGI~
Contig IV: AtH1
Contig II1:KG32
Contig I1:HY4
Contig II1:ve030-KG32
Contig I: ABP-m448
Contig IV: pCITd99-pCITd76
Contig II1:CC28C17
Contig II1:m518
Contig II1:AG-pCITd71
Contig IV: yUP24C6LE-yUP6D1LE
Contig Ill: mi465
Contig II1:mi330-Athsf1
Contig II1: mi198-EG15C8LE
Contig II1:B31
Contig IV: CC44H2-pCITd104
Contig II1:CC250 / PG11-DD1
Contig II1: CIC1H1LE
Contig II1:g2620-COP9
Contig II1:COP9--g10086
Contig II1: EW19E8LE
Contig II1: CIC1H1LE
Contig II1:yUP1F4LE-mi465
Contig II1:02213-2-g4513
Contig II1:EW9C3LE-CC250 / PG11
Contig II1:m326/455 / ve024-m580
Contig II1:mi422-UM415/555
Contig IV: g8300
Contig II1:CC10M20-CH42
Contig II1:CC250 / PG11-DD1
761
762
Renate Schrnidt et al.
Table 2. Chimaeric YAC clones carrying non-contiguous single-copy sequences of chromosome 4
YAC co-ordinate
Locations on chromosome 4
EG3A2
EG5D4
EG17C8/C9
EG23G8
EG23G9
EG24F9
EW8 E11
yUP4G1
yUP6B4
yUP10C1
yUP19H3
yUP20H8
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
II1:AGP66 / Contig II1:mi232 / I/dSpm64-g17340
II1:m210-g4108 / Contig II1:m326/455
IV: pCITdl04 / Contig IV: UM415/555
II1:TG1C8 / Contig II1:JGB9-LM117
II1:TG1C8 / Contig II1:JGB9-LM117 / Contig II1:UM415/555
II1:g4539 / Contig II1:CC10M20
I: mi51-g8802 / Contig II1: yUP17B7LE
II1:m518 / Contig II1:g3883-261aII1:mi128-g6837 / Contig II1:ve030-KG32 / Contig IV: g8300
I: g2616 / Contig II1: EW11E9LE-EW9C3LE
I: g8802-g6844 / Contig I: BIO206-mi233
II1: yUP13C7RE-yUP3F7RE / Contig II1:EW11E9LE-CC250 / PG11
Table 3. Chimaeric end fragments
YAC co-ordinate
Location of clone on chromosome 4
Unlinked end fragment
CIC5C3
EG1B10
EG1E3
EG2D2
EG4G7
EG5D4
EG6H10
EGTA4
EG11B7
EG11F9
EG11H6
EG15C10
EG17G9
EG23D9/E10
EW13F9
yUP7G10
yUP8A5
yUP10C1
yUP14B12
yUP15D11
yUP17F1
yUP20A4
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
Contig
RE
RE
LE
LE
RE
LE
LE
RE
RE
RE
RE
LE
LE
LE
LE
RE
LE
LE
RE
LE
LE
LE
I: mi87
II1:COP9-g10086
IV: yUP7A3LE-m214
II1: EG15C8RE
II1: yUP13CTRE
II1: m210-g4108; m326/455
IV: pCITd76-RNA-Polymerasell LS
IV: CC5P13 / CC7J19-g2486 / CC127
II1: yUP13CTRE
II1:CC250 / PG11
I: BIO219
II1:m326/455 / ve024
II1:g4108
II1:g14587-PRL1
II1: mi260-yUP3F7RE
I: I/dSpm27-g6844
IV: CC44H2-pCITd104
II1: EW11E9LE-EW9C3LE
II1: EW19E8LE
I: LD-GA1
I: CC50K21-1/dSpm41G
II1:Athsfl
have been genetically mapped we can estimate the size of
the gaps to be greater than 1 Mb. Thus the total size of
chromosome 4 is greater than 21.5 Mb. Measurements of
the synaptonemal complex length of chromosome 4 have
shown that this chromosome comprises 16.8% of the
genome (Albini, 1994). In the nucleolar organizing region
the synaptonemal complex is interrupted and it is not clear
how much of this region contributes to the entire length
of the chromosome established by this method. If we
assume that the nucleolar organizing region does not
contribute to the synaptonemal complex length at all or in
a much reduced way, then the minimum size of the nuclear
genome of Arabidopsis would be 107 Mb.
