DOI: 10.1093/brain/awh006
Advanced Access publication November 7, 2003
Brain (2004), 127, 120±132
Left and right hand recognition in upper limb
amputees
Daniele Nico,1,2,3 Elena Daprati,2,3 FrancËois Rigal,4 Lawrence Parsons5 and Angela Sirigu3
di Psicologia, UniversitaÁ `La Sapienza',
Fondazione S.Lucia, Roma, Italy, 3Institut de
Sciences Cognitive, Bron, 4HoÃpital des Massues, Lyon,
France and 5Research Imaging Center, UTHSCSA,
San Antonio, Texas, USA
1Dipartimento
2IRCCS
Summary
strongly affected by the side of limb loss: subjects who
underwent amputation of their preferred limb made
more errors and required greater latencies to respond as
compared with amputees of the non-dominant limb. In a
further analysis we observed that the habit of wearing
an aesthetic prosthesis signi®cantly interfered with the
ability to judge the corresponding hand. Our data lead
to three main conclusions: (i) loss of a single limb per se
does not prevent motor imagery but it signi®cantly
enhances its dif®culty; (ii) these subjects apparently perform the hand recognition task using a strategy in which
they initially mentally simulate movements of their
dominant limb; (iii) wearing a prosthesis, devoid of any
motor function, seems to interfere with motor imagery,
consistent with the view that only `tools' can be
incorporated in a dynamic body schema.
Keywords: hand recognition; amputation; motor imagery; hand preference; prosthesis
Abbreviations: ANOVA = analysis of variance; DP± = loss of dominant limb/not wearing prosthesis; DP+ = loss of
dominant limb/wearing prosthesis; NDP± = loss of non-dominant limb/not wearing prosthesis; NDP+ = loss of
non-dominant limb/wearing prosthesis; RT = response time
Introduction
In the past 20 years, varied and precise evidence has
accumulated for a remarkable correspondence between
properties of motor imagery and movement execution.
Psychophysical studies using mental chronometry in normal
subjects demonstrated how the time required to mentally
simulate an action closely matches that needed to execute the
corresponding motor act (Decety et al., 1989; Jeannerod,
1995). More precisely, motor simulations seem to obey the
same physical constraints (e.g. Fitt's law on speed/accuracy
trade-off) that apply to real movements (Sirigu et al., 1995,
1996). Such a parallelism is also found when the motor
system is impaired: indeed, some patients suffering from
somato-motor disorders show comparable perturbations in
their motor imagery. When required to imagine movements
of their hands, parkinsonian patients showed the same pattern
of slowness and limb asymmetry that was observed during
real motor execution (Dominey et al., 1995). Likewise,
patients suffering from hemiparesis as a consequence of
unilateral lesions of the motor cortex showed comparable
slowness when executing and mentally simulating movements of their affected arm (Sirigu et al., 1995). Furthermore,
recent neuroimaging studies demonstrate comparable activations during mental simulation and motor execution, suggesting the existence of a common neural substrate for the
accompanying multimodal sensory-motor information processing that includes the parietal and premotor cortex, the
Brain Vol. 127 No. 1 ã Guarantors of Brain 2003; all rights reserved
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Previous research suggests a close similarity in brain
activity between mental simulation of a movement and
its real counterpart. To explore this similarity, we aimed
to assess whether imagery is affected by the loss of a
limb or of its motor skills. We examined the performance of 16 adult, upper limb amputees (and age-matched
controls) in a left/right hand judgement task that implicitly requires motor imagery. The experimental group
included subjects who had suffered the amputation of
the dominant or the non-dominant limb. Although
responding well above chance, amputees as a group were
slower and less accurate than controls. Nevertheless,
their response pattern was similar to that of controls,
namely slower response times and more errors for
stimuli depicting hands in unnatural orientations, i.e.
postures dif®cult to reach with a real movement.
Interestingly, for all stimuli, amputees' performance was
Correspondence to: Angela Sirigu, Institute des Sciences
Cognitives, 67, Blv. Pinel, 69675 Bron, France
E-mail: sirigu@isc.cnrs.fr
Left-right hand recognition in amputees
could be crucial to the solution of an implicit motor imagery
task. Previous studies provide related and suggestive evidence. As discussed earlier, in a laterality judgement task,
response times (RTs) to a visually presented hand shape seem
to be strongly in¯uenced by the current posture of the
subject's own hands (Parsons, 1994). This effect has been
interpreted as a suggestion that subjects use their `®rstperson' experience in order to mentally simulate the movement. This hypothesis was recently con®rmed with an
imagery task that directly compared `®rst-person' and
`third-person' perspective (Sirigu and Duhamel, 2001),
suggesting a close link between the mentally represented
limb and its physical counterpart. Nevertheless, despite
strong similarities, a complete overlap between the processes
of action and mental simulation have been questioned.
Neuroimaging studies suggest that when mentally simulating
a movement, brain activity within the frontal lobe is more
anterior with respect to overt motor execution, whereas
activation within the parietal cortex shifts to more posterior
regions (Gerardin et al., 2000). Furthermore, psychophysical
studies demonstrate that patients who developed acute
hemiplegia following a cerebral vascular accident retain the
ability to use motor imagery of the paralysed limb in order to
decide whether an overhand or an underhand grip were more
appropriate to grasp a manipulandum (Johnson, 2000,
Johnson et al., 2002). In other words, these patients seem to
maintain the ability to mentally simulate movements of a
body part they can no longer use.
The present research aimed to clarify these issues and to
determine to what extent availability of a physical counterpart
is crucial to motor imagery. To this purpose, we recorded the
performance of a group of subjects who underwent amputation of the upper limb in a right/left hand-recognition task.
We assumed that if implicit motor imagery does not require
the presence of a physical counterpart, provided that a
complete body representation had been established through
prior experience, then amputees should be able to mentally
simulate the movement of a body part even if that body part
has been deleted. Alternatively, as several studies on
parkinsonian and hemiplegic patients seem to suggest
(Dominey et al., 1995; Sirigu et al., 1996), mental simulation
of a movement might depend on the actual state of the body.
If so, then the loss of a limb should interfere with the ability to
recognize it, and accordingly amputees should show performances signi®cantly different from that of normal controls. A secondary goal of the present study was to evaluate
whether the habit of wearing a prosthetic limb has any effect
on motor imagery. Tools are known to be functionally
incorporated in the body schema (Iriki et al., 1996, 2001),
therefore we were interested to evaluate whether and how an
external object attached to one's body in order to mimic a
speci®c part, such as a prosthesis, affects motor imagery of
the mimicked body part. With these two goals, we aimed to
gain a better understanding of how the natural or arti®cial
structural and functional integrity of one's body sets the
conditions for its motor-sensory imagery.