The information presented in this paper (the detailed
arrangement of the YAC clones in the YAC contigs, the
sizes of the YACs and known chimaeric YAC clones)
is available on the World Wide Web (WWW) at
both URL: http://genome-www.stanford.edu/Arabidopsis/
JIC-contigs.html and URL:http://nasc.nott.ac.uk/JIC-contigs/JIC-contigs.html. All available markers (i.e. all but
agp66, PETC and H2761) have been deposited at the
Arabidopsis Biological Resource Centre at Ohio. The majority of the YAC end probes were derived using IPCR and
were not cloned. However, they can easily be regenerated
using the protocols described in the Experimental procedures section. The density of RFLP markers mapped on the
genetic and physical maps of chromosome 4 now means
that any new mutation can be mapped to a small interval
both genetically and physically. These intervals vary in size
along the chromosome, most of them are between 50 and
500 kb. Given the availability of cosmid libraries built in
Agrobacterium binary vectors (Olszewski et al., 1988) and
YAC contigs on A r a b i d o p s i s c h r o m o s o m e 4
763
The YAC libraries were maintained as described previously
(Schmidt et al., 1992, 1994).The preparation of yeast colony filters,
the probe labelling, and the hybridization and washing conditions
were identical to the ones outlined before (Schmidt et aL, 1992).
For markers cloned in vectors with pYAC-homology the plant
DNA fragments were separated from the vector sequences prior
to their use as probes in colony hybridization experiments. For all
markers cloned in Lambda-vectors, the cosmid vectors Lorist or
pLAFR3, the complete clones were digested with a restriction
enzyme (four bp recognition sequence) and subsequently labelled.
were defined as those sequences which were adjacent to the
left arm of pYAC4 and its derivatives, while the right ends are
neighbouring the right arm. IPCR can be used to isolate both ends
of YAC DNA inserts. Yeast genomic DNA (0.5-1 ilg) was digested
with Alul, EcoRV and Hincll. Left ends were isolated with Alul and
EcoRV and right ends with Alul and Hincll. Use of these enzymes
guaranteed the isolation of suitable end fragments more than 50%
of the time. After an ethanol precipitation the fragments were
ligated at 4°C under dilute conditions to promote circle formation.
Heat inactivation of the ligase was followed by an ethanol precipitation. Samples for the left end YAC circles were then digested with
Nhel while right end YAC circles were linearized with Sspl. After
phenol extraction, samples were passed over a Sepharose CL 6B
spin column (in TE buffer). The resulting DNA solutions were used
in the PCR reactions. The PCR reactions contained 10-50 ng of
DNA, 10 mM Tris-HCI (pH 8.3), 50 mM KCI, 2 mM MgCI2, 0.01%
gelatin, 0.005% Tween 20, 0.005% NP40, 0.1 mM dATP, 0.1 mM
dCTP,0.1 mM dGTP,0.1 mM d'l-lP, 0.2 I~M of each of the appropriate
primers and 1.25 U of Taq DNA polymerase. The reaction volume
was 100 111.Thirty-five cycles of 1 min at 94°C, 1 min at 60°C and
2 rain at 72°C were followed by an additional 10-min incubation
at 72°C. The sequences of the PCR oligonucleotides used were:
Yeast genomic DNA preparation for restriction enzyme
digestion and YAC end-fragment isolation
Left-end (outer nest): D71 5'-TCCTGCTCGCTI'CGCTACTT-3'
C78 GCGATGCTGTCGGAATGGAC
Right end (outer nest): C69 CTGGGAAGTGAATGGAGACATA
C70 AGGAGTCGCATAAGGGAGAG.
the relatively facile in p/anta transformation procedure for
Arabidopsis (Bechtold et al., 1993), the isolation of genes
from chromosome 4 using map-based cloning should now
not be the limiting factor in the analysis of complex plant
biological processes.
Experimental procedures
Yeast colony hybridizations
Yeast colonies were removed from agar plates and resuspended
in 400 Id TE/SDS (10 mM Tris-HCI, pH 8.0, 1 mM EDTA, 0.1% SDS).