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
basal ganglia and the cerebellum (Decety et al., 1994;
Stephan et al., 1995; Grafton et al., 1996; Roth et al., 1996;
Gerardin et al., 2000).
The tight similarity between imagery and motor control
emerges also within tasks that implicitly activate motor
imagery. For instance, in order to judge whether a stimulus
presented in a picture is a left or a right hand, healthy subjects
use a set of mental transformations that closely match the
operations required for actual hand movements. Various
studies strongly support this hypothesis (see a review in
Parsons et al., 1998). In 1982, Sekiyama demonstrated that
when subjects were asked to decide whether a right or a left
hand was presented, their reaction times systematically varied
re¯ecting hand-speci®c joint constraints. Namely, for each
hand, greater reaction times were found for those positions
that the arm and the hand could not easily reach with a real
movement (Sekiyama, 1982). This response pattern revealed
a preference for `manageable directions' of actual movements, suggesting that subject's judgments are likely to be
based on a mental analogue that preserves kinaesthetic and/or
proprioceptive information relative to the real movements. In
another study, Parsons (1987a, b), using a task requiring the
left/right judgement of a hand or a foot, con®rmed that
reaction times increase as a function of rotation angle of the
stimulus. Interestingly, this effect is strongly in¯uenced by
the actual position of the subject's body during the task,
suggesting that subjects solve the task by mentally simulating
their own body-part movement rather than by imagining a
spatial transformation of a prototypical representation of a
hand (i.e. a right hand in dorsal view, ®ngers pointing up).
Thus, body representation appears to be the implicit functional base of motor activity also in the domain of mental
simulation (Jeannerod, 1995; Jeannerod and Decety, 1995).
In agreement with this hypothesis, it has been shown that
mentally simulated movements, like actual movements,
probably respect the principle of control by the contralateral
cerebral hemisphere. Parsons et al. (1998) investigated the
mental representation of the hand in two split-brain patients.
Subjects were required to judge whether a line drawing
depicted either a right or a left hand: stimuli were presented in
various orientations for 150 ms in either the left or right visual
hemi-®eld. Patients' performance was strongly affected by
laterality of the stimulus: patients' accuracy was equal to
healthy controls when the hand presented on the screen was
contralateral to the perceiving hemisphere, but did not rise
above chance level for stimuli ipsilateral to the perceiving
hemisphere. These results con®rm that mental operations on
body parts seem to depend on a contralateral cortical
representation for each hand, in close analogy to overt
motor control. A PET study on normal subjects with an
analogue of Parsons' experimental set, also con®rmed that
sensory-motor brain areas represent the mental simulation of
shape and movement of the contralateral hand (Parsons et al.,
1995).
To our knowledge, no study has directly addressed the
question whether physical availability of the motor effector
121
122
D. Nico et al.
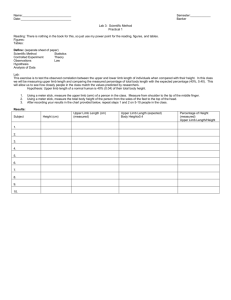
Table 1 Main clinical features of the experimental group
Patient Demographic data
Prosthesis
Phantom limb
Sex Age
Education Side and
(years) (years)
level
Time
Cause
(year,
month)
Use and
type
Time
After lesion
At test²
(year,
months)
Phantom Pain Therapy* Phantom Pain
M
M
M
F
F
F
M
M
M
M
M
M
M
M
M
M
2
7, 6
4
4
4
6
24
0, 6
6
1, 7
3, 4
0, 10
7, 4
3, 1
4, 7
9, 9
No
No
No
Aesthetic
Aesthetic
Myo-electric
Myo-electric
No
No
No
No
Mechanic
Aesthetic
Aesthetic
Aesthetic
Mechanic
±
±
±
4
4
5
2
±
±
±
±
0, 4
4
3
4
9
54
65
28
39
74
61
43
32
24
51
54
22
30
31
21
63
11
12
11
12
12
14
12
12
18
12
9
15
16
8
8
9
R arm
R forearm
R shoulder
R arm
R shoulder
R arm
R forearm
R arm
L shoulder
L forearm
L arm
L forearm
L forearm
L arm
L hand
L hand
Traumatic
Traumatic
Traumatic
Vascular
Therapeutic
Vascular
Traumatic
Traumatic
Therapeutic
Traumatic
Traumatic
Traumatic
Traumatic
Traumatic
Traumatic
Traumatic
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Pharm.
Surg.
Pharm.
surg.
Pharm.
Pharm.
No
Pharm.
Pharm.
Pharm.
Phys.
Pharm.
Pharm.
No
Pharm.
Pharm.
Yes³
Yes
Yes³
Yes³
Yes
Yes
Yes
No
No
Yes
Yes
No
Yes
Yes³
No
Yes³
Yes³
No
Yes
No
No
No
No
No
No
Yes
No
No
No
Yes³
No
Yes
*Pharm. = pharmacological; Surg. = surgical; Phys. = physiotherapy. ²Phantom limb sensation and/or pain elicited/enhanced by the task is
indicated by ³.
Methods
Participants
Sixteen subjects who had suffered amputation of the right or left
upper limb were recruited at the HoÃpital des Massues in Lyon
(France). None of them had a previous history of neurological or
psychiatric disorders. Seven among them (three women, four men;
mean age 52.0 6 16.1 years, range 28±74 years) had lost their
dominant limb, whereas nine (all men; mean age 36.4 6 15.5 years,
range 21±63 years) suffered from the amputation of the nondominant limb. According to the Edinburgh Inventory (Old®eld,
1971), all but one (A8) were right-handed. All had normal or
corrected to normal vision. The subjects' main demographic and
clinical data are summarized in Table 1 and in Supplementary data
(available at Brain Online). A group of seven control subjects was
recruited among relatives and medical staff (four women, three men;
mean age 39.7 6 15.3 years, range 24±64 years). All but one (C5)
were right-handed according to the Edinburgh Inventory. All
subjects had normal or corrected to normal vision. A preexperimental analysis of variance (ANOVA) showed no signi®cant
differences between control subjects and the two groups of amputees
with respect to mean age and educational level. As a control for
traumatic limb loss, three subjects presenting congenital limb
deletion of the left forearm (CD1±3, two women, one man, aged
22, 29 and 43 years) were also included in the study. To control for
the loss of limb functionality rather than for its physical absence, two
subjects having suffered a lesion of the right (PB1, right-handed
woman, 36 years old) and left brachial plexus (PB2, right-handed
man, 46 years old) were also tested. These subjects' main
demographic and clinical data are summarized in Supplementary
data (available at Brain Online). In accordance with the local ethical
committee, Comite Consultatif de Protection des Personnes dans la
Recherche BiomeÂdicale (CCPPRB) Centre LeÂon BeÂrard, Lyon,
which approved the study, all participants signed informed consent
before volunteering for this study.
Stimuli
Stimuli were line drawings of both right and left hands, derived from
Parsons (1987a, b) and were presented as single images on a
personal computer. Each drawing depicted one hand (approximately
one-third of the size of the real hand) presented in one of four
different viewpoints (see Fig. 1 for some examples). Viewpoints
included two frontal postures (back and palm) and two side views
(thumb side and pinkie side). For each viewpoint, hands were rotated
through 12 different angles (in a 30° steps, from an arbitrary starting
position with all ®ngers pointing up, corresponding to 0°/360°
orientations as shown in Fig. 1). As judged by 12 naive subjects, six
orientations corresponded to postures easily reached during normal
movements (right hand, from 30° to 240° counter-clockwise; left
hand, from 330° to 120° clockwise). The remaining six depicted
postures requiring unnatural/uncomfortable movements in order to
be matched (right hand: from 60° to 210° clockwise; left hand: from
300° to 150° counter-clockwise).
Procedure
Subjects sat comfortably in a dimly lit room and faced the screen of a
portable computer located ~30 cm from their frontal plane. They
positioned their hands over their thighs and were instructed not to
move them at all during the testing session. Subjects were required to
look carefully at each drawing of a hand that appeared on the screen
and to decide, as rapidly and accurately as possible, whether it was a
right or left hand. The examiner started each trial by pressing a
computer key. A ®xation point appeared in the middle of the blank
screen and remained visible for 200 ms. As soon as it disappeared,
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
A1
A2
A3
A4
A5
A6
A7
A8
A9
A10
A11
A12
A13
A14
A15
A16
Amputation
Left-right hand recognition in amputees
123
and pinkie side) and 12 orientations (six natural and six unnatural
postures).
Data analysis
Fig. 1 Stimuli. Examples of line-drawings of right and left hands
used as stimuli. Four different viewpoints were selected: back,
palm, little ®nger and thumb side, respectively. Each line-drawing
was presented at different angles (from 0° to 360°, following a 30°
step), corresponding to six natural and six unnatural orientations.
the image of one hand appeared in the same location; the drawing
lasted on the screen until onset of the verbal response. Subjects were
asked to answer by speaking aloud the words `droite' or `gauche'
(French for `right' and `left', respectively). A voice-key microphone
recorded response onset and terminated the trial by turning the
screen blank. Both time and verbal response to each trial were
recorded. RTs were computed as time elapsed between appearance
of the line drawing on the screen and verbal onset of the response.
RTs shorter than 300 ms and longer than 15000 ms were discarded
from analyses. The identity of each verbal response was manually
recorded by one experimenter. Two randomized sequences of 96
trials separated by a 15-min rest period were run in one testing
session. Each sequence included 48 drawings of right hands and 48 of
left hands presented in four different viewpoints (back, palm, thumb
Results
In an informal debrie®ng following the experimental session,
most participants reported to have solved the task by mentally
moving their own hand in order to reach the posture presented
in the line drawing (69%). Several subjects described
selecting what they considered the most plausible hand at
®rst, and then switching to the other in case of error. Several
amputees and subjects with congenital limb deletion, reported
to have attempted to mentally simulate the movement of the
present hand ®rst. Fewer subjects, in addition to the
aforementioned strategy, claimed to have based their
response also on the thumb's orientation relative to the
wrist (31%).
At the time of testing, 12 amputees (A1, A2, A3, A4, A5,
A6, A7, A10, A11, A13, A14, A16) and one subject with
brachial plexus lesion (PB2) reported the presence of
phantom sensations in their daily life. Interestingly, the
phantom limb sensation was elicited or enhanced by the hand
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Accuracy for each participant was computed as the proportion of
correct responses out of valid trials. This value was submitted to
arcsine transformation and used for parametric analysis. Only RTs
corresponding to valid trials were considered for analyses. RTs were
submitted to logarithmic transformation in order to control for the
effects of a skewed distribution and satisfy the conditions for
parametric statistical test. For the purpose of the analysis, for each
viewpoint, the 12 different orientations were grouped in two classes
according to the dif®culty of the real movement required to reach
that posture (natural orientations, unnatural orientations), as judged
by 12 naive subjects (see above). In order to assess whether our
paradigm produced results congruent with previous reports
(Sekiyama, 1982; Parsons, 1987a, b), two separate three-way
ANOVAs for repeated measures (factors: hand presented ± left,
right; view ± back, palm, thumb and pinkie side; orientation ±
natural, unnatural) were run on RTs and proportion of correct
responses of control subjects only. Then, two separate four-way
ANOVAs were run on proportion of correct responses and RTs to
compare the control group with the amputees. The between-subjects
factor was group (three levels: C, controls; D, subjects having lost
their dominant limb; ND, subjects having lost their non-dominant
limb); within-subjects factors were the same as in the previous
analyses (hand 3 view 3 orientation). Finally, in order to evaluate
the effect of wearing a prosthesis on motor imagery, amputees were
grouped according to the loss of their dominant/non-dominant limb
and to the habit of wearing/not wearing a prosthesis. Two separate
four-way ANOVAs were run on proportion of correct responses and
RTs, respectively. Between-subjects factor was group (®ve levels: C,
control subjects; DP±, loss of dominant limb/not wearing prosthesis;
DP+, loss of dominant limb/wearing prosthesis; NDP±, loss of nondominant limb/not wearing prosthesis; NDP+, loss of non-dominant
limb/wearing prosthesis) and the within-subjects factors were again
hand 3 view 3 orientation.
Newman±Keuls Test was used for post hoc analysis of signi®cant
interactions.