An equal volume of phenol/chloroform/isoamylalcohol (25:24:1,
v:v:v) was added, the preparations were mixed carefully and
subsequently incubated for 20-30 min at 65°C. The preparations
were again mixed thoroughly. After centrifugation, the supernatants were re-extracted with phenol/chloroform/isoamylalcohol.
Forty microlitres of 3 M NaAc (pH 5.4) were added to the preparations before they were precipitated with ethanol. The DNA pellets
were resuspended in 50 p.I of TE (10 mM Tris-HCI, 1 mM EDTA,
pH 8.0) and incubated overnight at 4°C. After centrifugation the
supernatants were extracted with an equal volume of phenol/
chloroform/isoamylalcohol. Five microlitres of 3 M NaAc (pH 5.4)
were added to the preparations and they were precipitated with
ethanol. The DNA pellets were resuspended in TE and used for
restriction enzyme digestion.
For Southern blot analysis, the yeast genomic DNA was digested
with EcoRI/BamHl. Gel transfer to Hybond-N and hybridization
conditions were according to manufacturer's instructions
(Amersham) with the modifications described previously (Schmidt
eta/., 1994).
Sizing of YACs
To size YACs, intact yeast chromosomal DNA was isolated and
separated by PFGE using concatemers of Lambda-DNA as a size
standard. Southern blots of the gels were hybridised using pYAC
vector as probe (Schmidt et al,, 1994).
YAC end-fragment isolation
Isolation of YAC end fragments was carried out by IPCR or plasmidrescue. The left arm of the pYAC4 vector carries Trp I, ARS I and
CEN 4 sequences as well as an origin of replication and an
antibiotic resistance gene functional in Escherichia coil, while the
right arm harbours the Ura 3 sequences. Left ends of YAC inserts
PCR products derived from A/ul circles could be reamplified with
inner nest primers (D72 / C77 for left end fragments and C72 / C71
for right end fragments). PCR products derived from EcoRVcircles
had to be reamplified with C78 and D72 (or D71) and PCR products
derived from Hincll circles could only be reamplified with C70 and
C72 (or C69), since oligonucleotides C77 and C71 are homologous
to vector sequences which are absent from the EcoRV and the
Hincll circles, respectively.
Left end (inner nest): D72 5'-CACTATCGACTACGCGATCA - 3 '
C77 GTGATAAACTACCGCA'I-I-AAAGC
Right end (inner nest): C72 CGAGTCGAACGCCCGATCTC
C71 AGAG CCTTCAACCCAGTCAG.
Details of the circles and PCR products generated have been
described elsewhere (Schmidt and Dean, 1996; Schmidt et al.,
1992).
Insert fragments adjacent to the left arm of the YAC vector could
also be isolated by plasmid-rescue. Yeast genomic DNA (1 ilg)
was digested with Xhol or Ndel. The DNA was extracted with
phenol/chloroform/isoamyl alcohol and then precipitated with
ethanol. The ligation of the fragments was carried out under dilute
conditions at 4°C to promote circularization. After heat inactivation
the DNA was precipitated with ethanol. Electroporation of aliquots
of the ligated DNA into competent DH5{x E. coil cells was carried
out using a Bio-Rad electroporator according to manufacturer's
instructions. Immediately after the electroporation 1 ml of growth
medium was added and the cells were grown for 1 h at 37°C
before the cells were spread on an agar plate containing ampicillin
(50 lig ml-1). Three clones were characterized from each of the
transformations.
To use the end fragments produced by IPCR or left end rescue
in colony hybridization experiments, all vector sequences had to
be removed. IPCR fragments and plasmid-rescue products were
cut with the enzyme which was used to digest the yeast DNA
prior to self-ligation (IPCR: e.g. Alul, Hincll, EcoRV, Plasmid-rescue:
Xhol, Ndel). Furthermore, products derived from the EG YAC
clones had to be cut with BamHI (cloning site in pYAC-41), while
764
Renate S c h m i d t et al.
products from CIC and yUP YAC clones had to be digested with
EcoRI (cloning site in pYAC4). Since the clones of the EW library
do not contain a restored YAC vector cloning site, a variety of
diagnostic digests were used on the plasmid-rescue derived clones
to identify an enzyme which was suitable to isolate at least part
of the YAC insert-specific fragment. IPCR products from EW YAC
clones were cut with Hhal (left-end products) and Sau3A (rightend products), since these restriction enzymes have recognition
sequences very close to the original cloning site in pYAC3.
resolved RFLP analysis and long range restriction mapping of
the DNA of Arabidopsis thaliana using whole YAC clones as
probes. Nucl. Acids Res. 20, 6201-6207.