124
D. Nico et al.
Table 2 Phantom phenomena in the subgroup of amputees experiencing modi®cations related to the task
Phantom limb type
Phantom pain
A1
Right arm (lower third) and
forearm (dominant limb)
amputation 2 years before
testing
Complete and normally
functioning upper limb,
present in daily life and
reported vividly while
performing the task
Soon after the accident acute Acute pain forcing frequent
breaks
pain persisting few months;
at the moment, painful sensation
appears only at rest and nighttime
A3
Complete limb loss including
shoulder joint (dominant
limb) 4 years before testing
Shortened limb present in
daily life and reported at the
moment of testing
Severe phantom pain soon
after the accident, reduced
following pharmacological
treatment; at present referred
as spasms and contractions of
the phantom limb
No painful sensations reported;
increased vividness of the
phantom limb that is perceived
as progressively elongating
A4
Right arm (lower third) and
forearm (dominant limb)
amputation 4 years before
testing
Sporadic perception of the
complete arm
Acute phantom pain soon
after the accident successfully
treated by brachial plexus
blockage
No painful sensation reported;
appearing of a vivid perception
of a static phantom arm while
solving the task
A14
Left arm (lower third) and
forearm (non-dominant limb)
amputation 3.1 years before
testing
Tightly contracted phantom
®ngers
Acute phantom pain since the
accident
Increased vividness of phantom
hand and ®ngers associated to
strong painful sensations
A16
Left hand and distal third of
forearm (non-dominant limb)
amputation 9.9 years before
testing
Moving phantom ®ngers;
the phantom palm has
progressively faded away
Phantom pain since the
accident, only partially
resolved by drugs; at present
episodic stump pain, more
frequent at rest
No painful sensation reported;
appearing of a vivid phantom
pinkie; the phantom hand does
not ®t prosthesis' location
recognition task in nearly half of those subjects (A1, A3, A4,
A14, A16), and in two of them (A1, A14) it was associated
with phantom pain (see Table 2 for details on phantom
sensations). In the latter two cases, subjects required several
breaks during the task in order to overcome the painful
sensation. A couple of subjects (A10, A11) reported actively
rotating their phantom limb as well as their present limb
during the experiment. Overall, subjective rating of task
dif®culty was higher among amputees than controls. Error
rate was low in both controls (2.7 6 2.2%) and amputees
(12.6 6 9.6%).
Control subjects
Results are summarized in Fig. 2 as the proportion of correct
responses (left panel) and RTs (right panel) for stimuli
depicting either a dominant (dark grey squares) or a nondominant hand (light grey squares) in the different views and
orientations.
Correct responses
As expected, a three-way ANOVA (hand 3 view 3
orientation) on proportion of correct responses of the control
group revealed a main effect of stimulus-hand [F(1,6) =
7.524, P < 0.004; dominant hand, 0.98 6 0.02; non-dominant
hand, 0.97 6 0.03] and orientation [F(1,6) = 5.736, P < 0.005;
natural orientation, 0.99 6 0.02; unnatural orientation, 0.97 6
0.02]. Control subjects gave signi®cantly more correct
responses to images of their dominant hand. Moreover,
Phantom sensations during the
task
natural orientations were more easily recognizable. The
interaction between view and orientation reached statistical
signi®cance [F(3,18) = 4.800, P < 0.02]. Post hoc analysis
showed that in the case of natural orientation, hands presented
in pinkie-side views were signi®cantly more dif®cult to
recognize than hands presented in more commonly adopted
postures, such as thumb (P < 0.05) or palm views (P < 0.05).
Response times
Analysis on RTs provided congruent results. An ANOVA
showed main effects of all factors: stimulus±hand [F(1,6) =
11.724, P < 0.02; dominant hand, 1191.39 6 124.94 ms; nondominant hand, 1272.72 6 172.12 ms]; view [F(3,18) =
11.331, P < 0.001; thumb side, 1102.57 6 108.14 ms; back,
1150.45 6 110.49 ms; palm, 1339.39 6 162.08 ms; pinkie
side, 1335.81 6 56.98 ms] and orientation [F(1,6) = 19.540,
P < 0.005; natural orientation, 1174.54 6 158.27 ms;
unnatural orientation, 1289.57 6 128.08 ms]. Namely, RTs
were faster when recognizing the dominant hand in the most
natural orientations and views. The interaction between view
and orientation was also signi®cant [F(3,18) = 4.619, P <
0.02]. As previously described for correct responses, post hoc
test showed that, for natural orientations, recognition of hands
presented in pinkie-side views required signi®cantly longer
RTs than that of hands presented in more usual perspectives,
such as thumb (P < 0.0003) or back views (P < 0.0005).
Parsons' study of movement time to these positions (for
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Subject Limb loss
Left-right hand recognition in amputees
125
blocks of trials when the handedness of the stimuli was
known by healthy subjects), showed that movement time to
the pinkie side was longer than to the other views (Parsons,
1994). Thus, while visual unfamiliarity may be a contributing
factor to the slower RTs (and somewhat less accurate
responses), joint constraints and unfamiliar movement trajectories are likely to be primarily responsible for the greater
mental simulation times and subsequent perceptual errors.
Moreover, for unnatural orientations palm views required
signi®cantly longer RTs than thumb (P < 0.02) and back
views (P < 0.04).
Summing up, in agreement with previous studies, control
subjects produced slower and less accurate responses when
judging a stimulus that depicted a hand in an unnatural
orientation. Independent of this effect, control subjects were
faster and more accurate judging stimuli portraying their
dominant hand.
Control subjects versus amputees: effect of side
of amputation
Correct responses
To examine whether the loss of the dominant limb versus the
non-dominant limb had different effects on motor imagery
and handedness judgment, amputees were grouped according
to laterality of limb loss. A four-way ANOVA on proportion
of correct responses showed main effects of group [F(2,20) =
4.304, P < 0.03; controls, 0.98 6 0.02; D amputees, 0.87 6
0.008; ND amputees, 0.90 6 0.06], view [F(3,60) = 5.283,
P < 0.003; thumb side: 0.93 6 0.12 back: 0.92 6 0.15, palm:
0.93 6 0.10 and pinkie side: 0.88 6 0.17] and orientation
[F(1,20) = 15.598, P < 0.001; natural orientation, 0.94 6
0.11; unnatural orientation, 0.89 6 0.16]. Namely, subjects
who had lost their dominant limb made signi®cantly more
errors in the task than controls (P < 0.02). On the contrary,
amputees of the non-dominant limb did not differ from
control subjects on overall measures. Unfamiliar postures, i.e.
pinkie-side view, and unnatural orientations produced signi®cantly more errors in all groups. Interaction between view
and orientation was also signi®cant [F(3,60) = 4.466, P <
0.007]. Post hoc test con®rmed that fewer correct responses
were reported by all participants for unnatural orientations
(for all views: pinkie, 0.87 6 0.20; thumb, 0.89 6 0.14; back,
0.89 6 0.17; palm, 0.90 6 0.12). Accuracy increased for
natural orientations, except for one condition: hands presented in pinkie side views (0.89 6 0.14) were signi®cantly
more dif®cult to recognize than hands presented in more
usual perspectives, such as thumb (0.97 6 0.08, P < 0.0002),
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Fig. 2 Control subjects. Proportion of correct responses (left panel) and corresponding response times (in ms, right panel) reported by
control subjects. Dark grey squares correspond to responses given to line-drawings depicting dominant hands, light grey squares to those
depicting non-dominant hands. The averages for the six natural and the six unnatural orientations are presented for each viewpoint. Error
bars represent standard deviations.
126
D. Nico et al.
back (0.95 6 0.12, P < 0.0005) and palm (0.95 6 0.07,
P < 0.003).
More interestingly, as suggested by the signi®cant interaction between group, view and orientation [F(6,60) = 2.318,
P < 0.05], a difference emerged among groups (see Fig. 3 for
details, left panel). When line drawings depicted pinkie-side
views and unnatural back views, subjects having suffered
amputation of the dominant limb were signi®cantly more
impaired than those having lost their non-dominant limb
(pinkie, P < 0.0003; back, P < 0.04) and control subjects
(pinkie, P < 0.0002; back, P < 0.0002). No difference
emerged between the latter two groups.
Response times
The same analysis on RTs showed main effects of all factors:
group [F(2,20) = 4.253, P < 0.03; controls, 1232.05 6
151.24 ms; D amputees, 2018.12 6 315.42 ms; ND amputees,
1616.61 6 181.32 ms], stimulus-hand [F(1,20) =15.383, P <
0.001; dominant hand, 1572.86 6 712.13 ms, non-dominant
hand, 1670.68 6 675.73 ms], view [F(3,60) = 11.419, P <
0.00001; thumb side, 1476.09 6 550.87; back, 1606.8 6
869.30; palm, 1687.74 6 665.42; pinkie side, 1713.70 6
640.95] and orientation [F(1,20) = 26.516, P < 0.0001;
natural orientation, 1501.35 6 561.83; unnatural orientation,
1742.19 6 789.77] (means and standard deviations are
summarized in Fig. 3, right panel). More precisely, subjects
having lost the dominant limb were signi®cantly slower than
controls (P < 0.02). Recognition of the non-dominant hand as
well as of unfamiliar postures, i.e. pinkie-side view, and
unnatural orientations required signi®cantly longer RTs in all
groups. This con®rms the ®nding that the movement time to
the natural orientations of the pinkie-side view is longer than
to the natural orientations of the other stimulus views, in
accordance with joint constraints and unfamiliar trajectories
(Parsons, 1994). Interaction between view and orientation
was signi®cant [F(3,60) = 10.109, P < 0.0001]. Post hoc test
on the signi®cant interaction con®rmed that pinkie-side views
were dif®cult to identify even in natural orientations and
required signi®cantly longer RTs (1696.85 6 1730.56) than
thumb-side (1371.38 6 500.23, P < 0.0001), back (1426.07 6
580.18, P < 0.0001) and palm views (1511.09 6 555.25, P <
0.01). As for unnatural orientations, thumb-side views
required shorter RTs (1580.79 6 583.97) than the other
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Fig. 3 Effect of dominant-limb loss. Proportion of correct responses (left panel) and corresponding response times (in ms, right panel)
given by amputees of the dominant limb (black circles), non-dominant limb (grey squares) and control subjects (grey area). Average for
the six natural and the six unnatural orientations are presented for each viewpoint. Error bars represent standard deviations.
Left-right hand recognition in amputees
127
perspectives (back, 1787.54 6 1060.55, P < 0.004; palm,
1869.86 6 732.03, P < 0.0001; pinkie 1730.56 6 709.84, P <
0.005), although they were more dif®cult to recognize
compared with the corresponding natural orientation (P <
0.0002).
In summary, subjects having suffered amputation of the
dominant limb made more errors and were signi®cantly
slower in solving the task compared with control subjects and
amputees of the non-dominant limb. In particular, as can be
seen in Fig. 3, amputees of the dominant limb were typically
more impaired in recognition of unnatural or uncommon
postures. Neither the age of subjects, nor elapsed time since
amputation, were correlated with performance in the task.
Control subjects versus amputees: effect of
wearing a prosthesis
Correct responses
To evaluate the role of the prosthesis in the mental simulation
of body-part movements required by the task, amputees were
grouped according to the habit of wearing (n = 9) or not
wearing a prosthesis (n = 7, see Table 1). A four-way
ANOVA on proportion of correct responses revealed a
signi®cant interaction between group and orientation [F(4,18)
= 4.325, P < 0.02]. In addition, there were the expected
effects of both view [F(3,54) = 5.634 P < 0.002; thumb 0.92 6
0.07; back 0.91 6 0.08; palm 0.92 6 0.06; pinkie 0.88 6
0.09] and orientation [F(1,18) = 20.719 P < 0.0003; natural
0.93 6 0.06; unnatural 0.87 6 0.09], as well as their
interaction [view 3 orientation: F(3,54) = 3.301, P < 0.03].
As can be seen in Fig. 4 (left panel), and is con®rmed by post
hoc analysis, an interesting effect of wearing a prosthesis was
found on subjects' responses: the number of correct responses
was signi®cantly reduced in subjects wearing a prosthesis
(®lled symbols) compared with controls (grey area). This
reduction was particularly striking for amputees of the
dominant hand when judging on unnatural postures (P <
0.0002), but it emerged also in amputees of the non-dominant
limb for both natural (P < 0.04) and unnatural postures (P <
0.04). Note that amputees of the non-dominant limb not
wearing any prosthesis did not differ from controls.
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Fig. 4 Effect of wearing prostheses. Proportion of correct responses (left panel) and corresponding response times (in ms, right panel)
given by amputees of the dominant limb (circles), non-dominant limb (squares) and control subjects (grey area). Filled symbols refer to
amputees wearing a prosthesis, empty symbols to amputees not wearing a prosthesis. The averages for the six natural and the six
unnatural orientations are presented. Error bars represent standard deviations.
128
D. Nico et al.
Table 3 Proportion of correct responses and RTs (in ms, second line) produced by subjects
with congenital limb deletion, subjects having suffered from brachial plexus lesion and
control subjects
Congenital
limb deletion
Present hand
Natural
Unnatural
Natural
Unnatural
CD1
0.93
1182.41
0.91
1469.15
1.00
1427.21
0.93
1617.19
0.96
1473.65
0.98
1646.87
1.00
1548.23
0.93
1646.98
0.98
1601.88
1.00
1499.15
1.00
1684.12
1.00
1571.69
CD2
CD3
Missing hand
Dexterous hand
Natural
Unnatural
Natural
Unnatural
PB1
0.88
2013.92
0.77
1355.11
0.79
2634.42
0.81
1507.16
0.75
2505.23
0.80
1203.26
0.71
2757.02
0.55
1526.99
PB2
Plegic hand
Control
subjects
Dominant hand
Natural
Unnatural
Natural
Unnatural
Mean
0.99
1149.58
0.04
265.07
0.98
1233.20
0.05
261.73
0.98
1199.50
0.05
285.32
0.96
1345.93
0.07
337.02
SD
Non-dominant hand
Data are presented for both stimulus-hands depicted in natural and unnatural orientations.