Bechtold, N., Ellis, J. and Pelletier, G. (1993) In planta
Agrobacterium-mediated gene transfer by infiltration of adult
Arabidopsis thaliana plants. C.R. Acad. ScL Paris, 316, 11941199.
Bell, C.J. and Ecker, J.R. (1994) Assignment of 30 microsatellite
loci to the linkage map of Arabidopsis. Genomics, 19, 137-144.
Bent, A.E, Kunkel, B.N, Dahlbeck, D., Brown, K.L., Schmidt, R.,
Giraudat, J., Leung, J. and Staskawicz, B.J. (1994) RPS2 of
Screening of cosmid libraries with whole YACs or end
fragments as probes
A gridded cosmid library carrying approximately 25 kb inserts of
Columbia ecotype DNA in pLAFR3 (Lister and Dean, unpublished
results) was plated at high density on selective medium and
grown overnight at 37°C. Up to 10 colony lifts were taken from
each agar plate. Colony filters were treated as described (Sambrook et aL, 1989). Hybridization and washing conditions were
the same as those used for Southern hybridization experiments,
however, the length of the washes was reduced when complete
YACs were used as probes. YACs were gel-purified using the
protocol described by Bancroft et al. (1992).
DNA probes
The repetitive DNA sequences (25S-18S rDNA, 5S-rDNA, chloroplast DNA, pAL1; Martinez-Zapater et aL, 1986) and most marker
DNA sequences used as probes to screen the YAC libraries have
been described previously (Schmidt et al., 1994, 1995). Several
end fragments provided by other laboratories were also incorporated in the YAC contig map, CICIHILE (B. Dietrich and J. Dangl,
University of North Carolina, Chapel Hill), EG21C9LE, EW22B3LE,
yUP3E9LE (B. Staskawicz, University of California, Berkeley),
EW14G1LE, EW9C3LE, EW11E9LE, yUP10B5LE, yUP11F11LE, yUP17B7LE (Bent et al., 1994) and yUP17E10LE (Pepper et al., 1994).
Acknowledgements
We acknowledge the generosity of the following people in sending
unpublished markers: B. Osborne, B. Baker (Plant Gene Expression
Center, Albany), B. Dietrich, J. Dangl (University of North Carolina,
Chapel Hill), B. Staskawicz (University of California, Berkeley), M.
Aarts, A. Pereira (CPRO, Wageningen), J. Glover, A. Chaudhury
and E. Dennis (CSIRO, Canberra) and S. Naito (Hokkaido University,
Sapporo). We thank Z. Lenehan (John Innes Centre, Norwich) for
help with the YAC mapping and D. Bouchez (INRA, Versailles),
P. Dunn, J. Ecker (University of Pennsylvania, Philadelphia) for
providing data on the YAC clones prior to publication. We also
thank David Flanders (Stanford AtDB) for construction of the Web
page and Mary Anderson (Nottingham Arabidopsis Stock Centre)
for setting up a mirror site. This work was supported by grants
from the European Community (BLOTCT 90-0207) and the BBSRC
(208/PG0608 and 208/PG01525) to C.D. and EC training fellowships
to A.B., G.C. and R.S.
References
Albini, S.M. (1994) A karyotype of the Arabidopsis thafiana genome
derived from synaptonemal complex analysis at prophase I of
meiosis. Plant J, 5, 665-672.
Bancroft, I., Westphal, L., Schmidt, R. and Dean, C. (1992) PFGE-
Arabidopsis thaliana: A leucine-rich repeat class of plant disease
resistance genes. Science, 265, 1856-1860.
Clack, 1".,Mathews, S. and Sharrock, R.A. (1994) The phytochrome
apoprotein family in Arabidopsis is encoded by five genes: the
sequences and expression of PHYD and PHYE. Plant Mol. BioL
25, 413-427.
Coulson, A., Sulston, J., Brenner, S. and Karn, J. (1986) Toward a
physical map of the genome of the nematode Caenorhabditis
elegans. Proc. Natl Acad. Sci. USA, 83, 7821-7825.