Response times
Analysis of RTs showed a signi®cant main effect of all
factors: group [F(4,18) = 3.485, P < 0.03; controls, 1232.05 6
151.24 ms; DP+ amputees, 2373.36 6 494.2 ms; NDP+
amputees, 1732.78 6 164.29; DP± amputees: 1544.46 6
146.85 ms; NDP± amputees, 1471.39 6 217.09 ms); stimulus
hand [F(1,18) = 15.601, P < 0.001; dominant hand, 1621.05 6
469.75 ms; non-dominant hand, 1720.56 6 468.72 ms]; view
[F(3,54) = 8.720, P < 0.0001; thumb side, 1667.66 6 613.88;
back, 1726.25 6 429.37; palm, 1762.54 6 447.82; pinkie
side, 1580.72 6 444.29 ms]; and orientation [F(1,18) =
30.184, P < 0.0001; natural orientation, 1542.16 6 344.88;
unnatural orientation, 1799.46 6 540.88 ms]. Namely,
subjects wearing a prosthesis were overall slower than
controls in responding: this was particularly true for amputees
of the dominant limb (®lled symbols) compared with controls
(grey area, P < 0.04, see Fig. 4).
Only the interaction between view and orientation was
signi®cant [F(3,54) = 8.771, P < 0.0001; thumb natural,
1371.38 6 500.23 ms; thumb unnatural, 1580.79 6
583.97 ms; back natural, 1426.07 6 580.18 ms; back
unnatural, 1787.54 6 1060.55 ms; palm natural, 1511.09 6
555.25 ms; palm unnatural, 1869.86 6 722.03; pinkie natural,
1696.85 6 571.24 ms; pinkie unnatural, 1730.56 6
709.84 ms).
In conclusion, presence of a prosthetic limb signi®cantly
degraded performance in the present task, as shown by the
increase of both errors and RTs. This effect was more
pronounced for those subjects who lost their preferred limb
and for responses to unnatural postures.
Control for limb loss
As a control for limb loss, we recruited three subjects
presenting congenital limb deletion or having suffered from a
lesion of the brachial plexus. Interestingly, those subjects,
who never experienced presence of one upper limb,
responded almost like control subjects (see Table 3 and
Fig. 5) and did not differ from them as for proportion of
correct hits. However, their RTs were overall slower than
those of controls (see Fig. 5). Interestingly, these subjects did
not show a tendency for longer RTs for unnatural postures for
the deleted hand, but did show that tendency for the present
hand. This suggests that the congenital absence of the limb
precludes the ability to produce joint-constrained mental
simulations for the deleted hand like those available for the
present hand. At the same time, the high accuracy of the
congenital deletion subjects may be a consequence of a
strategy in which they judge the stimulus by always
comparing it with their present hand: a mismatch in shape
(a discon®rmation) implies that the stimulus must be the other
hand. This interpretation is consistent with their subjective
reports. Use of a successful discon®rmation decision strategy
contrasts with the performance described in split brain
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Brachial
plexus lesion
Left-right hand recognition in amputees
129
patients (Parsons et al., 1998), who could judge with high
accuracy only hands from the side of the body contralateral
(and not ipsilateral) to the hemisphere viewing the stimuli.
This difference indicates that each hemisphere of the split
brain alone could not compare the hand for which it had
accurate motor imagery to the viewed stimulus, in order to
make successful use of a discon®rmation strategy.
An acquired peripheral loss of upper limb function, i.e.
following lesion of the brachial plexus, strongly reduced
performance in both the examined subjects (see Table 3 and
Fig. 6), to a larger extent than in case of amputees wearing a
prosthesis. The proportion of correct responses was quite
small in both subjects, especially for items depicting the
affected limb in unnatural postures. A consistent increase in
RTs to the paralysed limb was also found in one subject
(PB1).
Discussion
It has been demonstrated in a variety of ways that a strong
correlation exists between properties of motor imagery and
actual motor behaviour (for reviews, see Decety and Ingvar,
1990; Jeannerod and Decety, 1995; Crammond, 1997).
However, to our knowledge the nature of the exact relationship between accuracy in mental simulation of a movement
and actual state of the body is still poorly understood.
Neuropsychological data on patients suffering from motor
disabilities secondary to CNS damage suggest that motor
imagery may take into account, as well as ignore, alterations
affecting the motor system depending on the task (Dominey
et al., 1995; Sirigu et al., 1996; Johnson, 2000, Johnson et al.,
2002).
In the present study, we explored this issue by examining
the case of peripheral modi®cations of the sensorimotor
system. By contrast with previous studies, we assessed how
motor imagery is affected by the physical absence of a motor
equivalent in subjects who have no documented history of
neurological impairment or CNS damage. Our subjects, who
underwent amputation of either the dominant or nondominant upper limb, were required to judge whether the
image of a hand corresponded to a right or a left hand. It has
been shown that this task implicitly activates motor imagery
of the corresponding limb (Parsons, 1987a, b, 1994). Our
study provides new information that can be summarized as
follows. First, the loss of one limb does not prevent the ability
to judge handedness, although it signi®cantly increases task
dif®culty. Secondly, the loss of the dominant limb is a main
source of perturbation, because the task is signi®cantly more
dif®cult for amputees who have lost their preferred hand.
Thirdly, the everyday use of a prosthetic arm has a
detrimental effect on the left/right judgement of a hand.
Our ®rst ®nding con®rms that, even if no explicit
movement has to be performed, the handedness judgement
task activates the motor system. The reported re-activation of
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Fig. 5 Congenital limb loss. Proportion of correct responses (left panel) and corresponding response times (in ms, right panel) given by
subjects with congenital limb deletion (n = 3, grey triangles) and controls (n = 7, white squares). Average for the six natural and the six
unnatural orientations are presented. Error bars represent standard deviations. All subjects with congenital limb loss were missing the left
forearm, all controls subjects (except one) were right-handers.
130
D. Nico et al.
extinguished phantom limb sensations in some amputees
strongly support the claim that motor commands to the
missing limb are elicited by our task and are still effective.
This result is consistent with the report by Ramachandran and
colleagues that false visual feed-back provided by a mirror
image of the present limb can induce the feeling of motion in
a previously extinguished phantom limb (Ramachandran,
1996). In addition, our data show that absence of the real
effector reduces ef®ciency in motor mental simulation. There
are alternative explanations of this observation.
The ®rst possible explanation is that loss of a limb degrades
the performance of the neural mechanisms that normally
underlie movement and mental simulation. It is conceivable
that the mental operation required by the task elicits a motor
command that activates a predictive model of the ®nal state,
i.e. the posture that would eventually be achieved (Wolpert
et al., 1999). In the absence of one limb, even if streams of
motor commands can still be issued, no incoming information
from the periphery is available. Thus, the feed-forward
mechanism is no longer supported by information about
either initial or ®nal position of the missing hand. Without
this mechanism of support for mentally simulating the
movement of the missing limb, the subjects may resort to
using an alternative strategy for stimuli representing that
limb. If so, then the additional time required by amputees to
solve the task may re¯ect use of a `visual' imagery approach
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
Fig. 6 Brachial plexus lesion. Upper panels: proportion of correct responses (left) and corresponding response times (in ms, right) given
by subject PB1, who suffered brachial plexus lesion of the non-dominant limb (grey triangles), compared with non-dominant limb
amputees wearing a prosthesis (grey squares) and controls (grey area). Lower panels: proportion of correct responses (left) and
corresponding response times (right) given by subject PB2, who suffered brachial plexus lesion of the dominant limb (grey triangles),
compared with dominant limb amputees wearing a prosthesis (black circles) and controls (grey area). Average for the six natural and the
six unnatural orientations are presented. Error bars represent standard deviations.
Left-right hand recognition in amputees
a mental simulation of the real movement; namely, subjects
mentally rotate their limb in order to match the orientation of
the hand to be judged. It has been proposed that subjects
implicitly choose by `guessing' which hand to move ®rst.
After this automatic selection, a second explicit con®rmatory
phase would follow (Parsons, 1994; Parsons et al., 1995;
Gentilucci et al., 1998). Our data are consistent with the
possibility that, during the ®rst phase, our subjects automatically select by default their dominant, preferred hand. If so,
then when the subjects' dominant limb is missing, they need
to either switch to a visual imagery strategy, as previously
suggested, or to use motor simulation by the present limb.
Both of the latter operations would be likely to induce an
increase in RTs and incorrect judgments. This hand preference for motor imagery is consistent with reports that motor
asymmetries are present in the mental domain, affecting
mental simulation tasks (Maruff et al., 1999) and movement
attribution (Daprati and Sirigu, 2002). Moreover, in the
present experiment, this advantage for stimuli depicting the
dominant hand is supported by converging evidence: indeed,
control subjects respond faster to stimuli depicting their
dominant hand. Furthermore, subjects who suffered amputation of the non-dominant hand are not slower than controls.
A third possible explanation of the less ef®cient performance of the dominant-limb amputees is that loss and disuse of
long-standing sensorimotor processes for the dominant limb
may degrade in a general way the ef®ciency of both dominant
and non-dominant upper limb motor behaviour and imagery.
This is conceivable if the processes for the dominant limb,
which are localized primarily in the dominant cerebral
hemisphere, serve as the basis of abstract initial planning of
all limbs motor behaviour. These three alternative accounts
lead to predictions that could be readily tested in future
studies.
The last ®nding we report is a surprising effect on imagery
of wearing a prosthesis. In the nine amputees used to wearing
a prosthesis daily, performance was signi®cantly slower and
less accurate than for controls and amputees not wearing a
prosthesis. Most of these subjects used an aesthetic prosthesis, i.e. a rubber forearm and hand, or a mechanic device
allowing a pinch grip by means of a shoulder movement that
stretches a strip passing over the shoulder blade (see Table 1).
Only two subjects were equipped with a myo-electric
prosthesis allowing thumb opposition and wrist rotation by
means of the contraction of the residual forearm muscles.
Thus, this latter device affords movements that are biomechanic analogues of a few basic hand movements.
Interestingly, performance of these two subjects was slightly
better than the other amputees wearing prostheses.
Although conclusions must be drawn very cautiously from
such a small sample, our data suggest that prostheses interfere
with imagery when mental simulation of a movement is
required. However, when the prosthesis can be used as an
analogue of the missing hand, namely when it possesses a
natural functionality, interference can be reduced. In this
case, movement of the arti®cial hand re-establishes the
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
(i.e. a visual-spatial strategy rather than a motor-kinaesthetic
one). Thus, in order to match the most dif®cult orientations,
amputees may not use the strategy of mentally rotating their
own hand to match the stimulus and choose instead to rotate
the stimulus-hand as an object. However, on more familiar
hand orientations, they may use a simple visual matching
strategy. The latter possibility may be consistent with the
reduction of errors for stimuli depicting familiar positions.
An alternative explanation is that amputees are slower in
solving the task because of the change in body schema
produced by the amputation. Indeed, limb loss may increase
the dif®culty of recognizing the missing hand because visual
familiarity of the corresponding limb is no longer available.
Moreover, this handedness judgement task requires manipulation of the internal representation of a body part and is
known to activate brain areas devoted to somatic representation and body knowledge (Bonda et al., 1995; Parsons et al.,
1995; Kawamichi et al., 1998). Since most of our amputees
experienced phantom sensations in everyday life or during
the task, it is possible that these subjects' body image was still
being adjusted to re¯ect the peripheral changes in their body,
and perhaps this made it dif®cult to select the best strategy to
solve the task. However, both of these body schema
interpretations are contradicted by the ®ndings on subjects
with congenital limb deletion or brachial plexus lesion.
Indeed, even when one limb is absent from birth and
proprioceptive feed-back for movement has never been
experienced, subjects are slower in judging the missing
hand than the present one and show no prolonged RT effects
related to joint-constrained motor imagery to awkward
stimulus postures. This suggests that they are using alternative strategies, perhaps based on visual±spatial reasoning.
Similarly, the two subjects suffering from brachial plexus
lesion have lost the sensory-motor potential of their affected
limb, still have its visual experience and a continuous feedback of its presence, but are equally impaired in the task.
Taken together, the performance of the experimental group
emphasizes the effective role of availability of an intact motor
potential. This effect may derive from the apparent requirement for motor commands to be issued, which thereby elicit
failed or impoverished checks (on the basis of available
proprioception) for feed-forward commands (Blakemore
et al., 2002).