Creusot, F., Fouilloux, E., Dron, M. et aL The CIC library: a large
insert YAC library for genome mapping in Arabidopsis thaliana.
Plant J. 8, 763-770.
Ecker, J.R. (1990) PFGE and YAC analysis of the Arabidopsis
genome. Methods, 1, 186-194.
Grill, E. and Somerville, C. ( 1991) Construction and characterization
of a yeast artificial chromosome library of Arabidopsis which
is suitable for chromosome walking. Mo/. Gen. Genet. 226,
484-490.
Hauge, B.M., Hanley, S., Giraudat, J. and Goodman, H.M (1991)
Mapping the Arabidopsis genome. In Molecular Biology of Plant
Development (Jenkins, G.I. and Schuch, W., eds). Cambridge:
The Company of Biologists, pp. 45-56.
Hwang, I., Kohchi, T., Hauge, B.M. et aL (1991) Identification
and map position of YAC clones comprising one-third of the
Arabidopsis genome. Plant J. 1,367-374.
Konieczny, A. and Ausubel, E (1993) A procedure for quick
mapping of Arabidopsis mutants using ecotype specific
markers. Plant J. 4, 403-410.
Martinez-Zapater, J.M., Estelle, M.A. and Somerville, C.R. (1986)
A highly repeated DNA sequence in Arabidopsis tha/iana. Mo/.
Gen. Genet. 204, 417-423.
Meyerowitz, E.M. and Somerville, C.R. (eds) (1994) Arabidopsis.
Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Olszewski, N.E., Martin, F.B. and Ausubel, F.M. (1988) Specialised
binary vectors for plant transformation: expression of the AHAS
gene in Nicotiana tabacum. Nuc/. Acids Res. 16, 10 765-10 782.
Pepper, A., Delaney, T., Washburn, T., Poole, D. and Chory, J.
(1994) DET1,a negative regulator of light-mediateddevelopment
and gene expression in Arabidopsis, encodes a novel nuclearlocalized protein. Cell, 7, 109-116.
Reiter, R.S., Williams, J.G.K., Feldmann, K.A., Rafalski, J.A., Tingey,
S.V. and Scolnik, P.A. (1992) Global and local genome mapping
in Arabidopsis tha/iana by using recombinant inbred lines and
random amplified polymorphic DNAs. Proc. Nat/ Acad. ScL
USA, 89, 1477-1481.
Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Molecular
Cloning: A Laboratory Manual Cold Spring Harbor, NY: Cold
Spring Harbor Laboratory Press.
Schmidt, R. and Dean, C. (1996) Hybridization analysis of YAC
clones. In Methods Mo/. Cell. Biol., in press.
Schmidt, R., Chops, G., Bancroft, I. and Dean, C. (1992)
Construction of an overlapping YAC library of the Arabidopsis
tha/iana genome. Aust. J. Plant Physiol. 19, 341-351.
YAC contigs on A r a b i d o p s i s c h r o m o s o m e 4
Schmidt, R., Putterill, J., West, J., Chops, G., Robson, F., Coupland,
G. and Dean, C. (1994) Analysis of clones carrying repeated
DNA sequences in two YAC libraries of Arabidopsis thaliana
DNA. Plant J. 5, 735-744.
Schmidt, R., West, J., Love, K., Lenehan, Z., Lister, C., Thompson,
H., Bouchez, D. and Dean, C. (1995) Physical map and
organization of Arabidopsis thaliana chromosome 4. Science,
270, 480-483.
Ward, E.R. and Jen, G.C. (1990) Isolation of single-copy-sequence
765
clones from a yeast artificial chromosome library of randomlysheared Arabidopsis thaliana DNA. Plant MoL BioL 14, 561-568.
Wetzel, C.M., Jiang, C.-Z., Meehan, L.J., Voytas, D.F.and Rodermel,
S.R. (1994) Nuclear-organelle interactions: the immutans
variegation mutant of Arabidopsis is plastid autonomous and
impaired in carotenoid biosynthesis. Plant J, 6, 161-175.
Yamamoto, Y.T., Conkling, M.A., Sussex, I.M. and Irish, V.F. (1995)
An Arabidopsis cDNA related to animal phosphoinositidespecific phospholipase C genes. Plant Phys. 107, 1027-1030.