The second new ®nding here is that the left/right
handedness judgments are slower and less accurate after
loss of dominant limb than of non-dominant one. The longer
RTs for dominant-limb amputees could not be attributed to an
effect of the hand subjects used to respond, since no manual
response was required. One alternative possibility is that the
dominant-limb amputees used the visual±spatial strategy
discussed earlier, which is more cognitively demanding (thus
slower and more error prone). A second possible account is
that amputees solved the task by mentally simulating
movements of their preferred (missing) hand. Previous
research (Sekiyama, 1982; Parsons, 1987a, b) has demonstrated that the most common strategy to judge handedness is
131
132
D. Nico et al.
Acknowledgements
The authors wish to thank all the subjects who agreed to
participate in the present study, as well as to the medical and
technical staff of the HoÃpital des Massues (Lyon, France) for
their helpful assistance and Guillaume Fond for his help in
collecting data. The research was supported by CNRS.
References
Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of
action. Trends Cogn Sci 2002; 6: 237±42.
Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental
transformations of the body-in-space. Proc Natl Acad Sci USA 1995; 92:
11180±4.
Crammond DJ. Motor imagery: never in your wildest dream. Trends
Neurosci 1997; 20: 54±7.
Daprati E, Sirigu A. Laterality effects on motor awareness.
Neuropsychologia 2002; 40: 1379±86.
Decety J, Ingvar DH. Brain structures participating in mental simulation of
motor behavior: a neuropsychological interpretation. Acta Psychol (Amst)
1990; 73: 13±34.
Decety J, Jeannerod M, Prablanc C. The timing of mentally represented
actions. Behav Brain Res 1989; 34: 35±42.
Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, et al.
Mapping motor representations with positron emission tomography.
Nature 1994; 371: 600±2.
Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M. Motor imagery
of a lateralized sequential task is asymmetrically slowed in hemiParkinson's patients. Neuropsychologia 1995; 33: 727±41.
Gentilucci M, Daprati E, Gangitano M. Right-handers and left-handers have
different representations of their own hand. Brain Res Cogn Brain Res
1998; 6: 185±92.
Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, et al.
Partially overlapping neural networks for real and imagined hand
movements. Cereb Cortex 2000; 10: 1093±104.
Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp
representations in humans by positron emission tomography. II.
Observation compared with imagination. Exp Brain Res 1996; 112:
103±11.
Iriki A, Tanaka M, Iwamura Y. Coding of modi®ed body schema during tool
use by macaque postcentral neurones. Neuroreport 1996; 7: 2325±30.
Iriki A, Tanaka M, Obayashi S, Iwamura Y. Self-images in the video
monitor coded by monkey intraparietal neurons. Neurosci Res 2001; 40:
163±73.
Jeannerod M. Mental imagery in the motor context. Neuropsychologia 1995;
33: 1419±32.
Jeannerod M, Decety J. Mental motor imagery: a window into the
representational stages of action. Curr Opin Neurobiol 1995; 5: 727±32.
Johnson SH. Imagining the impossible: intact motor representations in
hemiplegics. Neuroreport 2000; 11: 729±32.
Johnson SH, Sprehn G, Saykin AJ. Intact motor imagery in chronic upper
limb hemiplegics: evidence for activity-independent action
representations. J Cogn Neurosci 2002, 14: 841±52.
Kawamichi H, Kikuchi Y, Endo H, Takeda T, Yoshizawa S. Temporal
structure of implicit motor imagery in visual hand-shape discrimination as
revealed by MEG. Neuroreport 1998; 9: 1127±32.
Maruff P, Wilson PH, De Fazio J, Cerritelli B, Hedt A, Currie J.
Asymmetries between dominant and non-dominant hands in real and
imagined motor task performance. Neuropsychologia 1999; 37: 379±84.
Old®eld RC. The assessment and analysis of handedness: the Edinburgh
inventory. Neuropsychologia 1971; 9: 97±113.
Parsons LM. Imagined spatial transformation of one's body. J Exp Psychol
Gen 1987a; 116: 172±91.
Parsons LM. Imagined spatial transformations of one's hands and feet.
Cognit Psychol 1987b; 19: 178±241.
Parsons LM. Temporal and kinematic properties of motor behavior re¯ected
in mentally simulated action. J Exp Psychol Hum Percept Perform 1994;
20: 709±30.
Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, et al. Use
of implicit motor imagery for visual shape discrimination as revealed by
PET. Nature 1995; 375: 54±8.
Parsons LM, Gabrieli JD, Phelps EA, Gazzaniga MS. Cerebrally lateralized
mental representations of hand shape and movement. J Neurosci 1998; 18:
6539±48.
Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom
limbs induced with mirrors. Proc R Soc Lond B Biol Sci 1996; 263:
377±86.
Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C,
et al. Possible involvement of primary motor cortex in mentally simulated
movement: a functional magnetic resonance imaging study. Neuroreport
1996; 7: 1280±84.
Sekiyama K. Kinesthetic aspects of mental representations in the
identi®cation of left and right hands. Percept Psychophys 1982; 32:
89±95.
Sirigu A, Duhamel JR. Motor and visual imagery as two complementary but
neurally dissociable mental processes. J Cogn Neurosci 2001; 13:
910±19.
Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y, et al.
Congruent unilateral impairments for real and imagined hand movements.
Neuroreport 1995; 6: 997±1001.
Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental
representation of hand movements after parietal cortex damage. Science
1996; 273: 1564±8.
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann
AO, Frith CD, et al. Functional anatomy of the mental representation of
upper extremity movements in healthy subjects. J Neurophysiol 1995; 73:
373±86.
Thobois S, Dominey PF, Decety PJ, Pollak PP, Gregoire MC, Le Bars PD,
et al. Motor imagery in normal subjects and in asymmetrical Parkinson's
disease: a PET study. Neurology 2000; 55: 996±1002.
Wolpert DM. Computational approaches to motor control. Trends Cogn Sci
1997; 1: 209±16.
Received April 24, 2003. Revised July 31, 2003.
Accepted August 1, 2003
Downloaded from http://brain.oxfordjournals.org/ by guest on October 2, 2016
possibility to update predictions issued by the motor out¯ow
(Wolpert, 1997), by showing (on-line) the effect of the
forearm muscles' contraction. In contrast, an aesthetic
prosthesis provides a visual feedback that emphasizes the
ineffectiveness of motor commands, thus interfering with
motor simulation. A similar effect is induced by the presence
of a deafferented limb (i.e. following brachial plexus lesion).
Indeed, our data show that performance of these subjects does
not differ from that of amputees wearing an aesthetic
prosthesis. This implies that a prosthesis can be incorporated
in the body schema (and eventually improve mental simulation of a movement) when it works as a tool. This result
would be in close agreement with studies on monkeys which
showed that tools can become part of the body, being
included in its representation (Iriki et al., 1996, 2001). These
®ndings have important implications for prostheses applications, which are currently emerging via technology-intensive
neural engineering approaches to assistive technologies.